Introduction
Diffuse intrinsic pontine glioma (DIPG) is one of
the most devastating pediatric cancers, and accounts for 10–15 of
pediatric brain and central nervous system (CNS) tumors (1,2). The
standard treatment for DIPG currently includes neurosurgery,
radiotherapy and chemotherapy. However, the prognosis for DIPG
remains poor, due to high relapse rates and rapid progression
(3). The 1-, 2- and 5-year survival
rates of patients with DIPG are approximately 30%, <10 and
<1%, respectively (2,4). Therefore, there is an urgent need to
identify novel DIPG-related molecular factors and therapeutic
targets for the treatment of DIPG.
It is now commonly accepted that at least 90% of the
human genome is actively transcribed, whereas only <2% encodes
proteins; the majority of the genome can be transcribed into
non-coding RNAs (ncRNAs) (5). ncRNAs
can be classified into two major classes based on transcript size:
Small ncRNAs (such as microRNAs) and long non-coding RNAs
(lncRNAs). To date, thousands of lncRNAs have been identified in
humans and other species (6). lncRNAs
are commonly defined as RNA molecules longer than 200 nucleotides
that are not necessarily translated into proteins (7). Accumulating evidence suggests that
lncRNAs play important roles in various biological processes by
negatively or positively regulating gene expression at the
epigenetic, transcriptional and post-transcriptional levels
(8–11). With advances in transcriptome
profiling, aberrant lncRNA expression has been observed in various
human diseases, including cancer. These dysregulated lncRNAs have
been implicated in cancer pathogenesis and development (12–17).
Recently, the regulatory roles of lncRNAs have been demonstrated in
the nervous system function, and their dysregulated expression is
involved in various pathologies of the CNS (18,19).
However, the expression patterns and prognostic roles of lncRNAs in
DIPG have not yet been systematically determined.
This study aimed to identify lncRNA expression
patterns in DIPG compared with brainstem low-grade glioma and
normal pediatric brainstem tissue, and identify the lncRNAs
associated with the survival of patients with DIPG.
Materials and methods
Datasets
The human microarray dataset GSE26576 (1) was downloaded from the NCBI Gene
Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/). The GSE26576 microarray
dataset was generated with the GPL570 platform (Affymetrix Human
Genome U133 Plus 2.0 Array) and included 26 DIPG samples, 6
brainstems low-grade glioma samples and 2 normal pediatric
brainstem samples.
lncRNA expression profiles
lncRNA expression profiles included in the GSE26576
dataset were obtained by repurposing microarray probes using
GATExplorer software, as previously described (20,21).
Briefly, a series of R packages in GATExplorer software were used
to map the data and annotate the lncRNA microarray probes. lncRNA
probes that mapped to the human and mouse genomes (derived from the
RNAdb database) (22) were retained.
Finally, 5635 lncRNAs were identified for further analysis.
Preprocessing and analysis of
expression profiles
The raw microarray dataset (CEL file) was obtained
from the GEO database and normalized using the Robust Multichip
Average (RMA) method, which involved three main steps: Background
correction, quantile normalization and log2-transformation. For
determination of lncRNA differential expression profiles, a
two-tailed T-test was used to identify differentially expressed
lncRNAs between patients with DIPG and normal controls, and between
patients with DIPG and low-grade glioma. lncRNAs with an adjusted
P<0.05 after FDR correction and a fold change of >2 or
<0.5 were considered as differentially expressed lncRNAs.
Hierarchical clustering analysis was performed for the expression
data of the differentially expressed lncRNAs using the R package
‘pheatmap’.
Statistical analysis
The association between the lncRNA gene expression
and patient survival was assessed by univariate Cox regression
analysis. The Kaplan-Meier method and two-sided log-rank test were
used to compare survival differences between low- and high-risk
groups. Multivariate Cox analysis was used to test whether the
lncRNA expression signature was independent of other clinical
features. Time-dependent receiver operating characteristic (ROC)
curves were used to compare the sensitivity and specificity of the
lncRNA expression signature for survival prediction.
Functional enrichment analysis
Expression correlation between protein-coding genes
and lncRNAs was measured using Pearson correlation coefficients.
Functional enrichment analysis was conducted for the protein-coding
genes co-expressed with the lncRNAs in GO and KEGG using the ClueGO
plugin (version 2.3.3) in Cytoscape (23), and DAVID (david.ncifcrf.gov/, version 6.8) (24). GO terms and KEGG pathways were
considered significantly enriched when P<0.05.
Results
Identification of differentially
expressed lncRNAs between patients with DIPG and normal
controls
To identify differentially expressed lncRNAs between
patients with DIPG and normal controls, we performed differential
expression analysis for lncRNAs using student's t-test. A total of
58 lncRNAs were identified as differentially expressed between
patients with DIPG and normal controls (Fold change >2 or
<0.5, P<0.05 after FDR adjustment). Of these, 41 lncRNAs were
upregulated, and 17 downregulated, in patients with DIPG.
Identification of differentially
expressed lncRNAs between patients with DIPG and low-grade
glioma
We performed a differential expression analysis of
lncRNA expression profiles between patients with DIPG and low-grade
glioma, and identified 197 differentially expressed lncRNAs using
student's t-test. (Fold change >2 or <0.5, P<0.05 after
FDR adjustment). Among the differentially expressed lncRNAs, 125
were upregulated and 72 were downregulated in patients with
DIPG.
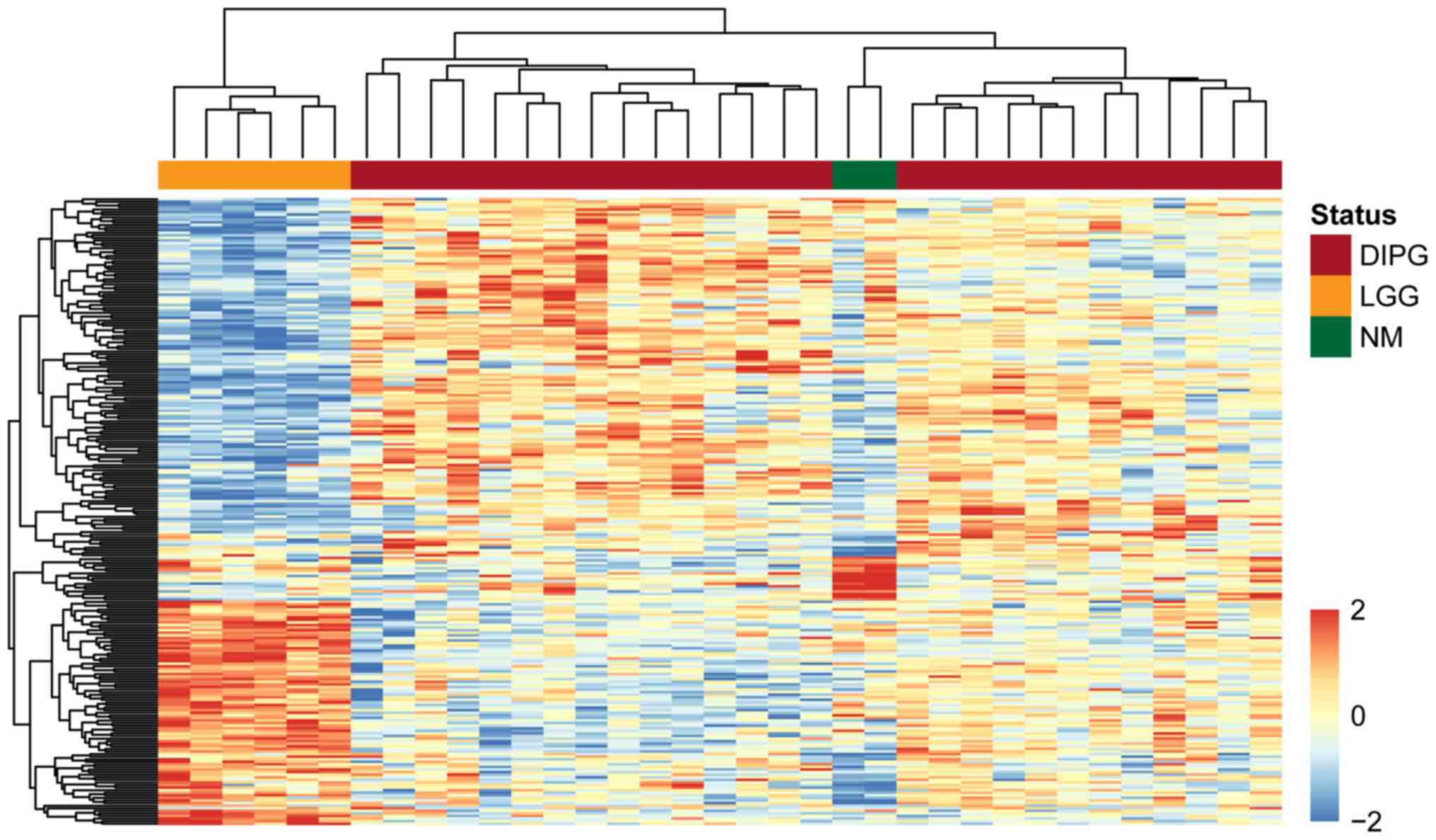
To demonstrate the significance of the dysregulated
lncRNAs in discriminating between patients with DIPG, normal
controls and patients with low-grade glioma, we performed an
unsupervised hierarchical clustering analysis for all samples
according to the expression values of the identified differentially
expressed lncRNAs. As shown in Fig.
1, three distinct sample clusters were obtained by hierarchical
clustering analysis, suggesting that the 255 differentially
expressed lncRNAs were closely associated with DIPG, and could be
used to distinguish DIPG from normal brainstem tissue and low-grade
glioma.
Identification of an lncRNA expression
signature for survival prediction in patients with DIPG
To identify survival-related lncRNAs, we performed
univariate Cox proportional hazards regression analysis for the
aforementioned 255 differentially expressed lncRNAs. A total of 14
lncRNAs were significantly associated with DIPG patient survival.
We conducted a multivariate Cox regression analysis for the 14
survival-related lncRNAs, and identified a set of 9 lncRNAs that
were independently associated with the DIPG patient survival time
(Table I). We constructed a lncRNA
expression signature as a classifier for survival prediction
according to the expression of the 9 lncRNAs weighted by the
multivariate Cox regression coefficient, as follows: Risk
Score=(−3.92)*AF086127 + 1.52*AF086217 + 2.42*AF086391 +
(−4.06)*AF119852 + 0.80*AK021535 + (−0.82)*AK022370 + 1.38*AL050068
+ (−0.89)*BC012548 + (−2.39)*BC041658. The risk score for each
patient was calculated based on the lncRNA gene expression
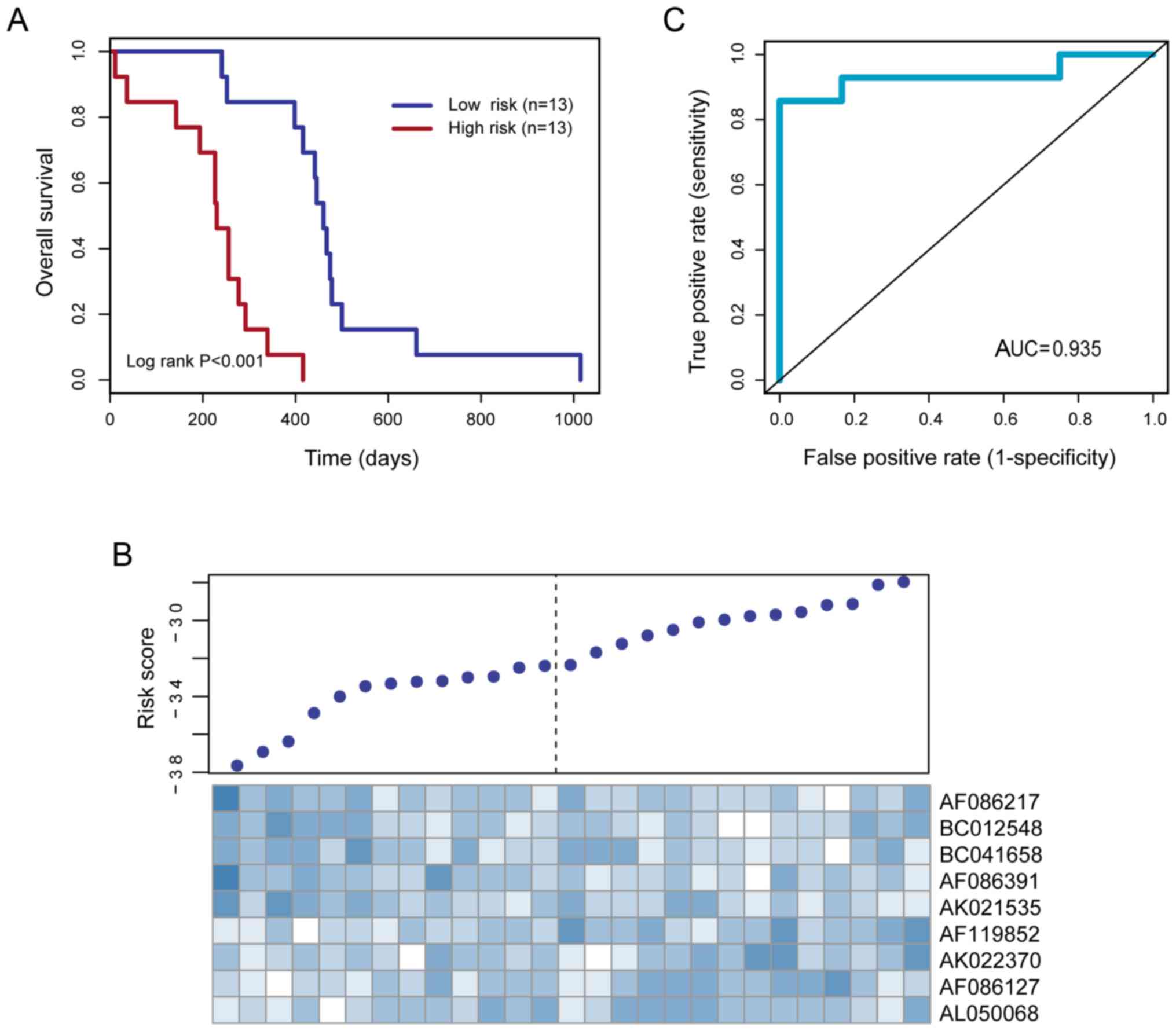
signature. Using the median risk score as the cutoff point
(−32.36), 26 DIPG patients were classified into the high- and
low-risk groups. Patients in the high-risk group exhibited a poorer
overall survival time than patients in the low-risk group (median
survival of 230 vs. 460 days, log-rank test P<0.001).
Kaplan-Meier curves for the high- and low-risk groups are shown in
Fig. 2A. The heatmap shows that five
protective lncRNAs exhibit a high expression level in the low-risk
group, while four risk lncRNAs exhibit a high expression level in
the high-risk group (Fig. 2B).
Analysis of time-dependent ROC demonstrated that the AUC value for
the lncRNA expression signature was 0.935 for 12-month survival
(Fig. 2C).
 | Table I.Nine long non-coding RNAs
significantly associated with the survival in the diffuse intrinsic
pontine glioma dataset. |
Table I.
Nine long non-coding RNAs
significantly associated with the survival in the diffuse intrinsic
pontine glioma dataset.
| Gene symbol | Coefficient | Hazard ratio | Z-score | P-value |
|---|
| AF086217 | 0.969 | 2.635 | 2.517 | 0.012 |
| AF086391 | 1.444 | 4.236 | 2.439 | 0.015 |
| AF119852 | −1.959 | 0.141 | −2.414 | 0.016 |
| AK021535 | 0.595 | 1.813 | 2.650 | 0.008 |
| AK022370 | −0.684 | 0.505 | −2.011 | 0.044 |
| AL050068 | −0.947 | 0.388 | −2.042 | 0.041 |
| BC012548 | 0.428 | 1.534 | 2.061 | 0.039 |
| BC041658 | 0.634 | 1.885 | 1.983 | 0.047 |
| AF086127 | −0.769 | 0.464 | −2.247 | 0.025 |
The 1-year survival rate in the high-risk group was
7.69%, whereas the corresponding rate in the low-risk group was
84.62%. The results of the univariate analysis indicated that the
hazard ratio of the high-risk score vs. the low-risk score for
survival was 2.72 [P<0.001; 95% confidence interval
(CI)=1.76–4.21] (Table II).
According to the multivariate analysis, including age, the hazard
ratio for the high-risk vs. the low-risk score for survival was
2.69 (P<0.001; 95% CI, 1.74–4.18) (Table II), indicating that the lncRNA
expression signature maintained an independent association with
survival.
 | Table II.Univariate and multivariate Cox
regression analysis of survival in the DIPG dataset. |
Table II.
Univariate and multivariate Cox
regression analysis of survival in the DIPG dataset.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable [DIPG
dataset (n=27)] | HR | 95% CI of HR | P-value | HR | 95% CI of HR | P-value |
|---|
| Risk score | 2.72 | 1.76–4.21 |
7.15×10−6 | 2.69 | 1.74–4.18 |
1.01×10−5 |
| Age | 1.04 | 0.93–1.16 | 0.53 | 1.01 | 0.90–1.14 | 0.84 |
Functional analysis of the lncRNA
expression signature
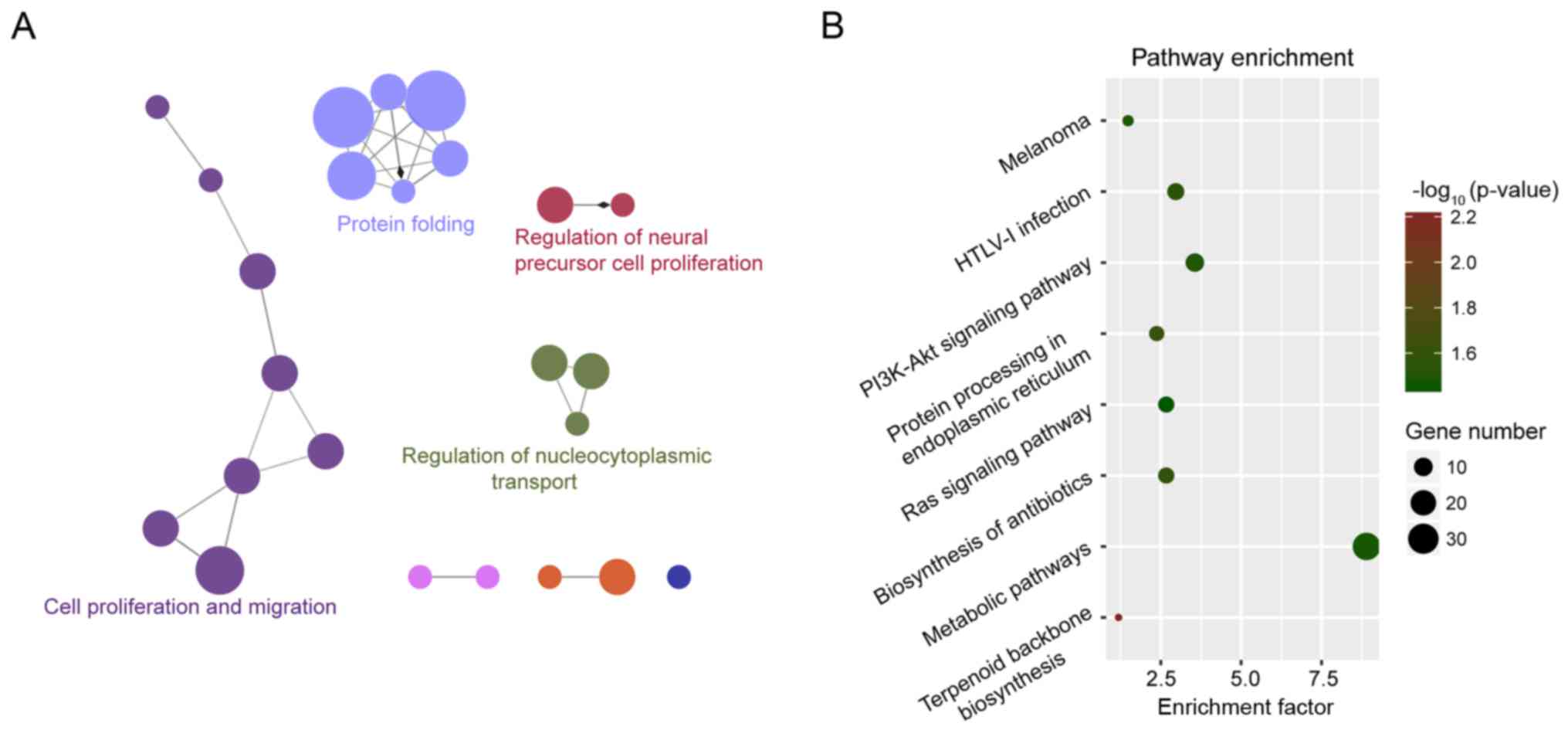
We performed GO and KEGG enrichment analyses for the
protein-coding genes which were co-expressed with the 9 lncRNAs in
the gene expression signature using DAVID and clueGO. The results
of GO enrichment analysis revealed four enriched GO functional
clusters, including ‘protein folding’, ‘cell proliferation’,
‘epithelial cell migration’ and ‘regulation of nucleocytoplasmic
transport’ (Fig. 3A). The results of
the KEGG enrichment analysis revealed eight enriched KEGG pathways,
including ‘terpenoid backbone biosynthesis’, ‘protein processing in
the endoplasmic reticulum’, ‘biosynthesis of antibiotics’, ‘HTLV-I
infection’, ‘PI3K-Akt signaling pathway’, ‘melanoma’, ‘metabolic
pathways’ and ‘Ras signaling pathway’ (Fig. 3B).
Discussion
DIPGs, representing 75–80% of pediatric brainstem
tumors, are the most common brainstem tumors in children (25). Previous studies have investigated the
molecular heterogeneity between DIPGs and adult high-grade gliomas
(HGGs) and between DIPGs and low-grade brainstem gliomas, to
improve our understanding of the molecular mechanisms and molecular
expression signatures underlying DIPG. Using polymerase chain
reaction-single strand polymorphism and nucleotide analyses, Zhang
et al (26) reported a p53
gene mutation in some DIPGs and inferred that DIPGs might be
associated with mutagenic or carcinogenic agents. Paugh et
al (1) performed genome-wide
analyses and demonstrated significantly different frequencies of
specific large-scale and local imbalances in gene expression
between DIPGs and nonbrainstem pediatric glioblastomas. Another
study performed by Lulla et al (27) studied the miRNA expression pattern in
TPG, and identified two distinct subgroups with differentially
expressed microRNAs. A recent study indicated that H3K27M-mutant
gliomas share similar histological features and an adverse
prognosis in adults and children (28). However, the above studies have focused
on genomic mutations, mRNAs or miRNAs. Recent studies have
suggested that lncRNAs, a new class of ncRNAs, is an important
component of disease biology, and the dysregulated expression of
lncRNAs has been observed in various human diseases (29). However, the expression patterns of
lncRNAs and their functional roles in DIPGs have not been
systematically studied yet.
In this study, we first obtained lncRNA expression
profiles of DIPG, brainstem low-grade glioma and normal pediatric
brainstem using the lncRNA-mining approach. We subsequently
performed differential expression analysis and identified 58 and
197 significantly differentially expressed lncRNAs between patients
with DIPG and normal controls, and between patients with DIPG and
low-grade glioma, respectively. To the best of our knowledge, our
study is the first to attempt to identify the dysregulated lncRNA
expression pattern in patients with DIPG compared with normal
controls and patients with low-grade glioma. We hypothesize that
these differentially expressed lncRNAs in patients with DIPG may be
involved in the pathogenesis and development of DIPG, and could be
used as candidates for the investigation of potential diagnostic or
prognostic biomarkers and/or therapeutic targets for the treatment
of DIPG. During the initial phase of marker discovery, we performed
univariate and multivariate Cox proportional hazards regression
analysis for these 255 differentially expressed lncRNAs, and
constructed a 9-lncRNA signature as a potential biomarker for
prognosis of DIPG. Kaplan-Meier curve analysis also demonstrated
that patients with the high-risk lncRNA signature had much poorer
survival than those with the low-risk lncRNA signature. As
radiotherapy and chemotherapy affect the prognosis of patients with
DIPG, whether the 9-lncRNA signature is affected by radiotherapy
and chemotherapy needs to be investigated in future studies.
Although a large number of lncRNAs have been
discovered in humans and animals, few lncRNAs have been
functionally characterized. It has been reported that it is an
effective method to infer the function of lncRNAs based on coding
genes that are co-expressed with these lncRNAs (30). Based on this assumption, we first
identified coding genes that are co-expressed with lncRNAs using
Pearson correlation coefficients. Functional enrichment analysis
was conducted for the protein-coding genes co-expressed with
lncRNAs to predict the functions of the 9-lncRNA signature.
Functional analysis suggested that the 9-lncRNA signature may be
involved in known cancer-related biological pathways and processes.
For example, altered Ras signaling has been detected in a variety
of cancers, including CNS tumors (31). The PI3K-Akt signaling pathway is well
known to be involved in various cellular functions, including
nutrient uptake, cell proliferation, growth, autophagy, apoptosis
and migration (32), and the
dysregulation of the PI3K-Akt signaling pathway is associated with
neurodevelopmental disorders (33).
In conclusion, we have identified some novel differentially
expressed lncRNAs in DIPG using previously generated microarray
data and identified a lncRNA signature comprising nine lncRNAs
(AF086127, AF086217, AF086391, AF119852, AK021535, AK022370,
AL050068, BC012548 and BC041658), which can be
collectively used as an independent prognostic marker of DIPG
patient survival. Our study provided basis for the further
investigation of the mechanisms underlying DIPG.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81641127), and the
Medical and Health Research Project of Health and Family Planning
Commission of Inner Mongolia Autonomous Region (grant no.
201701092).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ conceived and designed the experiments, and wrote
the paper. YL and HL performed the experiments and analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paugh BS, Broniscer A, Qu C, Miller CP,
Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS,
et al: Genome-wide analyses identify recurrent amplifications of
receptor tyrosine kinases and cell-cycle regulatory genes in
diffuse intrinsic pontine glioma. J Clin Oncol. 29:3999–4006. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korones DN: Treatment of newly diagnosed
diffuse brain stem gliomas in children: In search of the holy
grail. Expert Rev Anticancer Ther. 7:663–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jansen MH, van Vuurden DG, Vandertop WP
and Kaspers GJ: Diffuse intrinsic pontine gliomas: A systematic
update on clinical trials and biology. Cancer Treat Rev. 38:27–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bredlau AL and Korones DN: Diffuse
intrinsic pontine gliomas: Treatments and controversies. Adv Cancer
Res. 121:235–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein LD: Human genome: End of the
beginning. Nature. 431:915–916. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kornienko AE, Guenzl PM, Barlow DP and
Pauler FM: Gene regulation by the act of long non-coding RNA
transcription. BMC Biol. 11:592013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao J: The functional role of long
non-coding RNAs and epigenetics. Biol Proced Online. 16:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou M, Wang X, Li J, Hao D, Wang Z, Shi
H, Han L, Zhou H and Sun J: Prioritizing candidate disease-related
long non-coding RNAs by walking on the heterogeneous lncRNA and
disease network. Mol Biosyst. 11:760–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun J, Shi H, Wang Z, Zhang C, Liu L, Wang
L, He W, Hao D, Liu S and Zhou M: Inferring novel lncRNA-disease
associations based on a random walk model of a lncRNA functional
similarity network. Mol Biosyst. 10:2074–2081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou M, Wang X, Shi H, Cheng L, Wang Z,
Zhao H, Yang L and Sun J: Characterization of long non-coding
RNA-associated ceRNA network to reveal potential prognostic lncRNA
biomarkers in human ovarian cancer. Oncotarget. 7:12598–12611.
2016.PubMed/NCBI
|
|
13
|
Zhou M, Sun Y, Sun Y, Xu W, Zhang Z, Zhao
H, Zhong Z and Sun J: Comprehensive analysis of lncRNA expression
profiles reveals a novel lncRNA signature to discriminate
nonequivalent outcomes in patients with ovarian cancer. Oncotarget.
7:32433–32448. 2016.PubMed/NCBI
|
|
14
|
Zhou M, Zhao H, Wang Z, Cheng L, Yang L,
Shi H, Yang H and Sun J: Identification and validation of potential
prognostic lncRNA biomarkers for predicting survival in patients
with multiple myeloma. J Exp Clin Cancer Res. 34:1022015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou M, Zhao H, Xu W, Bao S, Cheng L and
Sun J: Discovery and validation of immune-associated long
non-coding RNA biomarkers associated with clinically molecular
subtype and prognosis in diffuse large B cell lymphoma. Mol Cancer.
16:162017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang JY, Lee JC, Chang YT, Hou MF, Huang
HW, Liaw CC and Chang HW: Long noncoding RNAs-related diseases,
cancers and drugs. ScientificWorldJournal. 2013:9435392013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou M, Zhang Z, Zhao H, Bao S and Sun J:
A novel lncRNA-focus expression signature for survival prediction
in endometrial carcinoma. BMC Cancer. 18:392018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qureshi IA, Mattick JS and Mehler MF: Long
non-coding RNAs in nervous system function and disease. Brain Res.
1338:20–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou M, Zhang Z, Zhao H, Bao S, Cheng L
and Sun J: An immune-related six-lncRNA signature to improve
prognosis prediction of glioblastoma multiforme. Mol Neurobiol.
55:3684–3697. 2017.PubMed/NCBI
|
|
20
|
Fan Y, Wang YF, Su HF, Fang N, Zou C, Li
WF and Fei ZH: Decreased expression of the long noncoding RNA
LINC00261 indicate poor prognosis in gastric cancer and suppress
gastric cancer metastasis by affecting the epithelial-mesenchymal
transition. J Hemat Oncol. 9:572016. View Article : Google Scholar
|
|
21
|
Hu Y, Chen HY, Yu CY, Xu J, Wang JL, Qian
J, Zhang X and Fang JY: A long non-coding RNA signature to improve
prognosis prediction of colorectal cancer. Oncotarget. 5:2230–2242.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang KC, Stephen S, Engstrom PG,
Tajul-Arifin K, Chen W, Wahlestedt C, Lenhard B, Hayashizaki Y and
Mattick JS: RNAdb-a comprehensive mammalian noncoding RNA database.
Nucleic Acids Res. 33(Database Issue): D125–D130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warren KE: Diffuse intrinsic pontine
glioma: Poised for progress. Front Oncol. 2:2052012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Feng X, Koga H, Ichikawa T, Abe S
and Kumanishi T: p53 gene mutations in pontine gliomas of juvenile
onset. Biochem Biophys Res Commun. 196:851–857. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lulla RR, Laskowski J, Goldman S,
Gopalakrishnan V and Fangusaro J: Microrna profiling reveals two
subgroups of diffuse intrinsic pontine glioma. Neuro-Oncology.
16:i40–i59. 2014.
|
|
28
|
Kleinschmidt-DeMasters BK and Mulcahy Levy
JM: H3 K27M-mutant gliomas in adults vs. children share similar
histological features and adverse prognosis. Clin Neuropathol.
37:53–63. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harries LW: Long non-coding RNAs and human
disease. Biochem Soc Trans. 40:902–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma H, Hao Y, Dong X, Gong Q, Chen J, Zhang
J and Tian W: Molecular mechanisms and function prediction of long
noncoding RNA. ScientificWorldJournal. 2012:5417862012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernandez-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang
X and Mo X: Brain development and Akt signaling: The crossroads of
signaling pathway and neurodevelopmental diseases. J Mol Neurosci.
61:379–384. 2017. View Article : Google Scholar : PubMed/NCBI
|