Introduction
Hepatocellular carcinoma (HCC) is the dominant form
of primary liver cancer, accounting for more than 80% of all liver
cancer cases, and is the third leading cause of cancer-related
mortality worldwide (1,2). Despite advances in prevention
techniques, screening and novel technologies used in the diagnosis
and treatment of HCC, incidence and mortality rates continue to
rise (1,2). Previous reports describe HCC as a type
of heterogeneous disease, and surgical resection and chemotherapy
are the common treatment methods; however, postoperative recurrence
and drug resistance pose challenges for the treatment of HCC
(1–3).
To date, sorafenib is the only drug approved by the US Food and
Drug Administration as a molecular targeting therapy for HCC, but
only extends the survival time of patients by 2–3 months (2,3). Research
has demonstrated that hyper-proliferation of tumor cells is the
main biological basis of tumor migration, diffusion, invasion,
postoperative recurrence and drug resistance (4–6). Although
progress has been made in research on the regulation underlying
tumor cell proliferation regulation, the mechanisms are still
largely unknown and need to be elucidated.
X-box binding protein 1 (XBP1), a member of the
basic leucine zipper family of transcription factors CREB/ATF
(cyclic adenosine monophosphate response element binding
protein/activation of transcription factors), is encoded by the
XBP1 gene and widely expressed in various tissues in adult mammals,
with particularly high expression in the exocrine glands and liver,
and is a necessary factor for liver cell differentiation and liver
formation (7). Dysregulation of XBP1
has been associated with tumor pathogenesis. In particular,
researches have reported that the inositol-requiring enzyme 1α
(IRE1α)/XBP1 pathway is involved in all processes associated with
the development and progression of tumors, including tumor cell
survival and proliferation, epithelial-mesenchymal transition,
transcription factor regulation, drug resistance and glycolysis
(8–13). In addition, several studies have
reported cyclin D to be a downstream effector of XBP1 (13–15), which
in turn is established as an important regulator of the cell cycle;
previous study demonstrated that inhibition of cyclin D induced
cell cycle arrest at G1 phase, suggesting that cyclin D is involved
in regulation of cell proliferation (13). However, the detailed mechanism for the
upstream regulation of cyclin D is yet to be fully elucidated.
MicroRNAs (miRNA/miRs) are a class of endogenous
non-coding small RNAs (20–24 nucleotides in length) that negatively
regulate protein expression via mRNA degradation or translation
inhibition, and are widely involved in cell proliferation,
differentiation and apoptosis, which can have important impacts in
the pathogenesis of tumors (16).
Previous studies demonstrated that the expression levels of several
miRNAs were significantly altered in HCC cancer cells, and
indicated involvement of the miRNAs in the pathophysiological
processes of HCC development; for instance miR-503a, −138, −199 and
−214 were associated with cell cycle regulation (16–19).
Preliminary experiments by our group found that miR-199 expression
was significantly decreased in HCC tumor cells, and implicated XBP1
as a putative target of miR-199 (unpublished data). Considering
XBP1 serves an important role in cell cycle regulation, we
speculate that miR-199 may participate in the process of HCC cell
proliferation by regulating the expression of XBP1, and in turn
cyclin D. However, there is a lack of research on the role of
miR-199/XBP1 in HCC at present.
The present study aimed to investigate whether
miR-199 is involved in the regulation of HCC tumor cell
proliferation and its potential underlying molecular mechanism. To
our knowledge, the present study is the first to investigate the
role of an miR-199/XBP1/cyclin D pathway in cell cycle regulation,
and may a provide novel theoretical basis for HCC treatment and
drug development.
Materials and methods
Clinical samples
Liver cancer and normal para-carcinoma tissue
samples from patients (including 6 females and 9 males enrolled
between September 2014 and November 2017; age range, 46–78 years)
diagnosed with HCC were provided by the Department of General
Surgery of Affiliated Changsha Hospital of Hunan Normal University,
Changsha, China. Prior to experiments, the tumor tissue and its
peripheral normal tissue were frozen in liquid nitrogen. The study
was performed following the guidelines of the Declaration of
Helsinki and the experiments were approved by the Hunan Normal
University Medical Ethics Committee, and all patients had signed
written informed consent.
The tissues were allocated to two groups (n=15 per
group): A control group (normal para-carcinoma tissues) and a HCC
group (cancer tissues). The frozen liver tissues were used evaluate
the expression of mRNA and protein.
Cell culture
The HCC cell line Hep3B2.1–7 was purchased from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). Cells were cultured with Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum in a
carbon dioxide incubator at 37°C in 5% CO2. A total of
1×104 cells were subcultured and seeded into 6 or
24-well plates for mimic and small interfering (si)RNA transfection
experiments. Following these, cells were digested with 0.2%
trypsinogen and the lysates collected for mRNA and protein
analyses.
Bioinformatics analysis
The target genes of miRNA and the cognate miRNAs of
a gene were predicted using the online systems TargetScan
(http://www.targetscan.org/vert_72/)
and miRBase (http://www.mirbase.org/). Focus was
on the upregulated XBP1 gene and the downregulated miR-199 in HCC
tissue, due to them being potential targets of each other,
according to the bioinformatics analysis.
miR-199 mimic/inhibitor
transfection
miR-199 mimic and inhibitor transfection experiments
were conducted with Lipofectamine 2000 according to the
manufacturer's instructions. Prior to transfection, a mix including
Lipofectamine 2000 and miR-199 mimic
(5′-CCCAGUGUUUAGACUAUCUGUUC-3′; 50 µM) and inhibitor
(5′-GGGUCACAAAUCUGAUAGACAAG-3′; 100 µM), or negative control (NC)
miRNA (5′-UCACAACCUCCUAGAAAGAGUAGA-3′; 50 µM) were prepared. The
mix was then added to the 6 or 24-well plates with cultured
Hep3B2.1–7 cells to make a final concentration of 80 nmol/l for
miR-199 mimics and NC miRNA, and 160 nmol/l for miR-199 inhibitors,
and the cells were transfected at 37°C for 6 h prior to a 24 h
normal culture at 37°C. Subsequently, the cells were used for
proliferation, mRNA and protein analyses.
Luciferase assay
The XBP1 sequence (Shanghai Genechem Co., Ltd.,
Shanghai, China) containing the seed sequence (5′-ACACUGGG-3′) of
the putative binding site for miR-199 or the identical sequence
with a mutation of the miR-199 seed sequence (5′-ACACCGGG-3′) was
inserted between the restriction sites of XhoI and NotI of a
pmiR-RB-Report™ luciferase vector (Promega Corporation, Madison,
WI, USA) and validated by sequencing. Hep3B2.1–7 cells were
transfected with the mix of Lipofectamine 2000 and the wild type or
mutated reporter vectors and miR-199 mimic or NC
(5′-UCACAACCUCCUAGAAAGAGUAGA-3′) at 37°C for 6 h. Lysates were
harvested 24 h after transfection using a specific report gene cell
lysis fluid (Beyotime Institute of Biotechnology, Shanghai, China).
Renilla luciferase activities were measured according to the
protocol of the Dual-Luciferase Assay System (Promega
Corporation).
XBP1 siRNA transfection
An XBP1 siRNA transfection experiment was conducted
according to the instructions of a XBP-1 siRNA(h) kit (sc-8015;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Prior to
transfection, a mix including transfection agent Entranster™-R4000
and XBP1 siRNA (sense, 5′-TGCCAATGAACTCTTTCCC-3′, and antisense,
5′-CCACATATATACCAAGCCC-3′) or NC siRNA (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′, and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) were prepared. The mix was then added
to the 6 or 24-well plates with cultured Hep3B2.1–7 cells to make a
final concentration of 2 pmol/l XBP1 siRNA or NC siRNA, and the
cells were transfected at 37°C for 48 h prior to a 24 h normal
culture at 37°C. Subsequently, the cells were collected for mRNA
and protein analyses.
Measurement of cell proliferation
To observe the proliferation of Hep3B2.1–7 cells
treated with miR-199 mimic or inhibitor, cell numbers were
determined with a Cell Counting Kit-8 (CCK-8) at 12, 24 and 36 h
after transfection, according to the manufacturer's instructions.
Optical density (OD) values were measured at 490 nm with a
microplate reader, with the OD values being proportional to the
number of cells.
5-Ethynyl-2′-deoxyuridine (EdU) and
DAPI double staining
EdU staining was conducted with a BeyoClick™ EdU
Cell Proliferation kit with Alexa Fluor 555 (Beyotime Institute of
Biotechnology). According to the manufacturer's instructions,
Hep3B2.1–7 cells were firstly labeled with 50 µM EdU solution and
incubated for 2 h, then fixed with 4% paraformaldehyde for 30 mins
and neutralized with 2 mg/ml glycine. Subsequently, 0.5% Triton
X-100 was added to increase cell membrane permeability, and then
the Edu staining reaction solution was added and incubated in the
dark at room temperature for 30 mins. Finally, 1X DAPI (Beyotime
Institute of Biotechnology) staining solution was added and
incubated in the dark for another 30 mins, and the Hep3B2.1–7 cells
were imaged with a confocal microscope.
Real-time reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the liver tissues
samples or Hep3B2.1–7 cells by using TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China) and the concentration and
purity of RNA were determined spectrophotometrically. A total 200
ng of RNA from each sample was used in the RT reaction, conducted
according to the instructions of a transcription kit (DRR037A;
Takara Biotechnology Co., Ltd.).
Real-time qPCR was used to analyze the mRNA levels
of XBP1 or levels of miR-199, with SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) on an ABI 7300 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR primers used for XBP1,
cyclin D and the internal control β-actin are listed in Table I. The primers for miR-199 were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Data
analysis was performed via the 2−ΔΔCq method (20) using the ABI software (V2.4; Thermo
Fisher Scientific, Inc.). The results were expressed as the ratio
of XBP1 or cyclin D to β-actin, or miR-199 to U6.
 | Table I.Primers for real-time reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for real-time reverse
transcription-quantitative polymerase chain reaction.
|
| Primer, 5′-3′ |
|
|---|
|
|
|
|
|---|
| Gene | Forward | Reverse | Product size, bp |
|---|
| XBP1 |
TCCTGTTGGGCATTCTGGAC |
GGCTGGTAAGGAACTGGGTC | 122 |
| Cyclin D |
TGGCAAGCGTGTCATTGTTG |
GGGGCTCACAGTAAGACTGG | 188 |
| β-actin |
CCGTTCCGAAAGTTGCCTTTT |
ATCATCCATGGTGAGCTGGC | 170 |
| miR-199 |
CTGAGTCCCAGTGTTCAGACT |
GTGCAGGGGTCCGAGGT | 24 |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT | 94 |
Western blot analysis
The total protein of the liver tissues samples or
Hep3B2.1–7 cells was extracted using a Cell lysis buffer for
Western and IP (Beyotime Institute of Biotechnology) supplemented
with 1% mmol/l phenylmethylsulfonyl fluoride to block protein
degradation by endogenous proteases. Supernatant including protein
was isolated by centrifugation at 1,200 × g, and protein
concentration was determined with a BCA Protein Assay kit (Beyotime
Institute of Biotechnology). A total of 40 µg protein (per lane)
was subjected to 10% SDS-PAGE, transferred to polyvinylidene
fluoride membranes, and incubated with rabbit anti-XBP1 (sc-8015)
or anti-CyclinD (sc-8396) (1:2,000 dilution; Santa Cruz
Biotechnology, Inc.) at 37°C for 16 h followed by horseradish
peroxidase-conjugated secondary antibodies (1:2,000; A0208;
Beyotime Institute of Biotechnology). Protein signals were
determined with an enhanced chemiluminescence kit (Amersham; GE
Healthcare, Chicago, IL, USA) on a Molecular Imager ChemiDoc XRS
System (Bio-Rad, Laboratories, Inc., Hercules, CA, USA).
Densitometric analysis was conducted with Image J 1.43 (National
Institutes of Health, Bethesda, MA, USA). To confirm equal loading,
blots were also incubated with primary mouse anti-β-actin
(sc-47778; 1:2,000; Santa Cruz Biotechnology, Inc.).
Statistical analysis
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Data were expressed as the mean
± standard error of the mean. Differences in measured values among
the groups were analyzed by one-way analysis of variance followed
by Bonferroni's multiple comparison tests. Differences were
considered significant when P<0.05.
Results
miR-199, XBP1 and cyclin D are
involved in HCC
Previous results suggest that abnormal expression of
numerous biological molecules, including α-fetoprotein, is
associated with the development of HCC (1,3,17). To investigate whether abnormal
expression of miR-199, XBP1 and cyclin D is associated with the
pathogenesis of HCC, the expression of these factors was first
measured in HCC tumor tissues by real-time RT-qPCR and western blot
analysis. The results indicated that miR-199 expression was
significantly decreased in tissues diagnosed as HCC when compared
with adjacent normal tissues (P<0.05; Fig. 1A). It was also determined that the
expression of XBP1 and cyclin D (mRNA and protein) was
significantly increased in HCC (P<0.05; Fig. 1B-E). These results suggested that
miR-199, XBP1 and cyclin D may serve important biological functions
in HCC. Bioinformatic analysis was subsequently performed to
identify the relationship between miR-199 and XBP1, and the results
indicated XBP1to be a direct target of miR-199 (Fig. 1F). Considering miRNA is a negative
regulator of gene expression and cyclin D is downstream of XBP1, we
speculated that a miR-199/XBP1/cyclin D pathway may have a key role
in the development of HCC.
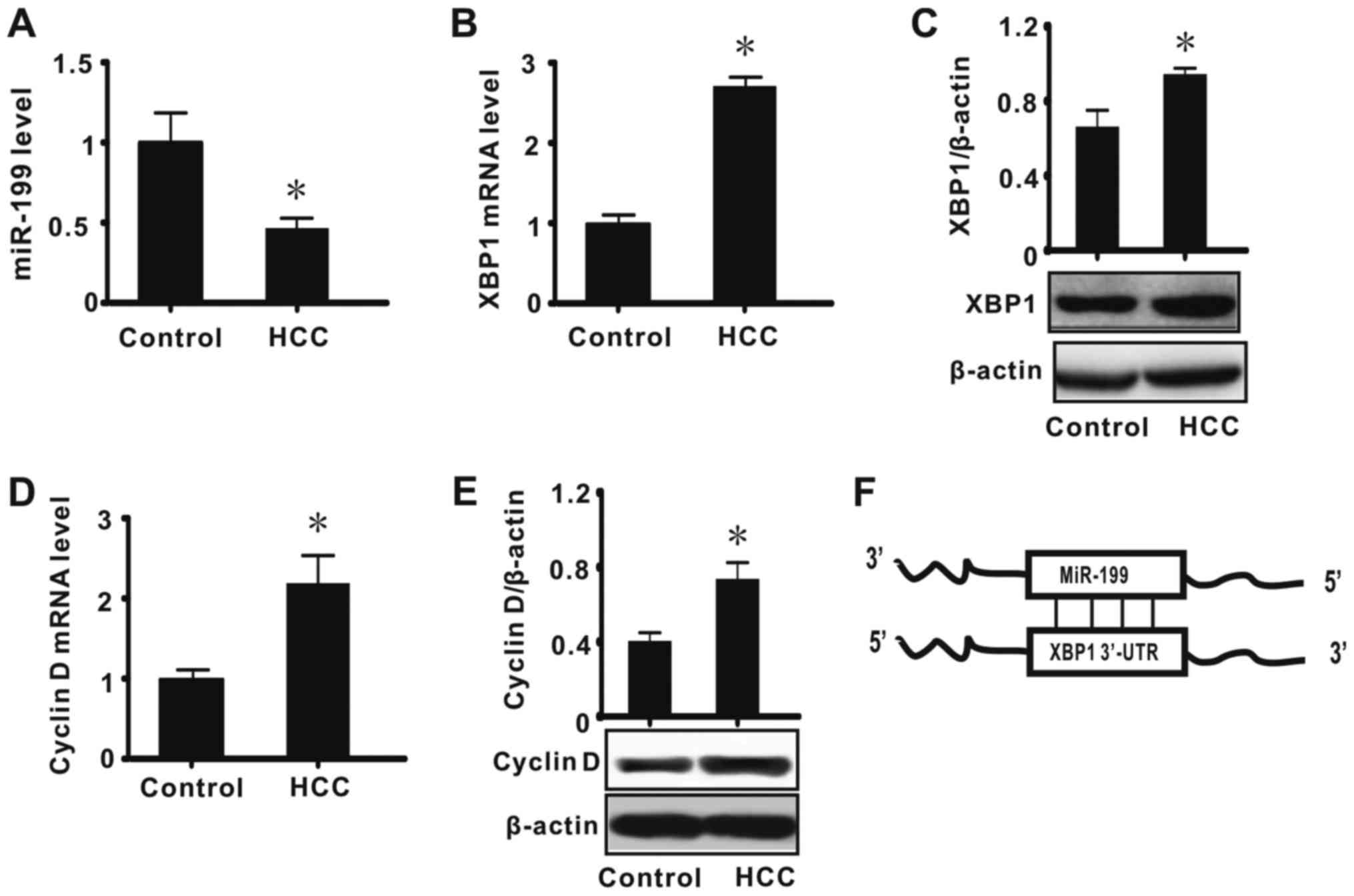 | Figure 1.miR-199, XBP1 and cyclin D are
involved in HCC. The data show the liver tissue expression levels
of (A) miR-199, (B) XPB1 mRNA, (C) XBP1 protein, (D) cyclin D mRNA
and (E) cyclin D protein, and (F) the results of bioinformatic
analysis. All values were expressed as means ± standard error of
the mean (n=15). miR and mRNA levels were expressed relative to U6
and β-actin expression, respectively. *P<0.05 vs. control. miR,
microRNA; XBP1, X-box binding protein 1; control, normal lung
tissue; HCC, hepatocellular carcinoma; UTR, untranslated
region. |
miR-199 directly suppresses the
expression of XBP1 by binding with the 3′untranslated region (UTR)
of XBP1
Subsequent experiments were conducted to examine the
association between miR-199 and XBP1. Hep3B2.1–7 cells were
transfected with miR-199 mimic or inhibitor to increase or decrease
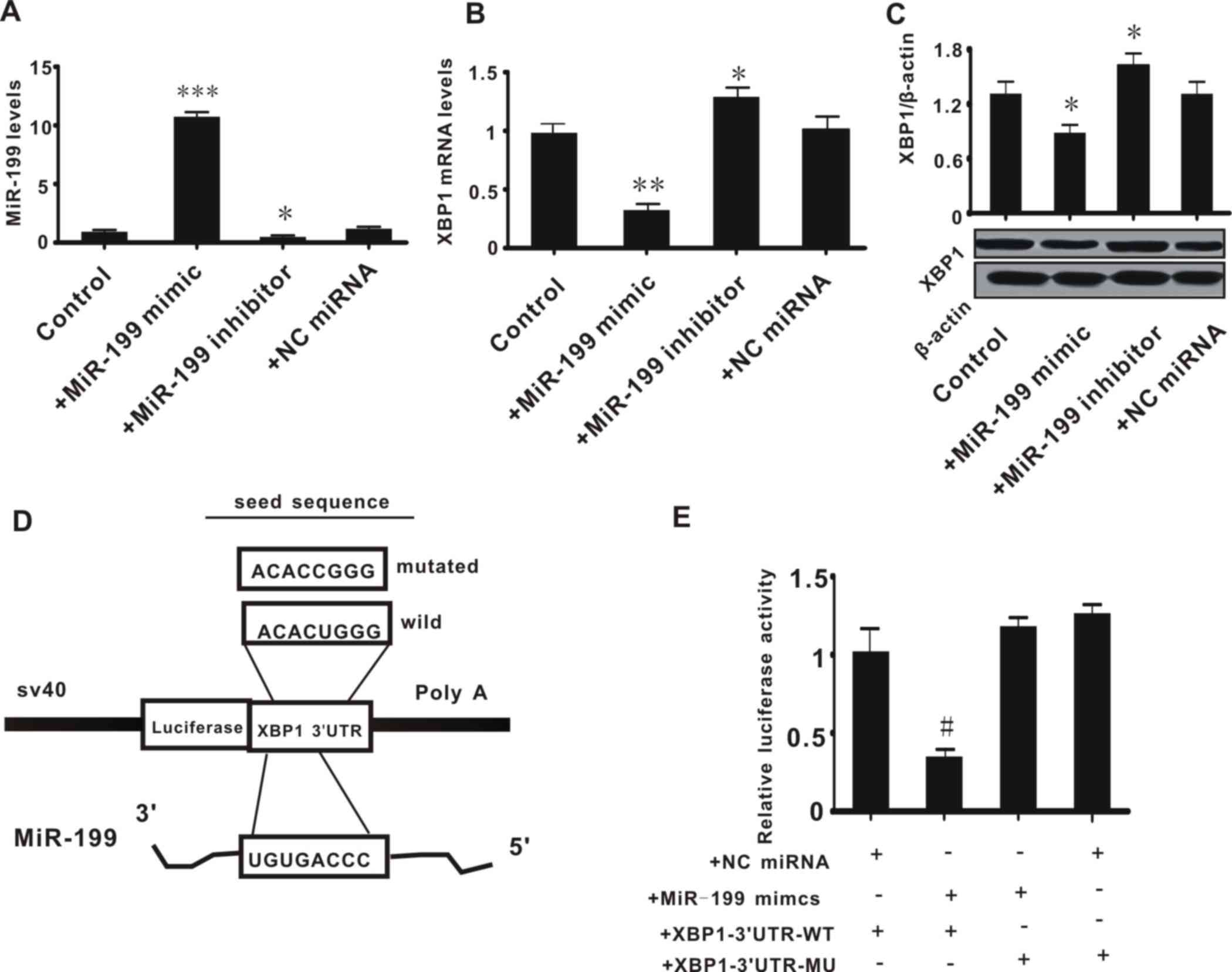
the expression of miR-199, respectively. The expression of miR-199
and XBP1 was then measured. It was observed that transfection with
miR-199 mimic or inhibitor significantly increased (P<0.001) or
decreased (P<0.05) the expression level of miR-199; whereas the
NC miRNA did not alter the level of miR-199 (Fig. 2A). This confirmed the effects of the
miR-199 mimic and inhibitor. The expression of XBP1 (mRNA and
protein) was significantly decreased in Hep3B2.1–7 cells treated
with miR-199 mimic, and was increased in cells transfected with
miR-199 inhibitor, when compared with control cells (P<0.05) or
cells treated with NC miRNA (P>0.05) (Fig. 2B and C). This preliminarily suggested
that miR-199 may negatively regulate the expression of XBP1.
Subsequently, to confirm that miR-199 directly
inhibits the expression of XBP1, a luciferase reporter plasmid was
constructed containing the seed sequence of the 3′UTR of XBP1
(XBP1-3′UTR-WT; Fig. 2D) or a mutated
seed sequence of the XBP1 3′UTR (XBP1-3′UTR-MT). The luciferase
reporter plasmids and miR-199 mimic were co-transfected into
Hep3B2.1–7 cells and the relative luciferase activity was measured.
The miR-199 mimic significantly decreased the relative luciferase
activity in cells transfected with XBP1-3′UTR-WT (P<0.05 vs. NC
miRNA + XBP1-3′UTR-WT group), but had no effect on the cells
treated with XBP1-3′UTR-MT (P>0.05 vs. NC miRNA + XBP1-3′UTR-MT
group; Fig. 2E). This suggested that
miR-199 directly inhibited the expression of XBP1.
Effect of the inhibition or knockdown
of XBP1 on the expression of cyclin D
In the present study, it was determined that there
is an association among miR-199, XBP1 and cyclin D. Considering
miR-199 appeared to suppress XBP1, we speculated that the
miR-199/XBP1 axis has roles in regulating cyclin D expression.
Thus, further experiments were conducted to observe the effect of
the inhibition or knockdown of XBP1 on the expression of cyclin D.
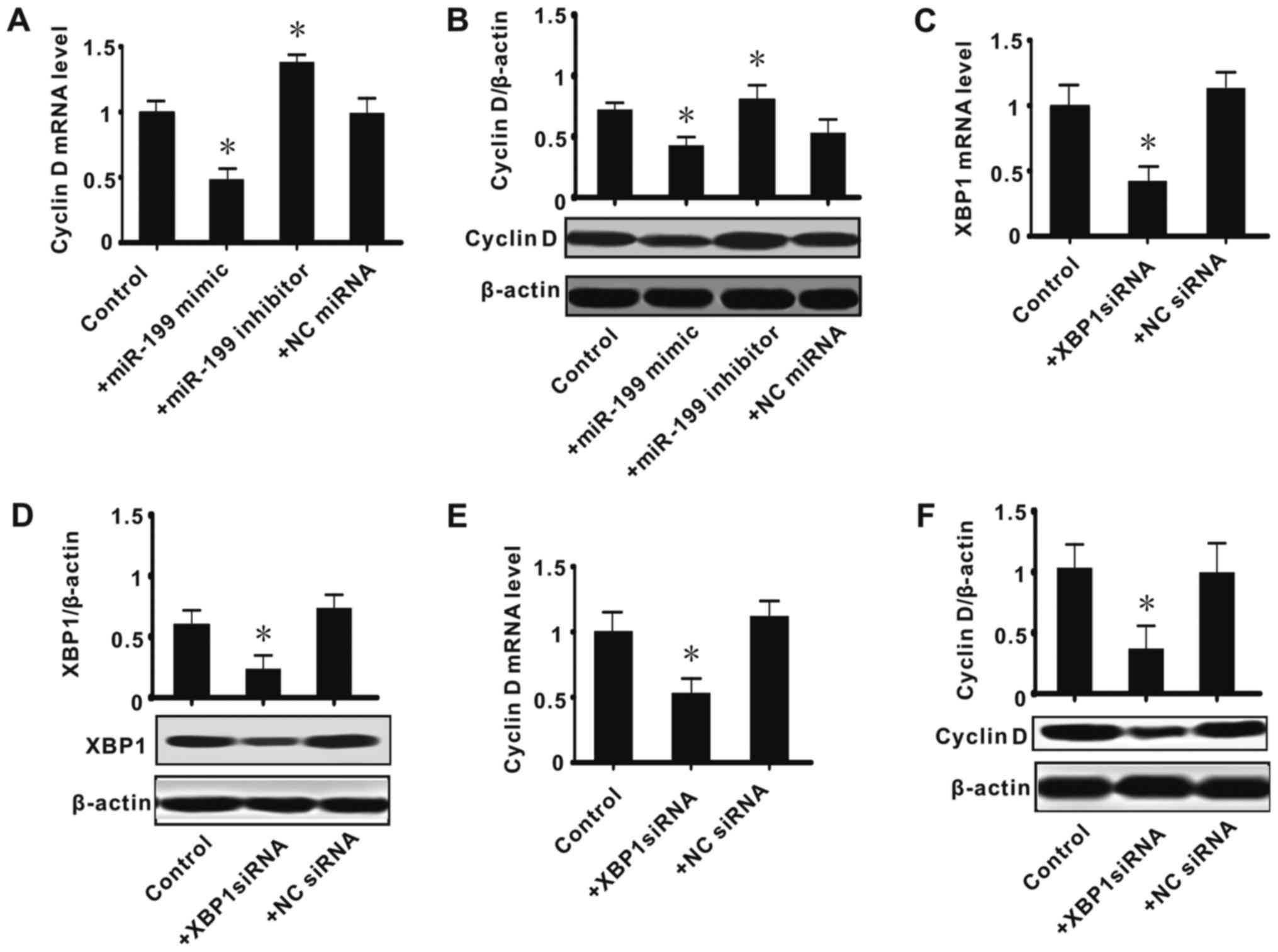
It was determined that exogenous miR-199 significantly inhibited
the expression of cyclin D (mRNA and protein; P<0.05), while
miR-199 inhibitor upregulated its expression (P<0.05; Fig. 3A and B). siRNA against XBP1
significantly suppressed the expression of XBP1 (mRNA and protein;
P<0.05), while the NC siRNA had no effect (Fig. 3C and D). This confirmed the effect of
the siRNA transfection. The effect of XBP1 knockdown on the
expression of cyclin D was next observed, and it was found that
siRNA against XBP1 significantly decreased the expression of cyclin
D (mRNA and protein; P<0.05; Fig.
3E-F). These data suggested that miR-199/XBP1 serve an
important role in cell proliferation.
Effect of exogenous miR-199 mimic or
inhibitor on the proliferation of Hep3B2.1–7 cells
Previous studies have demonstrated that XBP1 may
serve an important role in cell proliferation, and we found that
miR-199 directly suppressed the expression of XBP1. Thus, further
experiments aimed to investigate the effect of miR-199 on the
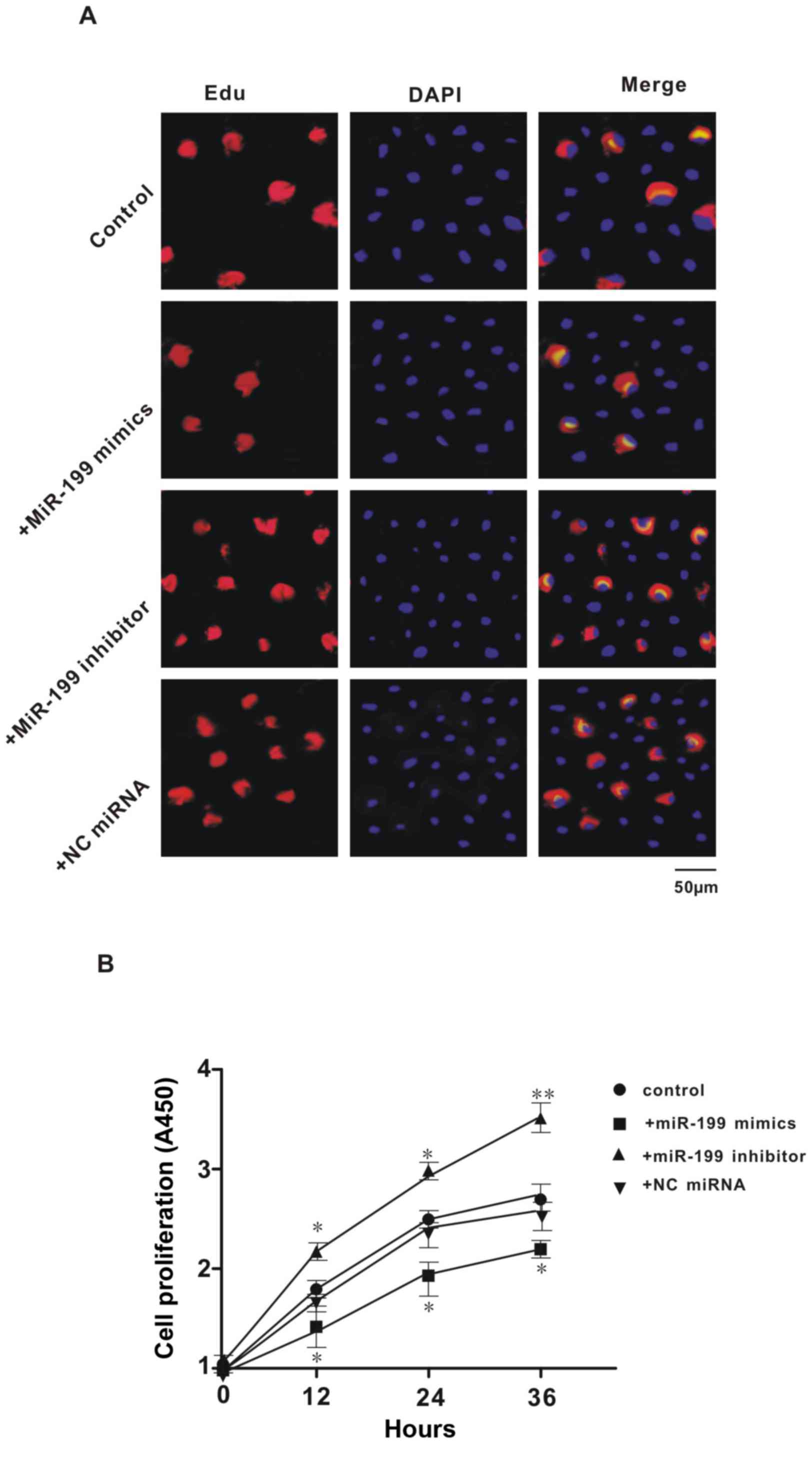
proliferation of Hep3B2.1–7 cells. Results of EdU and DAPI
double-staining indicated that transfection with miR-199 mimic
caused a reduction in the number of newly proliferated cells, while
treatment with miR-199 inhibitor caused an increase in newly
proliferated cells, when compared with controls (Fig. 4A). This indicated that miR-199
inhibited the proliferation of Hep3B2.1–7 cells. Consistent with
the results of EdU and DAPI-double staining, the data from the
CCK-8 assay showed that overexpression or inhibition of miR-199
significantly decreased or increased cell proliferation at all
three time points (12, 24 and 36 h; P<0.05), respectively;
whereas the NC miRNA had no effect (Fig.
4B). These data indicated that miR-199 serves an important role
in the proliferation of Hep3B2.1–7 cells.
Discussion
In the present study, it was demonstrated that
miR-199 may serve an important role in HCC. It was observed that
low expression of miR-199 in HCC tumor tissues was accompanied by
high expression of XBP1 and cyclin D. This suggested that miR-199
may be involved in regulation of the tumor cell cycle. Moreover,
the bioinformatics analysis indicated XBP1 to be a putative target
of miR-199. A luciferase reporter assay and gain- and lose-function
studies of miR-199 demonstrated that miR-199 inhibited the
expression of XBP1 by directly binding to its 3′UTR. As stated, the
expression level of XBP1 was negatively associated with the
expression of miR-199. Knockdown of XBP1 decreased the expression
of cyclin D in Hep3B2.1–7 cells. This confirmed cyclin D as a
downstream target of XBP1. Furthermore, overexpression of miR-199
markedly inhibited the proliferation of Hep3B2.1–7 cells, whereas
inhibition of miR-199 promoted proliferation. Thus the present
study, to our knowledge, is the first to demonstrate that a
miR-199/XBP1/cyclin D axis may be involved in tumor cell
proliferation in of HCC.
HCC is associated with distinct histological and
etiological features compared with other forms of primary liver
cancer (21), and 70–90% of patients
with HCC have an established background of chronic liver disease
and cirrhosis (1). Cirrhosis is a
main risk factor for the development of HCC, the major causes of
which are hepatitis B and hepatitis C viral infection (22,23).
Although 30–40% of patients with HCC are eligible for curative
treatments, which include surgical resection as the first-line
treatment, liver transplantation and percutaneous ablation, there
remains a high frequency of tumor recurrence following surgical
resection, and most cases of HCC appear resistant to conventional
chemotherapy and radiotherapy (1,2). This
suggests that the pathogenesis of HCC is a complex process. In
fact, HCC carcinogenesis may involve various modifications in a
number of molecular pathways as well as genetic alterations, which
ultimately lead to malignant transformation and HCC disease
progression (24). For example,
hepatocellular carcinoma (HCC) has been associated with increased
insulin-like growth factor (IGF) signaling, and sorafenib, a
multi-tyrosine kinase inhibitor, which directly targets the IGF
pathway, is the only chemotherapeutic option for patients with
advanced HCC (3). As is known, the
IGF system is involved in cellular proliferation in most cell
types, particularly in the hyper-proliferation of HCC cells
(3), which serves as indication of
abnormal proliferation being among the main causative factors of
HCC. Therefore, elucidating the mechanism responsible for tumor
cell proliferation in HCC may be key for improvement of HCC
treatment and novel drug development.
It is established that deregulations of cell cycle
genes exist in HCC. For example, retinoblastoma protein is
frequently abnormally expressed in many cell types in HCC, and the
four main cyclin types (D, E, A and B) have been observed to be
overexpressed in HCC and are associated with different outcomes
(25). In addition, dysregulation of
pleiotropic growth factors, receptors and their downstream
signaling pathway components represent a central protumorigenic
component of hepatocarcinogenesis (25). In particular, the IGF/IGF-1 receptor,
hepatocyte growth factor (HGF)/HGF receptor, Wingless/β-catenin,
transforming growth factor-α/epidermal growth factor receptor and
transforming growth factor (TGF)-β/TGF-β receptor pathways
contribute to the proliferative, anti-apoptotic and invasive
behaviors of HCC cells (26).
However, there is no single mechanism that can fully explain the
pathogenesis of tumor cell hyper-proliferation in HCC. Recent
studies revealed that XBP1 was closely linked with tumor occurrence
(4,8,10–12). Numerous researches have reported that
the IRE1/XBP1 pathway is involved in cell survival,
epithelial-mesenchymal transition, transcription factor regulation,
drug resistance and glycolysis in tumor cells (8–13). In
addition, XBP1 has been demonstrated to be necessary for cellular
proliferation (27) and its
underlying mechanism involves regulation of the expression of
matrix metalloproteinase-9 (28) and
phosphatidylinositol-4,5-bisphosphate 3-kinase/mechanistic target
of rapamycin (29). As is known, XBP1
is widely expressed in various tissues in mammals, particularly to
a high level in the liver, where it serves as a necessary factor
for liver cell differentiation and tissue formation (8). Therefore, it may be deduced that XBP1 is
an important molecule responsible for HCC cell proliferation.
Indeed this was indicated by the present experiments, since miR-199
inhibition and therefore increased expression of XBP1 was
positively associated with the proliferation of Hep3B2.1–7
cells.
Recent data have shown that epigenetic alteration is
an important cause of the development and progression of HCC, with
alterations including DNA methylation, acetylation and
post-transcriptional regulation of miRNAs (30). Among these, dysregulation of miRNAs
has been considered as one of the most attractive factors (as they
can be used as disease diagnosis biomarkers) associated with the
pathogenesis of HCC, with reports suggesting that miRNAs serve
critical roles in HCC progression. For example, deregulation of
miRNA may lead to abnormal cell proliferation, evasion of apoptotic
cell death, angiogenesis, and invasion and metastasis, by targeting
a number of protein-coding genes (30). Moreover, several studies on the role
of miRNAs in cell proliferation have found that mir-503a, mir-138,
mir-199 and mir-214 were involved in regulation of the cell cycle
in HCC cells (16–19). Consistently, the present study and
other studies (19,31) have determined that miR-199 expression
was significantly decreased in HCC tumor cells. Furthermore, in the
current study, it was indicated that low expression of miR-199
contributed to the hyper-proliferation of Hep3B2.1–7 cells by
directly suppressing XBP1. Taken together, these data indicate that
the miR-199/XBP1 pathway serves an important role in HCC.
Numerous studied are required to observe the role of
miR-199/XBP1 in HCC, particularly a study investigating of its role
in vivo, including observing the effect of miR-199 on
tumorigenesis when the Hep3B2.1–7 cells were injected into nude
mice. However, to our knowledge, the present study is the first to
demonstrate that low expression of miR-199 was associated with HCC,
and that the underlying mechanism of miR-199 involved direct
suppression of XBP1. This may provide novel theoretical foundation
for understanding the molecular mechanisms of HCC, as well as novel
targets for HCC treatment and drug development.
Acknowledgements
Not applicable.
Funding
The present work was supported by the HuNan
Provincial Science & Technology Department of the Affiliated
Changsha Hospital of Hunan Normal University (grant no. 2018JJ6126
to GHH) and the Education Department of HuNan Province of Changsha
Medical University (grant no. 15C0161 to ZL).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
requires.
Authors' contributions
ZL, YQG and XZ contributed to the acquisition of
data. GHH contributed to the design of the experiment and drafted
the manuscript. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
The use of materials in the present study was
approved by the Hunan Normal University Medical Ethics Committee,
and all patients had signed written informed consent.
Patient consent for publication
Patients were informed and consented to the
publication of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanyal AJ, Yoon SK and Lencioni R: The
etiology of hepatocellular carcinoma and consequences for
treatment. Oncologist. 15 Suppl 4:S14–S22. 2010. View Article : Google Scholar
|
|
2
|
Pievsky D and Pyrsopoulos N: Profile of
tivantinib and its potential in the treatment of hepatocellular
carcinoma: The evidence to date. J Hepatocell Carcinoma. 3:69–76.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enguita-Germán M and Fortes P: Targeting
the insulin-like growth factor pathway in hepatocellular carcinoma.
World J Hepatol. 6:716–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, Sharma D, et al: Sustained
proliferation in cancer: Mechanisms and novel therapeutic targets.
Semin Cancer Biol. 35:S25–S54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Derenzini M, Trerè D, Pession A, Govoni M,
Sirri V and Chieco P: Nucleolar size indicates the rapidity of cell
proliferation in cancer tissues. J Pathol. 191:181–186. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-Sáez JF, de la Torre C, Pincheira J
and Giménez-Martín G: Cell proliferation and cancer. Histol
Histopathol. 13:1197–1214. 1998.PubMed/NCBI
|
|
7
|
Glimcher LH: XBP1: The last two decades.
Ann Rheum Dis. 69 Suppl 1:i67–i71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marumoto Y, Terai S, Urata Y, Matsumoto T,
Mizunaga Y, Yamamoto N, Jin H, Fujisawa K, Murata T, Shinoda K, et
al: Continuous high expression of XBP1 and GRP78 is important for
the survival of bone marrow cells in CCl4-treated cirrhotic liver.
Biochem Biophys Res Commun. 367:546–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Iliopoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1α pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ming J, Ruan S, Wang M, Ye D, Fan N, Meng
Q, Tian B and Huang T: A novel chemical, STF-083010, reverses
tamoxifen-related drug resistance in breast cancer by inhibiting
IRE1/XBP1. Oncotarget. 6:40692–40703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Hou X, Liu M, Yang Z, Bi Y, Zou H,
Wu J, Che H, Li C, Wang X, et al: XBP1 silencing decreases glioma
cell viability and glycolysis possibly by inhibiting HK2
expression. J Neurooncol. 126:455–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chae U, Park SJ, Kim B, Wei S, Min JS, Lee
JH, Park SH, Lee AH, Lu KP, Lee DS and Min SH: Critical role of
XBP1 in cancer signalling is regulated by PIN1. Biochem J.
473:2603–2610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Lin DC, Guo X, Masouleh Kharabi B,
Gery S, Cao Q, Alkan S, Ikezoe T, Akiba C, Paquette R, et al:
Inhibition of IRE1α-driven pro-survival pathways is a promising
therapeutic application in acute myeloid leukemia. Oncotarget.
7:18736–18749. 2016.PubMed/NCBI
|
|
14
|
Diehl JA, Fuchs SY and Koumenis C: The
cell biology of the unfolded protein response. Gastroenterology.
14138(41–41): e1–2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D and Richardson DR: Endoplasmic
reticulum protein 29 (ERp29): An emerging role in cancer. Int J
Biochem Cell Biol. 43:33–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greene CM, Varley RB and Lawless MW:
MicroRNAs and liver cancer associated with iron overload:
Therapeutic targets unravelled. World J Gastroenterol.
19:5212–5226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao F, Zhang W, Chen L, Chen F, Xie H,
Xing C, Yu X, Ding S, Chen K, Guo H, et al: MicroRNA-503 inhibits
the G1/S transition by downregulating cyclin D3 and E2F3 in
hepatocellular carcinoma. J Transl Med. 11:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan Q, Wang X, Gong W, Ni L, Chen C, He
X, Chen F, Yang L, Wang P and Wang DW: ER stress negatively
modulates the expression of the miR-199a/214 cluster to regulates
tumor survival and progression in human hepatocellular cancer. PLoS
One. 7:e315182012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu MC, Yuan JM and Lu SC: Alcohol,
cofactors and the genetics of hepatocellular carcinoma. J
Gastroenterol Hepatol. 23 suppl 1:S92–S97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al:
Management of hepatocellular carcinoma in Asia: Consensus statement
from the Asian Oncology Summit 2009. Lancet Oncol. 10:1111–1118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monga SP: Role of Wnt/β-catenin signaling
in liver metabolism and cancer. Int J Biochem Cell Biol.
43:1021–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bisteau X, Caldez MJ and Kaldis P: The
complex relationship between liver cancer and the cell cycle: A
story of multiple regulations. Cancers (Basel). 6:79–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Breuhahn K, Longerich T and Schirmacher P:
Dysregulation of growth factor signaling in human hepatocellular
carcinoma. Oncogene. 25:3787–3800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa D, Calvo V, Avivar-Valderas A,
Lade A, Chou HI, Lee YA, Farias EF, Aguirre-Ghiso JA and Friedman
SL: Epithelial Xbp1 is required for cellular proliferation and
differentiation during mammary glanddevelopment. Mol Cell Biol.
35:1543–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia T, Tong S, Fan K, Zhai W, Fang B, Wang
SH and Wang JJ: XBP1 induces MMP-9 expression to promote
proliferation and invasion in human esophagealsquamous cell
carcinoma. Am J Cancer Res. 6:2031–2040. 2016.PubMed/NCBI
|
|
29
|
Yang J, Cheng D, Zhou S, Zhu B, Hu T and
Yang Q: Overexpression of X-Box binding protein 1 (XBP1) correlates
to poor prognosis and up-regulation of PI3K/mTOR in human
osteosarcoma. Int J Mol Sci. 16:28635–28646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li CW, Chang PY and Chen BS: Investigating
the mechanism of hepatocellular carcinoma progression by
constructing geneticand epigenetic networks using NGS data
identification and big database mining method. Oncotarget.
7:79453–79473. 2016.PubMed/NCBI
|
|
31
|
El-Abd NE, Fawzy NA, El-Sheikh SM and
Soliman ME: Circulating miRNA-122, miRNA-199a, and miRNA-16 as
biomarkers for early detection of hepatocellular carcinoma in
egyptian patients with chronic hepatitis c virus infection. Mol
Diagn Ther. 19:213–220. 2015. View Article : Google Scholar : PubMed/NCBI
|