Introduction
Osteosarcoma (OS) is one of most common types of
primary bone malignancy worldwide (1). Data analysis between 1973 and 2004 has
indicated that improvements occurring in the treatment of OS,
including surgical resection, chemotherapy and radiotherapy, have
prolonged the overall 5-year survival rate to ~60–70% in the US
(1,2).
However, due to the aggressive characteristics of rapid growth and
early metastasis associated with OS, the prognosis remains poor
(3,4).
Therefore, understanding the molecular mechanisms underlying OS is
essential.
According to recent studies, miRNAs (miR) were
demonstrated to regulate mRNA expression by binding to the
3′untranslated regions (3′UTR) of its targeted mRNAs in OS cells
(5). For example, miR-379 may
function as a tumor-suppressing miRNA via targeting
phosphoinositide kinase-1 in OS (6).
miRNA-494 inhibits the proliferation and metastasis of OS by
suppressing insulin receptor substrate-1 (7). Overexpression of miR-506 suppresses the
proliferation and promotes the apoptosis of OS cells by targeting
astrocyte elevated gene-1 (8).
Additionally, miR-491-5p was suggested to be involved in OS
development; the upregulation of microRNA-491-5p suppresses cell
proliferation and promotes apoptosis by targeting forkhead box
protein 4 (FOXP4) in human OS (9).
However, the biological roles and underlying mechanisms of
miR-491-5p in OS require exploration.
In the present study, it was identified that
miR-491-5p was downregulated in OS samples. Furthermore, miR-491-5p
inhibited the proliferation and colony formation abilities of OS
cells. In addition, it was demonstrated that pyruvate kinase,
muscle 2 (PKM2) was a direct target of miR-491-5p, and that
miR-491-5p suppressed proliferation in OS cells by targeting PKM2.
Therefore, miR-491-5p acts as a tumor suppressor in OS, which may
provide valuable insight for OS treatment.
Materials and methods
Clinical tissue specimens
A total of 36 pairs of OS tissues and adjacent
normal bone tissues were obtained from the Department of
Orthopedics, Shengjing Hospital of China Medical University. Tissue
samples were rapidly frozen in liquid nitrogen and stored at −80°C
following surgery. Written informed consent was obtained from all
patients. The study was approved by the Ethics Committee of
Shengjing Hospital of China Medical University.
Cell culture
A normal osteoblast cell line (Nhost) and four
different OS cell lines (KHOS, LM7, U2OS and MG-63) were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at
37°C in a humidified atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Takara Biotechnology, Co., Ltd.,
Dalian, China), following the manufacturer's protocol. RNA was
reverse transcribed to cDNA using a PrimeScript RT Reagent kit
(Takara Biotechnology, Co., Ltd., Dalian, China). miR-491-5p
expression was detected using a SYBR Premix Ex Taq™ kit (Takara
Biotechnology, Co., Ltd.). Relative mRNA expression was normalized
to that of β-actin and U6 small nuclear RNA. The RT-qPCR assays
were performed using an Applied Biosystems 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
were as follows: miR-491-5p forward, 5′-GGAGTGGGGAACCCTTCC-3′ and
reverse, 5′-GTGCAGGGTCCGAGGT-3′; PKM2 forward,
5′-CTGTGGACTTGCCTGCTGTG-3′ and reverse, 5′-TGCCTTGCGGATGAATGACG-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; β-actin forward,
5′-AGAAAATCTGGCACCACACC-3′ and reverse,
5′-TAGCACAGCCTGGATAGCAA-3′.
Cell proliferation assay
Cell proliferation was measured using a Cell
Counting Kit-8 (CCK8; Dojindo Molecular Technologies, Kumamoto,
Japan). U2OS or MG-63 cells (2×103 cells/well) were
seeded in 96-well plates. Cells were transfected with 100 nM miR-NC
(cat. no., QPG-04191), miR-491-5p mimics (cat. no., QPG-04192) or
miR-491-5p inhibitors (cat. no., QPG-04193) (sequence unavailable)
purchased by Shanghai Genepharma Co., Ltd. (Shanghai, China) using
the Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols at a final concentration of 100 nM.
Cells were incubated for 1, 2, 3, 4 and 5 days at 37°C. Then, 10 µl
CCK-8 solution was added to each well for 2 h at 37°C. Cell
viability was measured using a microplate reader and the absorbance
at 450 nm was recorded.
Cell colony formation assay
U2OS or MG-63 cells were transfected with 100 nM
miR-NC, miR-491-5p mimics or miR-491-inhibitors, as aforementioned.
Cells were incubated for 14 days, after which the cells were fixed
in 100% methanol for 20 min at room temperature, stained with 0.1%
crystal violet for 20 min at room temperature, and counted in 5
random fields of view using a light microscope (magnification,
200×).
Western blot analysis
Total protein was extracted from cells following
transfection with miR-NC, miR-491-5p mimics or miR-491-5p
inhibitors. The protein concentration was determined using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China), according to the manufacturer's protocol. Equal amounts of
protein (40 µg) were separated via 10% SDS-PAGE and
electrotransferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then incubated
with specific antibodies against PKM2 (dilution, 1:500; cat. no.,
sc-65178,) and GAPDH (dilution, 1:1,000; cat. no., sc-25778; both
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight. This was followed by incubation at room temperature with
horseradish peroxidase-conjugated anti-mouse IgG (dilution,
1:1,000; cat. no., 7074; Cell Signaling Technology, Inc., Danvers,
MA, USA). The protein bands were assessed using an Enhanced
Chemiluminescence Western Blotting kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein expression was analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Protein expression was normalized to that of GAPDH.
Prediction of the putative targets of
miR-491-5p
The putative targets of miR-491-5p were predicted by
the online software Targetscan (http://www.targetscan.org/vert_71/) and miRanda
(http://www.microRNA.org).
Dual-luciferase assay
The wild-type 3′-UTR region of the PKM2 mRNA
(3′UTR-PKM2-WT) containing a putative miR-491-5p-binding site and
the corresponding mutant type 3′-UTR region of the PKM2 mRNA
(5′-CCCCAC-3′ to 5′-GGGGUG-3′) (3′UTR-PKM2-MUT) were generated by
Guangzhou RiboBio Co., Ltd., (Guangzhou, China). U2OS cells were
seeded into a 96-well plate (1×104 cells/well) and
co-transfected with 3′UTR-PKM2-WT/MUT plasmids (Promega
Corporation, Madison, WI, USA), along with miR-491-5p or the
miR-negative control (NC), using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.). Relative luciferase
activity was measured at 48 h after transfection using a
Dual-Luciferase Reporter Assay System (Promega Corporation).
Firefly luciferase was used to normalize the luciferase
activity.
Statistical analysis
All data were assessed using SPSS v.20.0 software
(IBM Corp., Armonk, NY, USA) and are presented as the means ±
standard deviation from ≥3 independent experiments. Differences
between two groups were compared using a two-tailed paired
Student's t-test; one-way analysis of variance (ANOVA) was used for
comparisons between multiple groups. The Student Newman-Keuls test
was used as a post-hoc test following ANOVA. The association
between PKM2 expression and miR-491-5p in OS tissues was determined
by Spearman's correlation analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
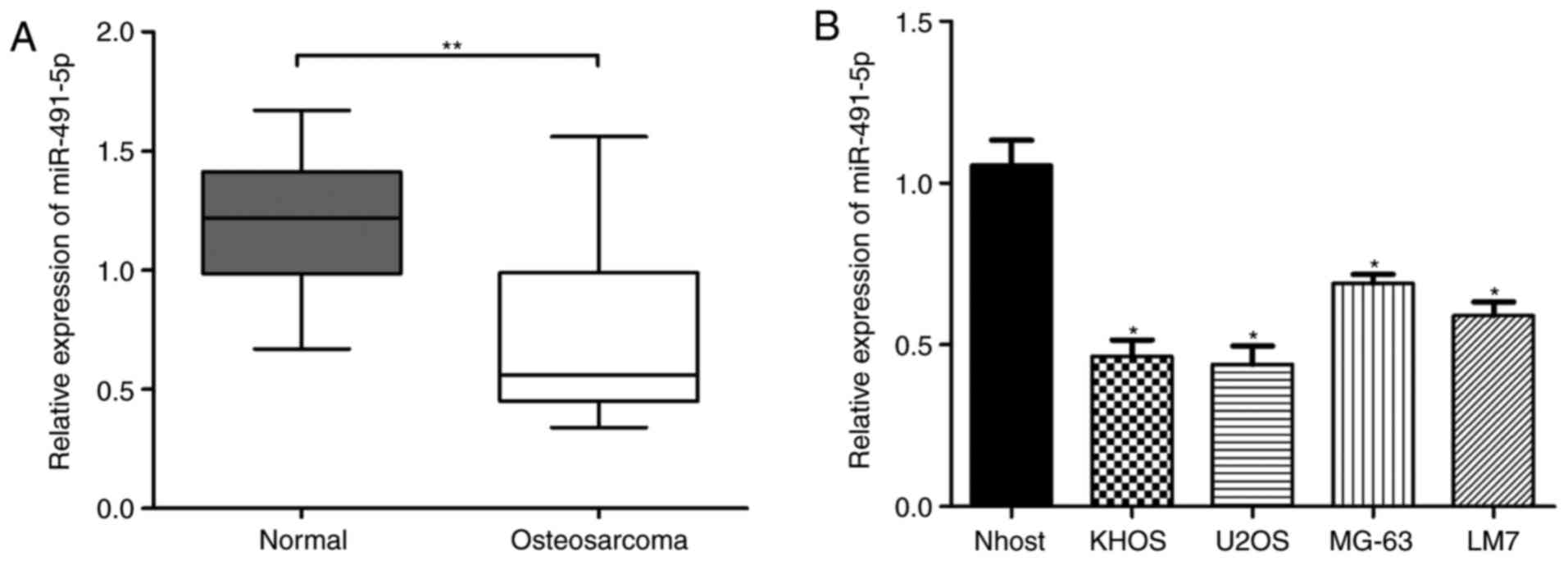
Relative expression of miR-491-5p is
significantly downregulated in OS tissues and cells
Firstly, the relative expression of miR-491-5p in 36
paired of OS tissues and adjacent normal tissue samples was
analyzed via RT-qPCR. The results demonstrated that miR-491-5p
expression was significantly decreased in OS tissues compared with
in the adjacent normal tissues (Fig.
1A; P<0.01). Next, relative miR-491-5p expression levels in
Nhost cells and 4 different OS cell lines (KHOS, LM7, U2OS, and
MG-63) were examined. miR-491-5p expression was significantly
decreased in the 4 OS cell lines compared with in the Nhost cells
(Fig. 1B). Therefore, these results
indicated that miR-491-5p expression is significantly downregulated
in OS tissues and cells.
miR-491-5p inhibits cell proliferation
in vitro
To investigate whether miR-491-5p expression affects
the biological function of OS cells, according to the expression of
miR-491-5p in the 4 OS cells, gain- and loss-of-function assays
were performed using miR-491-5p mimics and miR-491-5p inhibitors,
respectively, in U2OS and MG-63 cells. CCK8 assays indicated that
cells transfected with miR-491-5p mimics had significantly
inhibited proliferation abilities compared with the control;
however, the miR-491-5p inhibitor promoted cell proliferation
abilities, compared with the control groups in U2OS and MG-63 cells
(Fig. 2A and B; P<0.05).
Concurrently, cell colony formation assays demonstrated that
miR-491-5p mimics reduced the number of colonies formed compared
with the control, while the miR-491-5p inhibitor exhibited the
opposite effect, in U2OS and MG-63 cells (Fig. 2C and D; P<0.05). These results
indicated that miR-491-5p inhibited proliferation in OS cells.
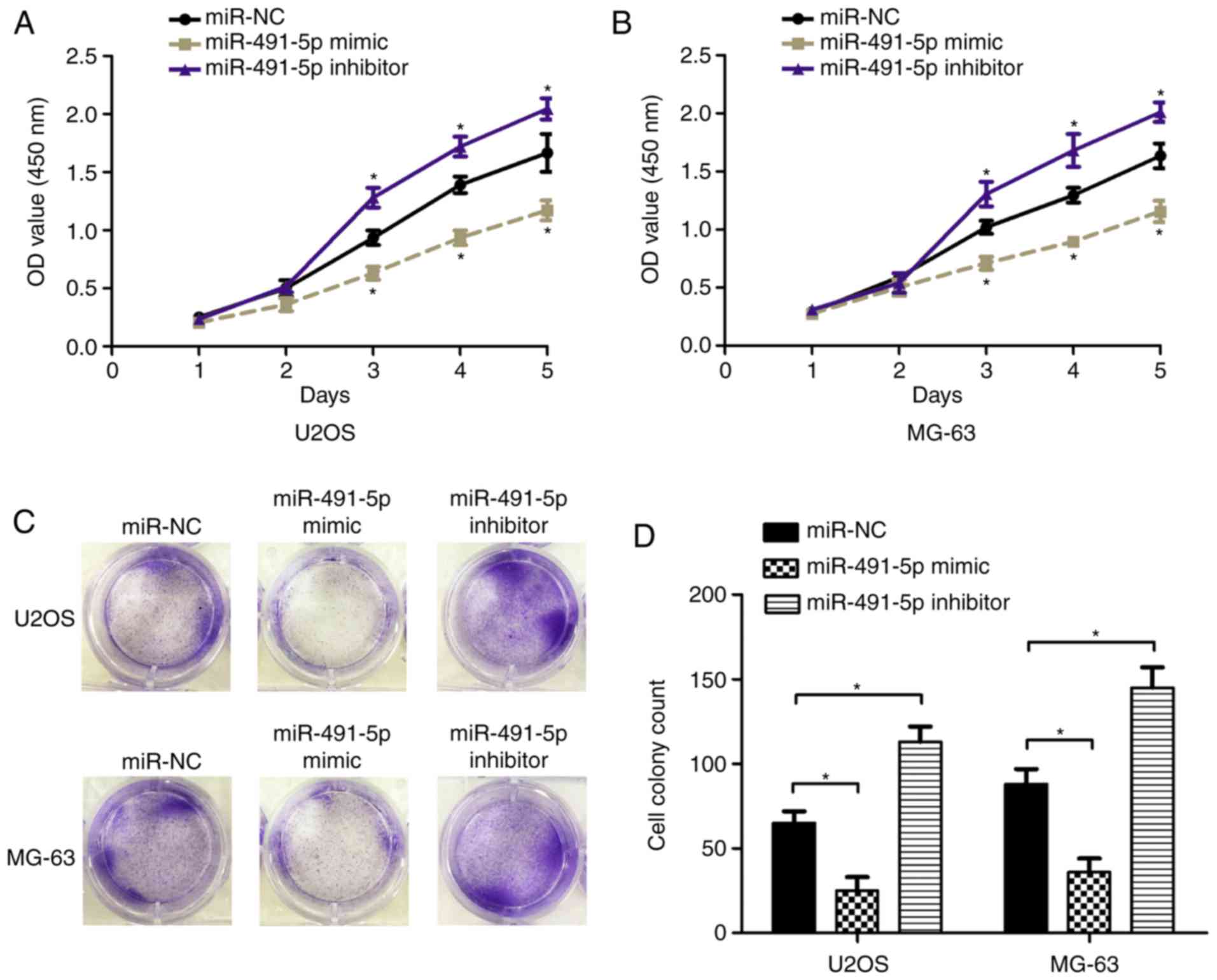 | Figure 2.miR-491-5p suppresses proliferation in
osteosarcoma cells. CCK8 assay was used to evaluated the cell
proliferation rate following transfection with miR-NC, miR-491-5p
mimics or miR-491-5p inhibitors in (A) U2OS and (B) MG-63 cells.
The miR-NC group was used as the control at 1, 2, 3, 4 and 5 days,
*P<0.05 compared with control. Cell colony formation assays were
performed and cell colony number was analyzed following
transfection with miR-NC, miR-491-5p mimics or miR-491-5p
inhibitors into (C) U2OS and (D) MG-63 cells, *P<0.05. miR,
microRNA; NC, negative control; OD, optical density. |
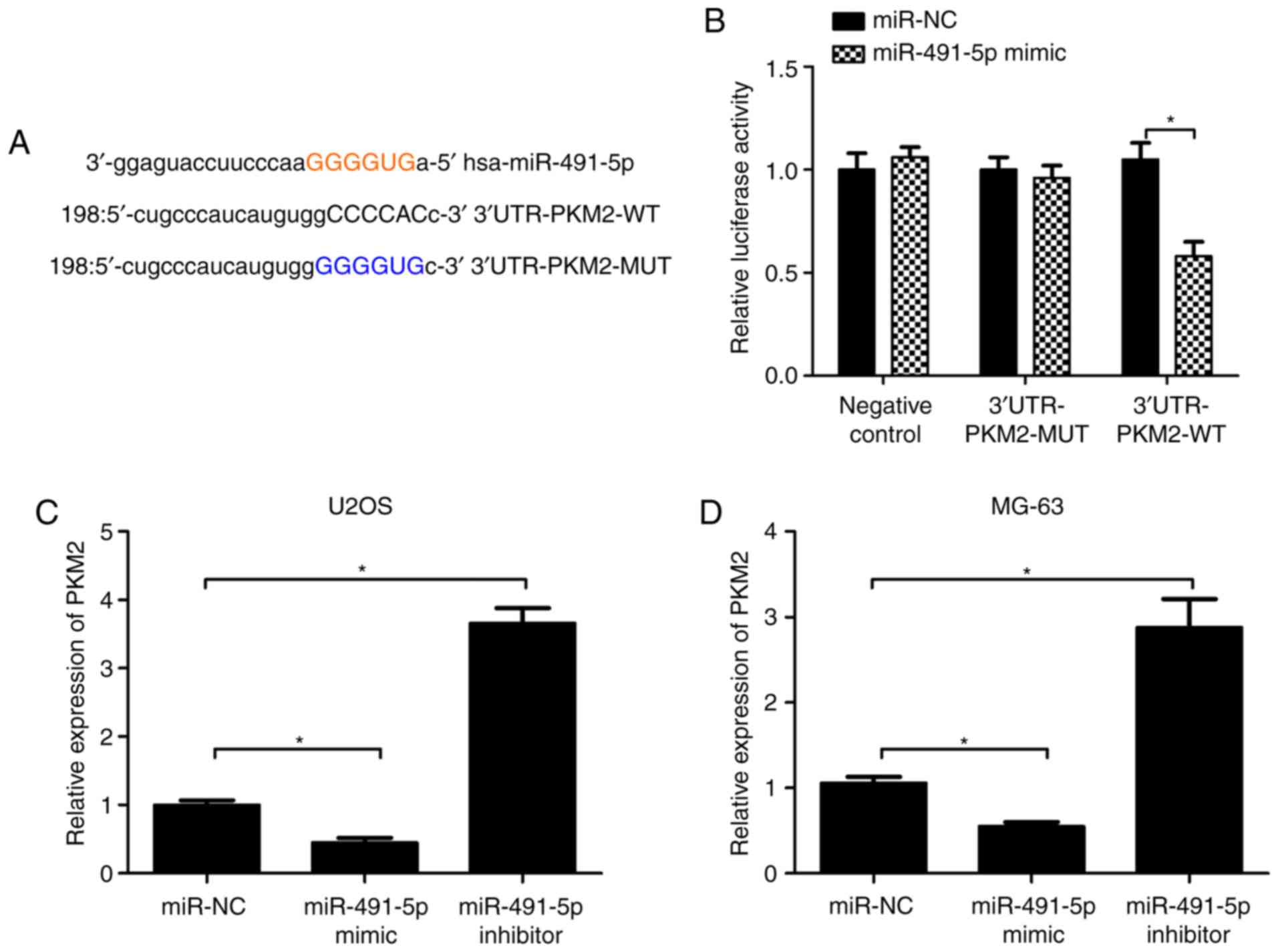
PKM2 is a target of miR-491-5p
Using miRanda and TargetScan target prediction
software, PKM2 was identified to be a potential target of
miR-491-5p (Fig. 3A). Furthermore,
the luciferase reporter vector containing the putative 3′-UTR PKM2
target site and the mutant site for miR-491-5p was constructed
(Fig. 3A). To demonstrate that PKM2
was a target of miR-491-5p, U2OS cells were co-transfected with
3′-UTR PKM2-WT or 3′-UTR PKM2-MUT and miR-491-5p mimic or miR-NC.
Luciferase activity analysis results indicated that the miR-491-5p
mimic significantly suppressed the luciferase activity of the
3′-UTR PKM2-WT reporter vector, but that of 3′-UTR PKM2-MUT was not
altered (Fig. 3B). Furthermore, it
was demonstrated that PKM mRNA expression levels were significantly
reduced with miR-491-5p mimics, but increased with miR491-5p
inhibitors, compared with the miR-NC in U2OS and MG-63 cells
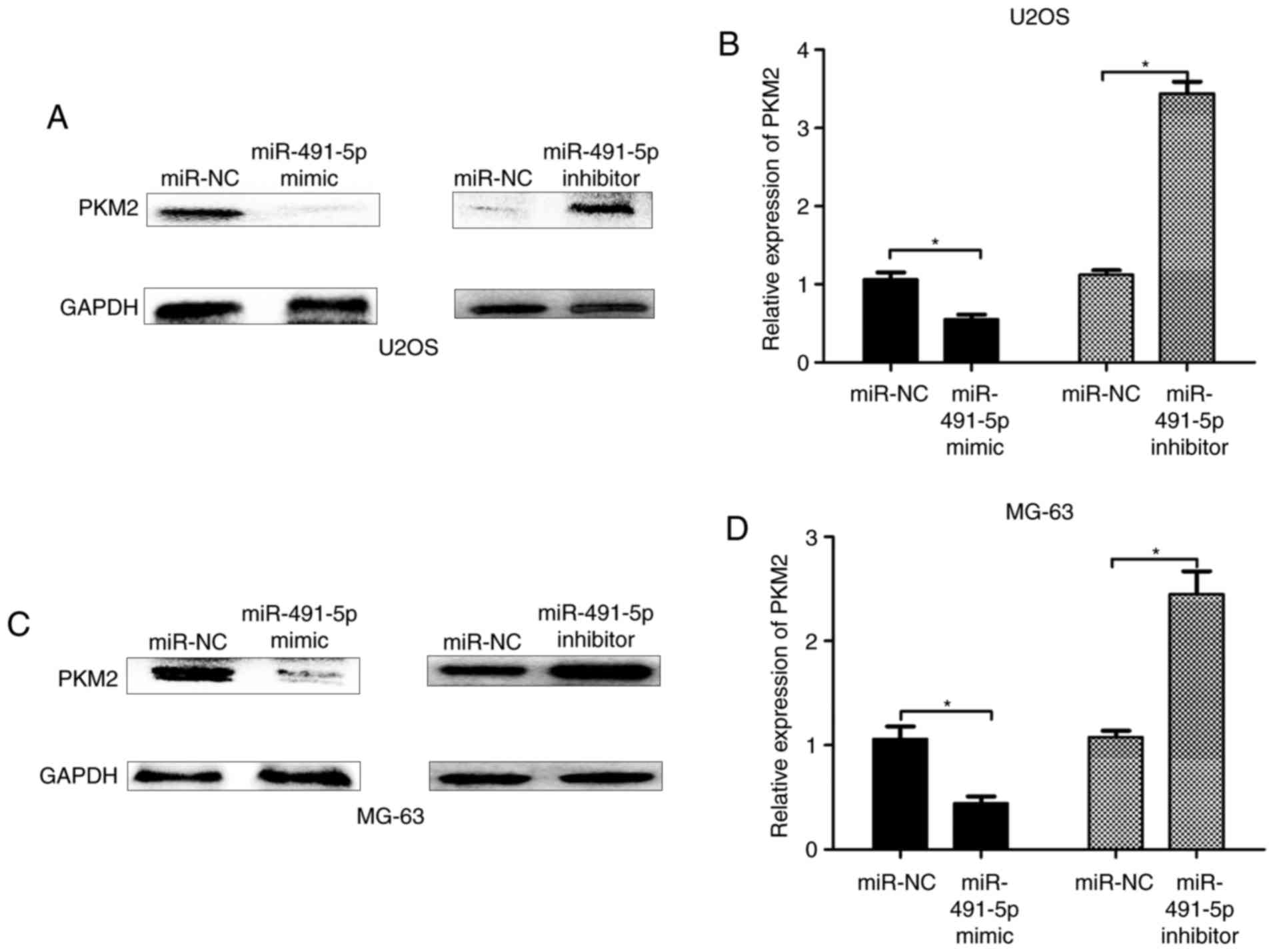
(Fig. 3C and D). Consistently, the
protein expression levels of PKM2 were significantly reduced in the
miR-491-5p mimic group, but increased in the miR491-5p inhibitor
group, compared with the miR-NC group in U2OS and MG-63 cells
(Fig. 4A-D). Therefore, these results
indicated that PKM2 was a direct target of miR-491-5p.
PKM2 overexpression reverses the
proliferation-inhibiting effects of miR-491-5p in OS cells
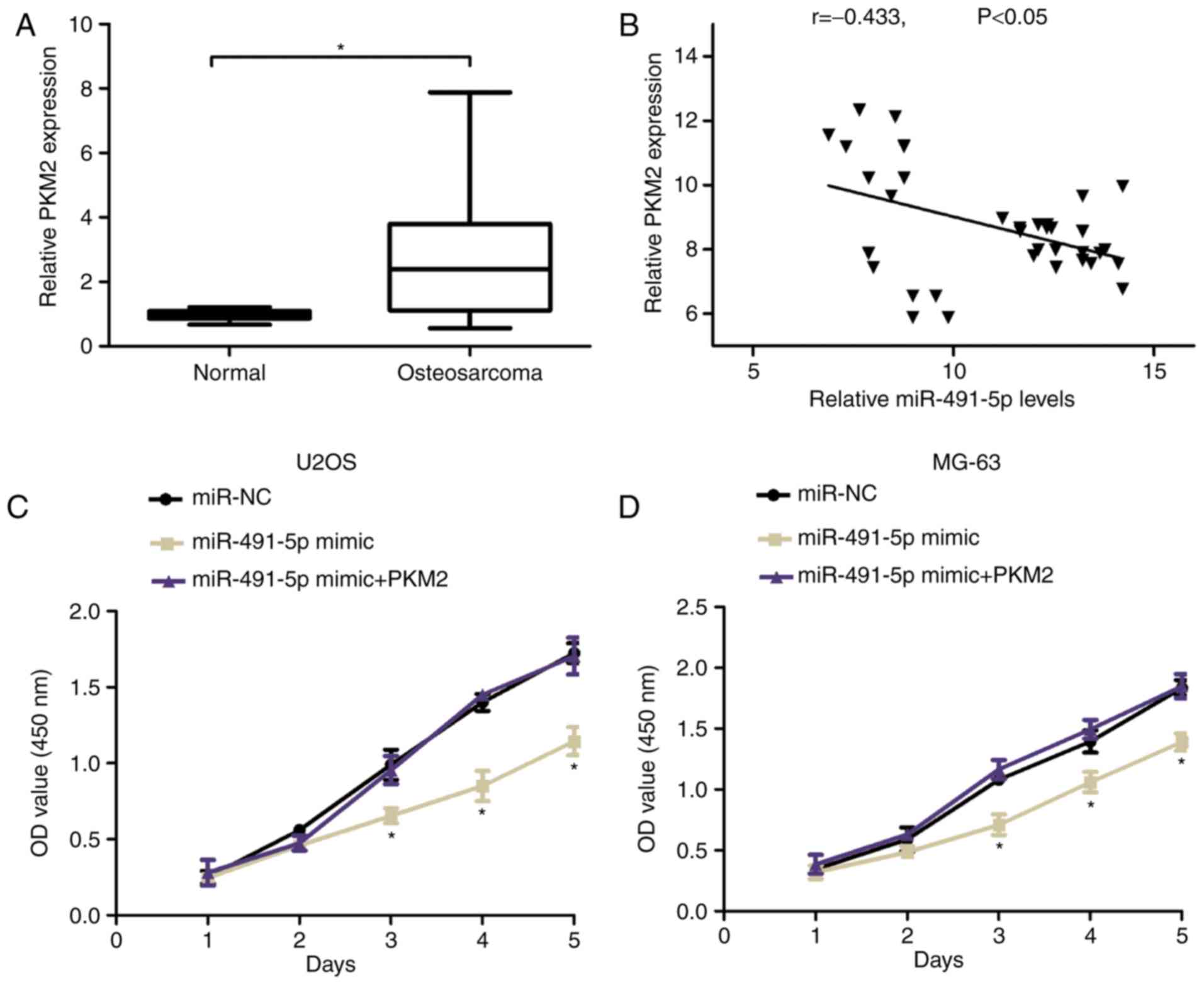
Furthermore, the expression of PKM2 was analyzed and
identified that PKM2 expression was increased in OS tissues and
adjacent normal tissue samples using the RT-qPCR assay (Fig. 5A). Increased PKM2 expression was
negatively associated with miR-491-5p in OS tissues as determined
by Spearman's correlation analyses (r=−0.433, Fig. 5B). To investigate whether PKM2
mediated the proliferation-inhibiting effects of miR-491-5p in OS,
CCK8 assays were used and demonstrated that the miR-491-5p mimic
suppressed cell proliferation compared with the control group, but
was reversed by co-transfection with pcDNA-PKM2 plasmid in U2OS and
MG-63 cells (Fig. 5C and D). These
results indicated that PKM2 overexpression reversed the
proliferation-inhibiting effects of miR-491-5p in OS cells.
Discussion
Previous studies have indicated that miRNAs function
as oncogenes or tumor suppressors to regulate the expression of
cancer-associated genes (10,11). Certain miRNAs have been demonstrated
to be involved in OS progression: miRNA-503 suppresses cell
proliferation and invasion in OS via targeting insulin-like growth
factor 1 receptor (12); miR-187
inhibits tumor growth and invasion by directly targeting mitogen
activated protein kinase 12 in OS (13); miRNA-199a-5p promotes tumor growth by
dually targeting protein inhibitor of activated signal transducer
and activator of transcription 3 and cyclin-dependent kinase
inhibitor 1B in human OS (14). In a
previous study, performed by Yin et al (9), miR-491 was identified to be
downregulated in OS and to serve as a tumor suppressor, revealing
that the upregulation of miRNA-491-5p suppressed proliferation and
promoted apoptosis by targeting FOXP4 in human OS. Serum level of
miR-491 has potential as a biomarker for predicting the prognosis
of overall survival in patients, and inhibits OS lung metastasis
and chemoresistance by targeting αB-crystallin (15). miR-491-3p suppresses the growth and
invasion of OS cells by targeting tetraspanin 1 (16). However, the underling regulatory
mechanisms for miR-491-5p in OS require additional
investigation.
In the present study, it was indicated that
miR-491-5p expression was significantly decreased in OS tissues
compared with adjacent normal tissues. Furthermore, using gain- and
loss-of-function in vitro assays, it was demonstrated that
miR-491-5p overexpression significantly suppressed OS cell
proliferation and colony formation abilities, while the miR-491-5p
inhibitor promoted the proliferation and colony formation
abilities. Additionally, it was revealed that PKM2 was a direct
target of miR-491-5p. RT-qPCR and western blot analysis
demonstrated that miR-491-5p overexpression suppressed the relative
expression of PKM2 in OS cells. In a previous study, PKM2 was
suggested to be involved in OS development; Liu et al
(17) confirmed that the
overexpression of PKM2 predicts a poor prognosis for patients with
OS. Metformin increases the sensitivity of OS stem cells to
cisplatin by inhibiting expression of PKM2 (18). In the present study, it was
demonstrated that miR-491-5p inhibited OS cell proliferation
ability, but that this was reversed by introducing pcDNA-PKM2,
which suggested that PKM2 overexpression rescued the
proliferation-inhibiting effects of miR-491-5p in OS cells.
In conclusion, the present study demonstrated that
miR-491-5p was downregulated in OS and that upregulated miR-491-5p
levels inhibited cell proliferation of OS, while the miR-491-5p
inhibitor promoted the proliferation of OS cells. Furthermore, it
was revealed that miR-491-5p inhibited OS cell proliferation by
targeting PKM2. Therefore, these data suggest the value of
miR-491-5p acts as a target in OS treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TC, YuL and WC collected patient data and performed
cell experiments. WC and YaL performed PCR, western blotting and
other molecular experiments. WC contributed to study design and
manuscript writing.
Ethics approval and consent to
participate
The present study was approved by Ethic Committee of
Sheng Jing Hospital of China Medical University (Sheng Jing, China)
and each patient provided written informed consent.
Patient's consent for publication
All patients gave informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyers PA, Heller G, Healey J, Huvos A,
Lane J, Marcove R, Applewhite A, Vlamis V and Rosen G: Chemotherapy
for nonmetastatic osteogenic sarcoma: The memorial sloan-kettering
experience. J Clin Oncol. 10:5–15. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakamoto A and Iwamoto Y: Current status
and perspectives regarding the treatment of osteo-sarcoma:
Chemotherapy. Rev Recent Clin Trials. 3:228–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rytting M, Pearson P, Raymond AK, Ayala A,
Murray J, Yasko AW, Johnson M and Jaffe N: Osteosarcoma in
preadolescent patients. Clin Orthop Relat Res. 373:39–50. 2000.
View Article : Google Scholar
|
|
5
|
Kushlinskii NE, Fridman MV and Braga EA:
Molecular mechanisms and microRNAs in osteosarcoma pathogenesis.
Biochemistry (Mosc). 81:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Shen J, Chan MT and Wu WK:
MicroRNA-379 suppresses osteosarcoma progression by targeting PDK1.
J Cell Mol Med. 21:315–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhi X, Wu K, Yu D, Wang Y, Yu Y, Yan P and
Lv G: MicroRNA-494 inhibits proliferation and metastasis of
osteosarcoma through repressing insulin receptor substrate-1. Am J
Transl Res. 8:3439–3447. 2016.PubMed/NCBI
|
|
8
|
Yao J, Qin L, Miao S, Wang X and Wu X:
Overexpression of miR-506 suppresses proliferation and promotes
apoptosis of osteosarcoma cells by targeting astrocyte elevated
gene-1. Oncol Lett. 12:1840–1848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Z, Ding H, He E, Chen J and Li M:
Up-regulation of microRNA-491-5p suppresses cell proliferation and
promotes apoptosis by targeting FOXP4 in human osteosarcoma. Cell
Prolif. 50:doi: 10.1111/cpr.12308.
|
|
10
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Zheng C, Jiang K, He J, Cao X and
Wu S: MicroRNA-503 suppresses cell proliferation and invasion in
osteosarcoma via targeting insulin-like growth factor 1 receptor.
Exp Ther Med. 14:1547–1553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng Y, Luan F, Zeng L, Zhang Y and Ma K:
MiR-429 suppresses the progression and metastasis of osteosarcoma
by targeting ZEB1. EXCLI J. 16:618–627. 2017.PubMed/NCBI
|
|
14
|
Wang C, Ba X, Guo Y, Sun D, Jiang H, Li W,
Huang Z, Zhou G, Wu S, Zhang J and Chen J: MicroRNA-199a-5p
promotes tumour growth by dual-targeting PIAS3 and p27 in human
osteosarcoma. Sci Rep. 7:414562017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SN, Luo S, Liu C, Piao Z, Gou W, Wang
Y, Guan W, Li Q, Zou H, Yang ZZ, et al: miR-491 inhibits
osteosarcoma lung metastasis and chemoresistance by targeting
αB-crystallin. Mol Ther. 25:2140–2149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan J, Liu J, Liu Y, Huang B and Rao L:
miR-491-3p suppresses the growth and invasion of osteosarcoma cells
by targeting TSPAN1. Mol Med Rep. 16:5568–5574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu ZX, Hong L, Fang SQ, Tan GH, Huang PG,
Zeng Z, Xia X and Wang XX: Overexpression of pyruvate kinase M2
predicts a poor prognosis for patients with osteosarcoma. Tumour
Biol. 37:14923–14928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang D, Wu J, Guo L, Xu Y, Liu L and Lu
J: Metformin increases sensitivity of osteosarcoma stem cells to
cisplatin by inhibiting expression of PKM2. Int J Oncol.
50:1848–1856. 2017. View Article : Google Scholar : PubMed/NCBI
|