Introduction
Hepatocellular carcinoma (HCC) is the most common
primary hepatic malignancy and is the third leading cause of
cancer-associated mortality globally (1). HCC is a remarkably heterogeneous entity,
at the molecular and clinical level, with a variety of pathogenetic
mechanisms that have not been completely elucidated (2). Therefore, it is necessary to identify
the underlying mechanisms driving HCC, in order to identify
appropriate treatment targets.
Numerous genetic alterations result in the
dysfunction of cell growth and migration pathways and have been
demonstrated to be associated with HCC progression (3). An increasing number of studies
demonstrated that liver kinase B1 (LKB1) loss and mutations are
identified in various cancer types, including lung, mammary gland
and ovarian cancer, melanoma and HCC (4–6). However,
the mechanism by which LKB1 functions is not completely
understood.
Epithelial-mesenchymal transition (EMT) is a key
process for cancer invasion and metastasis (7). EMT is a process that is activated by
EMT-inducing transcription factors such as SNAIL, Twist and Zinc
Finger E-Box Binding Homeobox (ZEB). Previous studies have
demonstrated the potency of ZEB1 (8,9). ZEB1 is
associated with aggressive behavior, invasion, drug resistance and
poor prognosis in various cancer types, including lung, pancreatic
and breast cancer (7,10).
The Yes-associated protein (YAP) is a
transcriptional factor of the Hippo signaling pathway (11). A number of studies have supported a
critical role for YAP in different types of cancer, including
breast and lung cancer and pancreatic ductal adenocarcinoma (PDAC)
(12,13). YAP has been demonstrated to be an
essential promoter of the mutant KRAS oncogenic program,
specifically inducing the expression of secreted factors such as
CTGF and CYR61, and interacting with FOS, a proto-oncogene, to
regulate the expression of EMT-associated genes such as E-cadherin,
SLUG, SNAIL and Vimentin (13,14). These
pieces of evidence suggest that YAP serves an important role in
EMT. Recent studies have reported that YAP is activated in
LKB1-mutant tumors (15–19). Further functional studies have
reported that YAP as an oncogenic driver is often hyper activated
in a wide number of human cancer types, including HCC and melanoma
(14,19,20).
However, the role of YAP in HCC progression in the context of
LKB1-deficient HCC remains elusive.
The present study demonstrated that the
overexpression of LKB1 in the HCC cell line Hep3B leads to the
decreased motility and invasiveness of cells. Functional evidence
is presented that suggests ZEB1 is an important mediator of
LKB1-loss-induced EMT progression. Further study indicated YAP as a
downstream coactivator of ZEB1 in LKB1-deficient HCC malignant
progression. The present study indicated ZEB1 as a direct mediator
of YAP and determined a critical role of YAP in HCC.
Materials and methods
Plasmids
Plasmids were constructed using the following
primers: LKB1 forward, 5′-GCGCTAGCATGGAGGTGGTGGACCCG-3′ and
reverse, 5′-GCGCGGCCGCTCACTGCTGCTTGCAGGCC-3′; ZEB1 forward,
5′-GCGCTAGCATGGCGGATGGCCCCAGG-3′ and reverse,
5′-GCGCGGCCGCTTAGGCTTCATTTGTCTTTTCTTCAG-3′; The PCR product was
purified and cloned into a pCDH lentivirus vector, and positive
clones were sent for DNA sequencing to confirm there were no
mutations. PCDH lentivirus vector was used as the empty vector
control. For RNAi, the LKB1 RNAi sequence was as follows:
5′-UAGUUGAAUUUCCUUCUUCUU-3′; ZEB1 RNAi, 5′-GGUAGAUGGUAAUGUAAUA-3′;
Scramble sequence, 5′-CATGATGATGGGCTCCATA-3′. For luciferase
activity assay, the promoter (−2,000+ 5′UTR) of YAP was cloned into
a pGL3-Basic vector (Promega Corporation, Madison, WI, USA).
pGL3-SV40-Renilla plasmid (Promega Corporation) was used as
the internal control.
Cell culture
The CRL-11233 cell line (American Type Culture
Collection, Manassas, VA, USA) was cultured in bronchial epithelia
growth medium (BEGM) supplemented with a BEGM Bullet kit (Lonza
Group, Ltd., Basel, Switzerland). The human hepatocellular
carcinoma cell line Hep3B (American Type Culture Collection) was
cultured in Eagle's minimum essential medium (EMEM cat, no.
30-2003; American Type Culture Collection) +2 mM glutamine +1%
Non-Essential Amino Acids (NEAA) +10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cultures
were maintained in a humidified chamber at 5% CO2 and
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA of CRL-11233 and Hep3B cells treated as
indicated was extracted using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.). First strand cDNA was synthesized
using a Reverse Transcription kit (iScript™ Reverse
Transcription Supermix for RT-qPCR; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocols. Gene
expression of cells treated as indicated was detected with a QPCR
kit (iQ™ SYBR®-Green Supermix; Bio-Rad
Laboratories, Inc.), according to the manufacturer's protocols. All
qPCR was performed at 95°C for 3 min, and then 40 cycles of 95°C
for 15 sec and 60°C for 1 min using the CFX 96 realtime PCR machine
(Bio-Rad Laboratories, Inc.). The specificity of the reaction was
verified by melt curve analysis (21). The relative quantitation of each gene
was performed using the comparative ∆∆Cq method (21). Primer sequences were as follows:
LKB1 forward, 5′-AGTCCAACATCACCATGCAG-3′ and
reverse, 5′-TTCCCTTTCCTCGAACTGATTT-3′; SNAIL forward,
5′-ACAAGCACCAAGAGTCCG-3′ and reverse, 5′-ATGGCAGTGAGAAGGATGTG-3′;
SLUG forward, 5′-ACTGCTCCAAAACCTTCTCC-3′ and reverse,
5′-TGTCATTTGGCTTCGGAGTG-3′; TWIST1 forward,
5′-CTCAGCTACGCCTTCTCG-3′ and reverse,
5′-ACTGTCCATTTTCTCCTTCTCTG-3′; ZEB1 forward,
5′-ACCCTTGAAAGTGATCCAGC-3′ and reverse,
5′-CATTCCATTTTCTGTCTTCCGC-3′; ZEB2 forward,
5′-GCCATCTGATCCGCTCTTATC-3′ and reverse,
5′-ACCTGTGTCCACTACATTGTC-3′; AXL forward,
5′-TTTATGACTATCTGCGCCAGG-3′ and reverse,
5′-TGTGTTCTCCAAATCTTCCCG-3′; CTGF forward, 5′-ACCAATGACAACGCCTCC-3′
and reverse, 5′-TTGGAGATTTTGGGAGTACGG-3′; SDPR forward,
5′-TCAAAGAGCGCATGGATAGG-3′ and reverse, 5′-TGGCAGGGATCTCATTTTCC-3′;
YAP forward, 5′-AAGCTGCCCGACTCCTTCTTCAAG-3′ and reverse,
5′-TGAGCTCGAACATGCTGTGGAGTCAG-3′; GAPDH forward,
5′-AGGTGAAGGTCGGA-3′ and reverse, 5′-TTGAGGTCAATGAAG-3′.
Western blot analysis
CRL-11233 and Hep3B cells were washed in PBS and
lysed in radioimmunoprecipitation assay buffer [25 mM Tris (pH
7.4), 150 mM NaCl, 0.5% sodium deoxycholate and 1% Triton X-100]
with the addition of proteinase inhibitors (Pierce; Thermo Fisher
Scientific, Inc.). The protein concentration determination was
performed using bicinchoninic acid method (Pierce; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Samples (30 µg) were loaded per well. Polyacrylamide gels (4–12%)
were used and the proteins in the gels were transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked with 5% non-fat milk in TBS with 0.05%
Tween-20 (TBST) at room temperature for 1 h, and the indicated
primary antibodies were incubated at 4°C overnight whilst shaken.
Membranes were washed in TBST for 10 min 3 times and incubated with
secondary antibody at room temperature for 1 h, and then the
membranes were washed in TBST for 10 min 3 times. The blots were
detected using Pierce™ ECL Western Blotting Substrate (cat. no.
32106, Thermo Fisher Scientific, Inc.). Antibodies were as follows:
Rabbit anti-LKB1 (ab199970; Abcam, Cambridge, UK), rabbit anti-ZEB1
(HPA027524; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), mouse
anti-YAP (sc-101199), goat anti-CTGF (sc-14939), rabbit anti-ZEB2
(sc-48789), β-actin (sc-47778; all Santa Cruz Technology, Inc.,
Dallas, TX, USA), rabbit anti-AXL (cat. no. 8661), rabbit
anti-SNAIL (cat. no. 3879), rabbit anti-SLUG (cat. no. 9585) and
rabbit anti-Twist (cat. no. 46702; all Cell Signaling Technology,
Inc., Danvers, MA, USA). The β-actin signal was used as a loading
control. Secondary Antibodies were: Donkey anti-rabbit, horseradish
peroxidase (HRP)-conjugate (sc-2313); donkey anti-mouse,
HRP-conjugate (sc-2314); and donkey anti-goat, HRP-conjugate
(sc-2020) (1:5,000; Santa Cruz Technology, Inc.). The densitometric
analysis was conducted with ImageJ software (ImageJ bundled with
64-bit Java 1.8.0_112; National Institutes of Health, Bethesda, MD,
USA).
In vitro Transwell™ assay
CRL-11233 and Hep3B cells were starved overnight and
then seeded (1×105 cells/well) in upper 8-µm-pore
membrane chambers coated with (invasion assay) or without
(migration assay) Matrigel® (BD Biosciences, Franklin
Lakes, NJ, USA). The complete medium (BEGM supplemented with a BEGM
Bullet kit for CRL-11233 cells and EMEM +2 mM glutamine +1% NEAA
+10% FBS for Hep3B cells, respectively) was loaded in the lower
chamber. After 16 h of incubation in a humidified chamber at 5%
CO2 and 37°C, cells that passed through the membrane
were fixed in 4% paraformaldehyde at 4°C for 15 min, washed once
with PBS, stained with 1% crystal violet at room temperature for 20
min, washed with PBS three times, and then the cell invasion was
quantified by counting the number of cells passing through the
pores from five different fields per sample at magnification, ×100
under a light microscope selected in a random manner.
Luciferase reporter assay
CRL-11233 and Hep3B cells (1×105
cells/well) were seeded in 24 wells overnight and transfected with
indicated amounts of plasmids encoding ZEB1 or the corresponding
empty vector controls using FuGENE HD transfection reagent (Promega
Corporation), according to the manufacturer's protocol. Cells were
collected after 48 h and luciferase activity was measured using a
Dual-Luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's instruction. Relative expression
values were normalized to the respective values of
Renilla.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). For
multiple comparisons, one-way ANOVA with Bonferroni's post-hoc test
was applied. Data are presented as the mean ± standard deviation of
the mean (SEM). All experiments were repeated at least three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HCC cells show LKB1 downregulation and
EMT progression
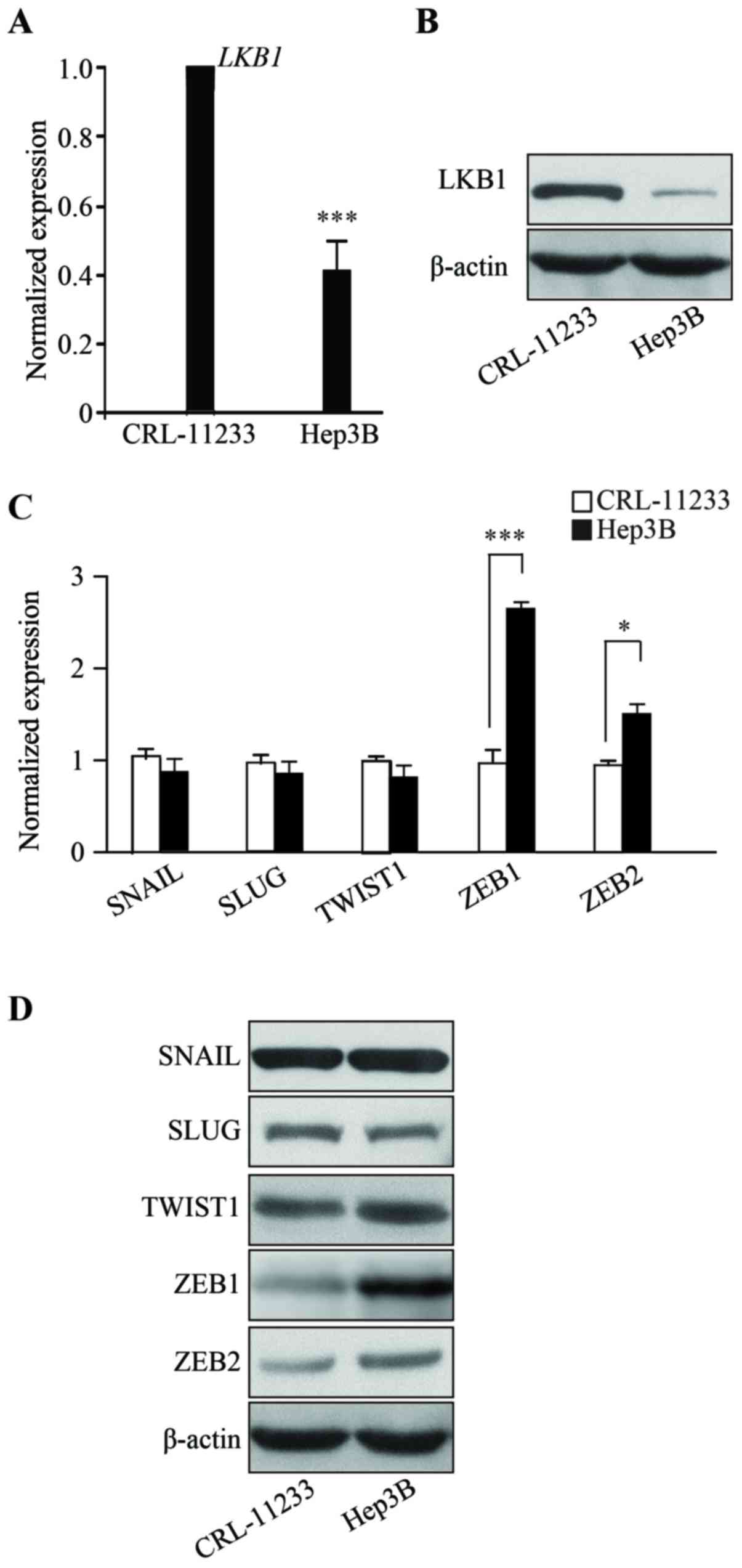
To characterize HCC, the present study examined the
expression level of LKB1. RNAs from human the HCC line Hep3B were
isolated and RT-qPCR was performed which demonstrated that,
compared with the non-malignant normal liver cell line CRL-11233,
the mRNA expression of LKB1 in Hep3B cells is significantly
downregulated (Fig. 1A) (P<0.001).
Consistent with the RT-qPCR data, LKB1 protein levels are decreased
in Hep3B cells (Fig. 1B). The RT-qPCR
and western blotting results demonstrated that the expression of
EMT markers, namely SNAIL, SLUG, TWIST1, ZEB1 and ZEB2, are
upregulated in Hep3B cells, when compared with the normal liver
cell line CRL-11233 (Fig. 1C and D).
These results demonstrated that LKB1 is downregulated in HCC and
suggest that EMT may be involved in the progression of HCC.
LKB1 downregulates the expression of
the EMT marker ZEB1 in Hep3B cells
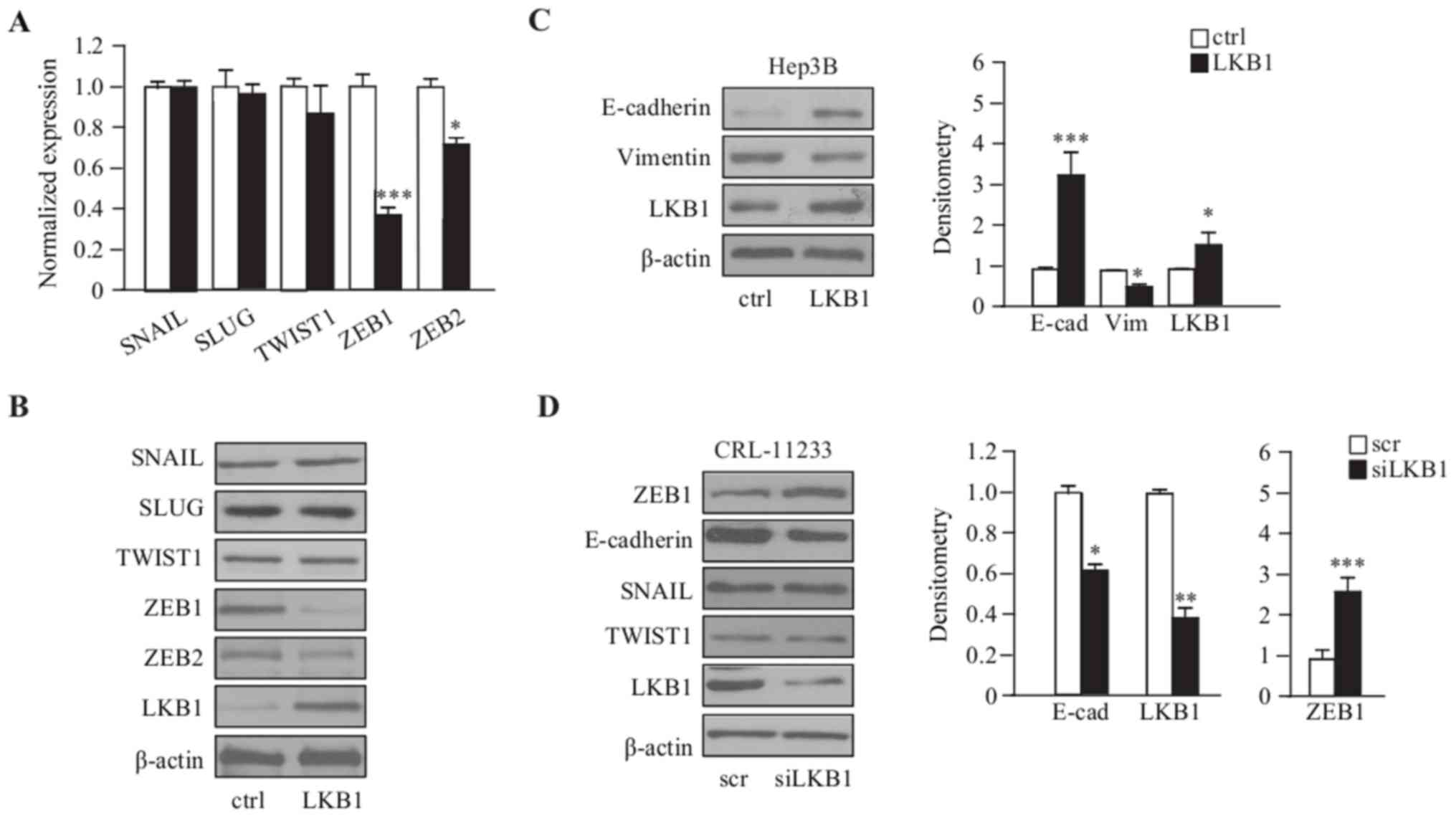
To illustrate the correlation of EMT and LKB1, LKB1
was transiently overexpressed in Hep3B cells and the expression of
EMT markers examined; for SNAIL, SLUG, TWIST1, ZEB1 and ZEB2, the
RT-qPCR and immunoblot results demonstrated that the expression of
ZEB1 and ZEB2 were significantly downregulated in
LKB1-overexpressing Hep3B cells, compared with the empty vector
control cells (Fig. 2A and B).
Consistently, the epithelial marker E-cadherin was upregulated, and
the mesenchymal marker Vimentin was downregulated, as demonstrated
by western blot analysis (Fig. 2C).
Furthermore, LKB1 depletion by siRNA in the normal liver cell line
CRL-11233, exhibited an upregulation of ZEB1; however, this was not
observed with additional EMT markers such as SNAIL and TWIST1, and
the downregulation of E-cadherin (Fig.
2D). Taken together, these results suggest that LKB1 primarily
regulates the expression of ZEB1 in order to regulate EMT process
in HCC cells.
EMT induced by LKB1 loss promotes HCC
migration and invasion through ZEB1
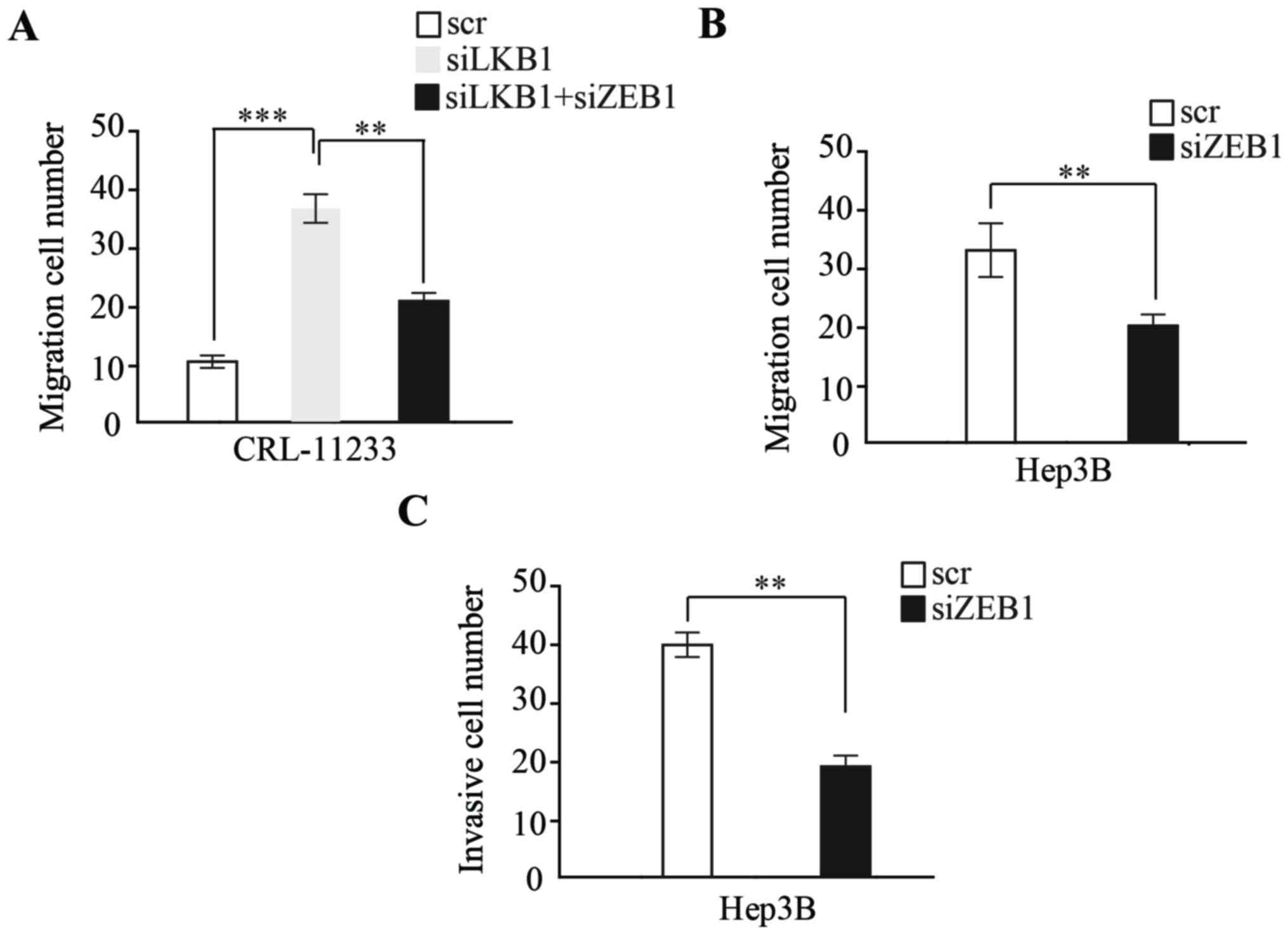
To examine whether EMT induced by LKB1 regulates HCC
migration and invasion, the present study examined LKB1-depleted
CRL-11233 cells, which demonstrated a high migration capability;
additionally, the depletion of ZEB1 by siRNA significantly
inhibited the migration capability of CRL-11233 cells (Fig. 3A). Furthermore, the expression of ZEB1
by siRNA was depleted in Hep3B cells, with a migration and invasion
assay demonstrating that, compared with a scramble RNA control,
ZEB1 knockdown significantly inhibited the migration and invasion
capability of HCC cells (Fig. 3B and
C).
ZEB1 regulates the expression of YAP
to promote YAP signaling
The present study then attempted to further
determine the mechanism by which ZEB1 functions in HCC cells. It
has been reported that upregulation of Hippo signaling regulates
tumor progression in various cancers, such as NSCLC, breast,
pancreatic and colon cancer (12). In
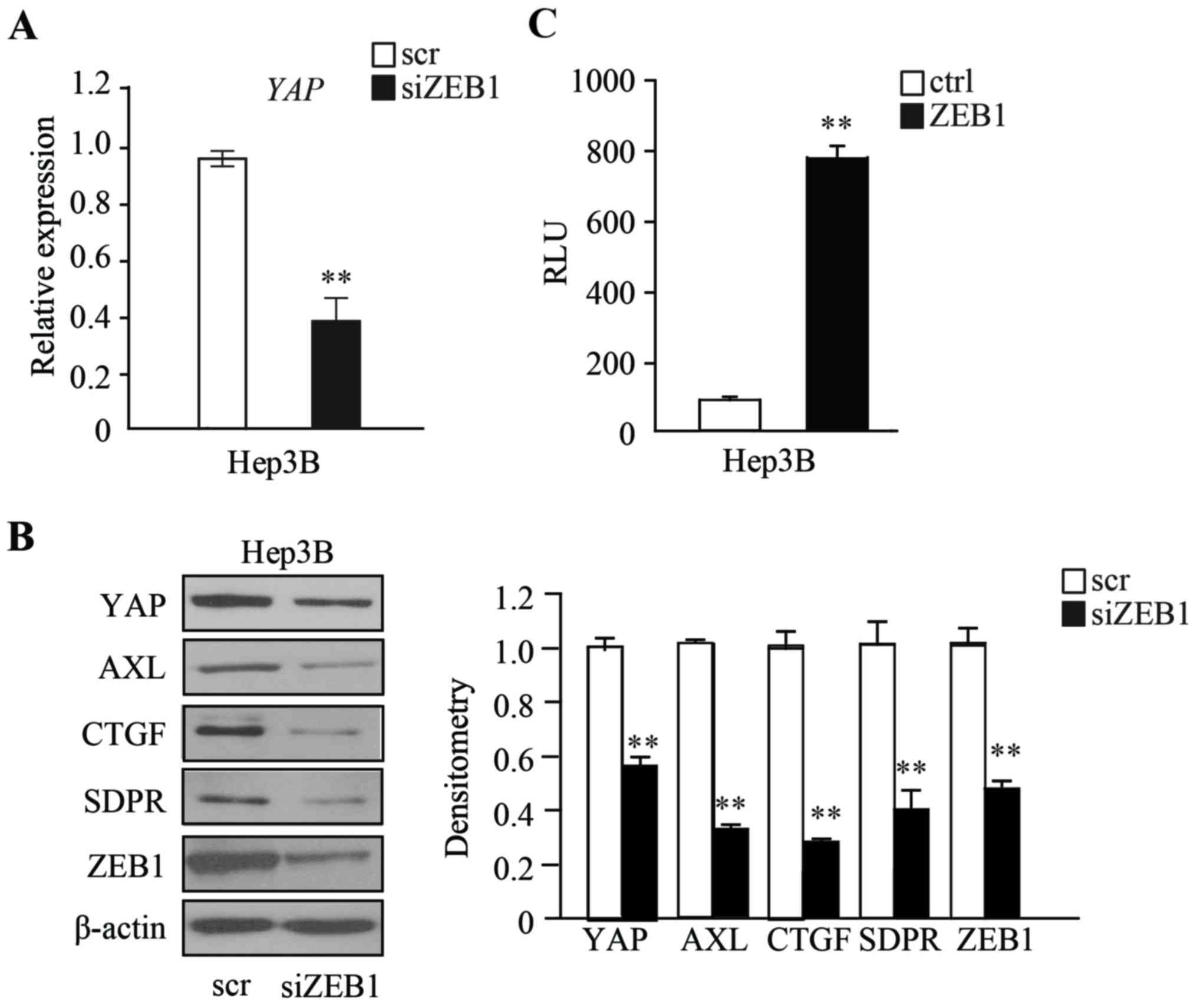
order to examine whether ZEB1 regulates the expression of YAP, the
expression of ZEB1 was transiently depleted in Hep3B cells and the
expression of YAP was examined. The RT-qPCR and western blot
analysis results demonstrated that the expression of YAP is
significantly downregulated in ZEB1-depleted Hep3B cells, compared
with scramble RNA control cells (Fig. 4A
and B). YAP and ZEB1 have been reported to co-localize in the
nucleus (10); therefore, the present
study investigated whether the expression of YAP is directly
regulated by ZEB1, by performing a luciferase reporter assay. The
promoter and 5′UTR region of YAP were cloned into the promoter
region of luciferase genes using a pGL3-Basic plasmid. The plasmid
was then co-transfected with ZEB1-expressing plasmids into Hep3B
cells, and the luciferase reporter assay was performed after 48 h.
Relative Luminometer Units (RLU) results demonstrated that Hep3B
cells expressing ZEB1 had a markedly higher RLU when compared with
cells transfected with an empty vector, indicating that ZEB1 could
directly bind to the promoter and 5′UTR region of YAP gene
(**P<0.01) (Fig. 4C). The
expressions of downstream target genes that are regulated by YAP
were then examined. Western blot analysis demonstrated that AXL,
CTGF, and SDPR are downregulated in ZEB1-depleted Hep3B cells,
compared with in scramble RNA control cells (Fig. 4B).
Taken together, these results demonstrated that the
loss of LKB1 promotes the expression of EMT marker ZEB1, which in
turn promotes EMT and the Hippo signaling pathway by interacting
with YAP, resulting in HCC progression.
Discussion
The results of the present study demonstrate that
ZEB1, an activator of EMT induced by LKB1-loss, promotes HCC
progression by activating YAP signaling. LKB1 is a frequently
mutated tumor suppressor in HCC (22). It is indicated that LKB1 may regulate
cell growth, metabolism, polarity and mobility. The loss of LKB1
contributes to a more aggressive phenotype in tumor cells,
including EMT (14). LKB1
inactivation triggers EMT in lung cancer cells through the
induction of ZEB1 (4).
ZEB1 is a tumor-specific molecule, and the elevated
expression of ZEB1 has been observed in numerous types of cancer,
including lung, breast and pancreatic head cancer (23,24). ZEB1
has been reported as a LKB1 target, due to a marked increase in
ZEB1 levels observed upon LKB1 depletion in mice (24,25).
A previous study demonstrated that deletion of YAP
in liver cells suppresses hepatomegaly and hepatocyte hyperplasia
induced by acute deletion of LKB1 in mice (26). YAP is a well-studied oncogene in the
liver that drives liver enlargement as well as liver tumor
formation; a previous study revealing that ectopic expression of
YAP resulted in accelerated liver tumor progression (14). In addition, depletion of YAP
attenuates the metastasis potential of cells (27). The results of the present study
demonstrated that YAP activation promotes liver cancer progression.
Notably, ZEB1 was identified as a crucial regulator of YAP in
LKB1-deficient HCC progression; however, the present study only
examined the function of ZEB1 and YAP in vitro, and future
studies should use a mouse model is required in order to
characterize the function and association of ZEB1 with HCC in
vivo.
In conclusion, the present study determined a
functional link between YAP and ZEB1 in the context of
LKB1-deficient HepB3 cells. Therefore, these findings demonstrated
that ZEB1 not only regulates the expression of YAP, but also serves
as a co-activator of YAP in regulating the expression of downstream
target genes to promote malignant progression. Furthermore,
considering the prevalence of LKB1 mutations in various cancer
types, the data may also suggest a novel target for the treatment
of other LKB1-deficient cancer cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BQ conducted the experiment and collected the data,
WW designed the project. JZ, GF and DL analyzed the data and
drafted the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2011. View
Article : Google Scholar
|
|
2
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roy BC, Kohno T, Iwakawa R, Moriguchi T,
Kiyono T, Morishita K, Sanchez-Cespedes M, Akiyama T and Yokota J:
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of
human lung cancer cells. Lung Cancer. 70:136–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rhodes LV, Tate CR, Hoang VT, Burks HE,
Gilliam D, Martin EC, Elliott S, Miller DB, Buechlein A, Rusch D,
et al: Regulation of triple-negative breast cancer cell metastasis
by the tumor-suppressor liver kinase B1. Oncogenesis. 4:e1682015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Z, Zang M and Guo W: AMPK as a
metabolic tumor suppressor: Control of metabolism and cell growth.
Future Oncol. 6:457–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Tilló E, Siles L, de Barrios O,
Cuatrecasas M, Vaquero EC, Castells A and Postigo A: Expanding
roles of ZEB factors in tumorigenesis and tumor progression. Am J
Cancer Res. 1:897–912. 2011.PubMed/NCBI
|
|
9
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lehmann W, Mossmann D, Kleemann J, Mock K,
Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP and
Brabletz T: ZEB1 turns into a transcriptional activator by
interacting with YAP1 in aggressive cancer types. Nat Commun.
7:104982016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thongon N, Castiglioni I, Zucal C, Latorre
E, D'Agostino V, Bauer I, Pancher M, Ballestrero A, Feldmann G,
Nencioni A, et al: The GSK3β inhibitor BIS I reverts YAP-dependent
EMT signature in PDAC cell lines by decreasing SMADs expression
level. Oncotarget. 7:26551–26566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao DD, Xue W, Krall EB, Bhutkar A,
Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et
al: KRAS and YAP1 converge to regulate EMT and tumor survival.
Cell. 158:171–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohseni M, Sun J, Lau A, Curtis S,
Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al: A
genetic screen identifies an LKB1-MARK signalling axis controlling
the Hippo-YAP pathway. Nat Cell Biol. 16:108–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Li J and Sun S, Chen X, Zhang H, Li
B and Sun S: YAP/TAZ-mediated activation of serine metabolism and
methylation regulation is critical for LKB1-deficient breast cancer
progression. Biosci Rep. 37:BSR201710722017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Gao Y, Li F, Tong X, Ren Y, Han
X, Yao S, Long F, Yang Z, Fan H, et al: YAP promotes malignant
progression of Lkb1-deficient lung adenocarcinoma through
downstream regulation of survivin. Cancer Res. 75:4450–4457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao Y, Zhang W, Han X, Li F, Wang X, Wang
R, Fang Z, Tong X, Yao S, Li F, et al: YAP inhibits squamous
transdifferentiation of Lkb1-deficient lung adenocarcinoma through
ZEB2-dependent DNp63 repression. Nat Commun. 5:46292014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher ML, Grun D, Adhikary G, Xu W and
Eckert RL: Inhibition of YAP function overcomes BRAF inhibitor
resistance in melanoma cancer stem cells. Oncotarget.
8:110257–110272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barbier-Torres L, Delgado TC,
García-Rodríguez JL, Zubiete-Franco I, Fernández-Ramos D, Buqué X,
Cano A, Gutiérrez-de Juan V, Fernández-Domínguez I, Lopitz-Otsoa F,
et al: Stabilization of LKB1 and Akt by neddylation regulates
energy metabolism in liver cancer. Oncotarget. 6:2509–2523. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Lu X, Huang L, Wang W, Jiang G,
Dean KC, Clem B, Telang S, Jenson AB, Cuatrecasas M, et al:
Different thresholds of ZEB1 are required for Ras-mediated tumour
initiation and metastasis. Nat Commun. 5:56602014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bronsert P, Kohler I, Timme S, Kiefer S,
Werner M, Schilling O, Vashist Y, Makowiec F, Brabletz T, Hopt UT,
et al: Prognostic significance of Zinc finger E-box binding
homeobox 1 (ZEB1) expression in cancer cells and cancer-associated
fibroblasts in pancreatic head cancer. Surgery. 156:97–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo J, Wu Y, Yang L, Du J, Gong K, Chen W,
Dai J, Li X and Xi S: Repression of YAP by NCTD disrupts NSCLC
progression. Oncotarget. 8:2307–2319. 2017.PubMed/NCBI
|