Introduction
Breast cancer is one of the most common human
neoplasms, accounting for ~1/4 of cases of cancer in females
worldwide and 27% of cancer cases in developed countries (1). Breast cancer can also occur in men;
however, it is more common in women (2). It is the leading cause of mortality in
women with a global incidence of >1,000,000 cases annually
(3). It typically has a poor
prognosis due to due to the majorty of diagnoses ocuring at an
advanced stage of disease (4). As a
complex disease, alteration of cellular signals serves important
functions in breast cancer development. In order to reduce the
mortality rate of breast cancer, molecular-targeted therapy is
emerging as an alternative treatment (5). In previous decades, efforts have been
concentrated on the discovery of molecular targets; numerous
molecular targeted therapies approved by the Food and Drug
Administration (FDA), have demonstrated remarkable clinical success
in the treatment of breast cancer (6). However, breast cancer associated
incidence and mortality has been steadily increasing from 2000
through to 2011 (7,8). Studies to identify molecular targets
that may improve therapeutic strategies and clinical outcomes are
required.
Centromere protein U (CENPU) encodes for a
putative transcriptional repressor whose deregulation has been
associated with the development of several types of cancer,
including glioblastoma, and prostate and luminal breast cancer
(9–12). It is localized to human chromosome
4q35.1 and is expressed in the nuclei and cytoplasm of the cells of
a number of tissues, including fetal liver, bone marrow, thymus and
testicular tissue (13). The function
and mechanism of CENPU deregulation in breast cancer is
poorly understood. In the present study, CENPU expression
was examined in human breast cancer tissue and cell lines. Using
the lentiviral mediated RNA interference approach; the effects of
CENPU expression inhibition on the cell proliferation,
apoptosis and cell cycle progression of breast cancer MDA-MB-231
cells were investigated.
Materials and methods
Patients and tissue samples
Paired tumor and normal breast tissue specimens were
obtained from 15 patients with breast cancer who were surgically
treated at Zhejiang Hospital (Hangzhou, China) between January 2016
and December 2017. Written informed consent was obtained from each
of the patients, in compliance with the Declaration of Helsinki,
and the study was approved by the Medical Ethics Committee of
Zhejiang Hospital (approval no. 2017-19K). The normal breast
tissues were removed a distance of >3 cm from the tumor margin.
All specimens were fixed in 10% neutral formalin at room
temperature for 24–36 h prior to analysis.
Immunohistochemical analysis
All tissues were formalin-fixed and
paraffin-embedded. Tissues were sectioned into 4-µm slices. Then
the sections were deparaffinized in xylene and rehydrated in a
descending ethylalcohol series (100, 95, 85 and 75%) followed by
distilled water. Immunohistochemical staining was performed using
an enhanced labeled polymer method (14). In brief, tissue sections were
subjected to a boiling antigen retrieval procedure in Ethylene
Glycol Tetraacetic Acid buffer (EGTA, pH 9.0) for 25 min. Following
blocking of endogenous peroxidase activity with 3% hydrogen
peroxide (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) at room temperature for 10 min, the sections were incubated
overnight at 4°C with a polyclonal rabbit anti-human CENPU antibody
(dilution, 1:50; cat. no. ab117078; Abcam, Cambridge, UK). Negative
control sections were treated with PBS (Zhongshan Golden Bridge
Biotechnology Co., Ltd) instead of the primary antibody. Following
incubation with the EnVision System-Horseradish Peroxidase
(PV-8000, Zhongshan Golden Bridge Biotechnology Co., Ltd) at 37°C
for 20 min, the reaction products were visualized by
3,3′-diaminobenzidine staining (Zhongshan Golden Bridge
Biotechnology Co. Ltd) and counterstaining with hematoxylin at room
temperature for 1 min. Immunohistochemical scoring was analyzed by
Carl Zeiss Microscope (at ×400 and ×200 magnification), and this
was conducted by two pathologists by consensus without knowledge of
the clinicopathological information. CENPU expression levels were
scored semi-quantitatively by assessing the intensity of staining
(0, no staining; 1, mild staining; 2, moderate staining; and 3,
strong staining) and by the percentage of positively stained cells
(0, <30%; 1, 30–49%; 2, 50–69%; and 3, ≥70%). The index sum was
the result of totaling the staining intensity and percentage
scores. Based on the results of previous studies (15,16), a
specimen with a final score >4 was considered as positive, while
the others were considered to be negative.
Cell culture
The normal breast MCF-10A cell line was purchased
from the cell bank of GuanDao Biotechnology (http://shgdbio.company.lookchem.cn/; Shanghai, China),
and the breast cancer MDA-MB-231 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA) and the T-47D
cell line was obtained from the cell bank of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured and passaged in
Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% Fetal
Bovine Serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C in a 95%
relative humidified atmosphere containing 5% CO2. The
medium was changed every 2 days for all cell lines.
The Cancer Genome Atlas (TCGA) gene
expression data analysis
Public data of CENPU gene expression for patients
with breast cancer were obtained from the Cancer Genome Atlas
Project (TCGA; http://cancergenome.nih.gov/) and the Firebrowse
dataset (http://firebrowes.org/) (17). The matched tumor and non-tumor
specimens were available for a total of 106 patients.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA of all three cell lines was extracted using a
TRIzol® kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. RNA purity and concentration were
assessed using a NanoDrop 2000c spectrophotometer (Thermo Fisher
Scientific, Inc.). Reverse transcription was performed with the
M-MLV RTase cDNA Synthesis kit (Promega Corporation, Madison, WI,
USA). First, 2.0 µg total RNA and 1 µl Oligo dT (0.5 µg/µl;
Shanghai Sangon, China) were added to a PCR vial, and then RNA-free
H2O was added to reach a total vial volume of 10 µl. The
vial was mixed gently, instantaneously centrifuged, placed in a
70°C warm bath for 10 min and then immediately transferred to an
ice-water bath (0°C). Then, a RT reaction system, including 5×RT
buffer (4.0 µl), RNA-free H2O (2.6 µl), 10 mM dNTPs (2.0
µl; Promega, USA), RNasin (0.4 µl) and M-MLV-RTase (1.0 µl), was
run for 1 h at 42°C, then transferred to a 70°C water bath for 10
min to inactivate the RTase. The RT-qPCR cDNA product was placed at
−20°C for use. PCR was performed using 1.0 µl cDNA with SYBRGreen
PCR Master mix kit (Takara Bio, Inc., Otsu, Japan), according to
the manufacturer's protocol. The RT-qPCR product was detected using
an Agilent MX3000p qPCR system (Agilent Technologies, Inc., Santa
Clara, CA, USA) with GAPDH as an internal control. The primer
sequences were as follows: CENPU forward,
5′-ATGAACTGCTTCGGTTAGAGC-3′ and reverse,
5′-TATTTCGCAGATGGCTTTCGG-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The PCR reaction was performed with an
initial denaturation step of 95°C for 15 sec, followed by 45 cycles
of 95°C for 5 sec and 60°C for 30 sec. Following all the cycles,
95°C denaturation was performed for 1 min, followed by cooling at
55°C for 30 sec. Next, from 55°C, the temperature was increased in
0.5°C increments and maintained for 4 sec at each step for
absorbance readings until a final temperature of 95°C was achieved.
The relative mRNA expression was calculated using the
2−ΔΔCq method (18), with
GAPDH mRNA expression being used for normalization.
shRNA expression lentiviral vector
construction and cell transfection
A short hairpin RNA targeting CENPU sequences
(5′-CAGGTATGAGCTATAATAA-3′) and a control with scrambled
non-silencing RNA (5′-TTCTCCGAACGTGTCACGT-3′) were designed and
synthesized by Shanghai Genechem Co., Ltd., (Shanghai, China). The
synthesized fragments at a final concentration of 100 M were
inserted into the lentiviral vector GV115 at the
AgeI/EcoRI sites to form the recombinant lentiviral
CENPU shRNA expression vector and its control vector. The accuracy
of the constructed vectors was verified via DNA sequencing. The
verified lentiviral vectors carrying CENPU shRNA and non-silencing
RNA were packaged by co-transfecting with Helper 1.0 and Helper 2.0
plasmids (Shanghai Genechem Co., Ltd., Shanghai, China) in 293(T)
cells. The resulting recombinant lentiviruses were then harvested
by centrifugation (4°C, 10 min, 4,000 × g) and purification after
48-h cell culture and designated shCENPU and shCtrl. For lentivirus
transfection, the MDA-MB-231 cell culture in the logarithmic phase
was treated with trypsin (0.25%, pH=8.0, Shanghai Chemical Reagent
Co., Ltd., Shanghai, China) at 37°C for 1–2 min and then
re-suspended in DMEM (Gibco; Thermo Fisher Scientific, Inc.). The
cell suspension (3–5×104 cells) was seeded onto a
six-well plate and incubated at 37°C in 5% CO2 until it
reached 30% confluence. A total of 2×106 TU/ml
lentivirus was then added to the wells according to the
multiplicity of infection (obtained from a preliminary experiment).
The transfection efficiency was measured by green fluorescent
protein (GFP) fluorescence (Shanghai Genechem Co., Ltd., Shanghai,
China), and CENPU gene expression of the transfected cells
was evaluated using RT-qPCR for mRNA and western blotting for
protein 3 days after the transfection.
Western blotting
The transfected shCENPU cells and shCtrl cells were
harvested and lysed in 2X SDS Lysis buffer (Shanghai Chemical
Reagent Co., Ltd.). The protein determination was performed
following the BCA Protein assay kit (Shanghai Beyotime Biotech Co.,
Ltd.) Equal amounts of protein (20 µg) were separated using 10%
SDS-PAGE and electrotransferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA), according to the
standard operating procedure (19–21). The
membranes were then blocked with 5% dry skimmed milk in
Tris-buffered saline with Tween-20 (0.1%) at room temperature for 1
h and incubated with a primary rabbit anti-human CENPU polyclonal
antibody (dilution, 1:500; cat. no. ab117078; Abcam, Cambridge, UK)
at 4°C overnight. A mouse anti-GAPDH monoclonal antibody (dilution,
1:2,000; cat. no. sc-32233; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) was used as the loading control. Subsequent to washing
with TBST (Shanghai Chemical Reagent Co., Ltd.) at room temperature
for 8 min for 4 times, the membranes were incubated with a
horseradish peroxidase-conjugated goat-anti rabbit or goat
anti-mouse secondary antibody (dilution, 1:2,000; cat. no. sc-2004,
2005, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
visualized using an Enhanced Chemiluminesence Western Blotting
Substrate kit (Pierce; Thermo Fisher Scientific, USA).
Cell proliferation assay
The transfected shCENPU and shCtrl cells were plated
in five 96-well plates at equal cell densities (2,000 cells/100 µl
in each well) and cultured at 37°C with 5% CO2 for 24 h.
The plates were then analyzed for GFP expression in each well using
the Celigo Imaging Cytometer (Nexcelom Bioscience, Lawrence, MA,
USA) over a 5-day period. Statistical data mapping and construction
of cell proliferation curves were then performed on the dataset by
GraphPad software (version 5.1, GraphPad Software, Inc., La Jolla,
CA, USA).
An MTT assay was conducted using a commercial assay
kit according to the manufacturer's protocol (GenView, Lausanne,
Switzerland). In brief, the shCENPU and shCtrl transfected cell
cultures were harvested from the primary cultures via trypsin
treatment (0.25%, 37°C, 1–2 min), followed by resuspension in DMEM
(Gibco; Thermo Fisher Scientific, Inc.). The cells were seeded at
equal density (2,000 cell/100 µl in each well) in five 96-well
plates and incubated at 37°C in 5% CO2 for continuous
detection over a 5-day period. The first plate was analyzed after
24-h culture. The culture was terminated by adding 20 µl MTT (5
mg/ml) to each well. After 4 h incubation, the medium was
aspirated, and 100 µl dimethyl sulfoxide (Shanghai Pharmaceutical
Group Co., Ltd., Shanghai, China) was added to each well to
dissolve the purple formazan. The plates were then oscillated for 5
min and the optical densities at 490 nm of each well were measured
using a M2009PR microplate reader (Tecan Group, Ltd., Mannedorf,
Switzerland).
Cell cycle assay
The shCENPU and shCtrl cell cultures were treated
with trypsin and resuspended in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) after achieving 80% confluence. Subsequent to
washing with D-Hanks (Shanghai Genechem Co., Ltd.), the cells were
counted by a hemocytometer to ensure a sufficient number of cells
(≥1×106/well with three wells per experimental group)
were present and pelleted via centrifugation (200 × g) at room
temperature for 5 min. The cell pellet was washed in pre-cooled
D-Hanks (pH, 7.2–7.4), fixed in 70% ethanol at 4°C for 2–3 h, and
stained with 0.6–1 ml propidium iodide solution [40× PI liquor (2
mg/ml; cat. no. P4170; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), 100× RNase A liquor (10 mg/ml; cat. no. EN0531;
Fermentas; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA),
and 1× D-Hanks (Shanghai Genechem Co., Ltd.)]. The suspension was
then filtered through a 300-mesh nylon mesh and analyzed by flow
cytometry using a FACS Calibur instrument (EMD Millipore) at a flow
rate of 300–800 cells/sec. Data analysis was performed using FlowJo
Version 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Apoptosis detection
The shCENPU and shCtrl cell cultures were treated
with trypsin (0.25%, 37°C, 1–2 min) and resuspended in the DMEM
(Gibco; Thermo Fisher Scientific, Inc.) after reaching 85%
confluence. Following washed with D-Hanks (Shanghai Chemical
Reagent Co., Ltd.), the apoptosis status of the cell population was
evaluated using the Annexin V-APC Apoptosis Detection kit
(eBioscience; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. In brief, cells were resuspended in
binding buffer (Shanghai Chemical Reagent Co., Ltd., Shanghai,
China) at a density of 5×105 cells/ml. A total of 200 µl
of this suspension was transferred to a 5 ml tube with 10 µl of the
Annexin V-APC reagent added. Following gentle mixing, the cells
were incubated at room temperature in dark for 15 min. The cells
were then analyzed by flow cytometry using a FACS Calibur
instrument (EMD Millipore), Data analysis was performed using
FlowJo Version 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19; IBM Corp., Armonk, NY, USA). Bar graphs
represent the mean of the data from triplicate experiments with
error bars indicating standard deviations (mean ± standard
deviation). Data analysis for multiple pairs of samples was
performed using the generalized linear model (GLM) using the edgeR
software package (22). Comparisons
between groups were performed using one-way analysis of variance
and the χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CENPU expression in breast cancer
tissues, adjacent normal tissues and cell lines
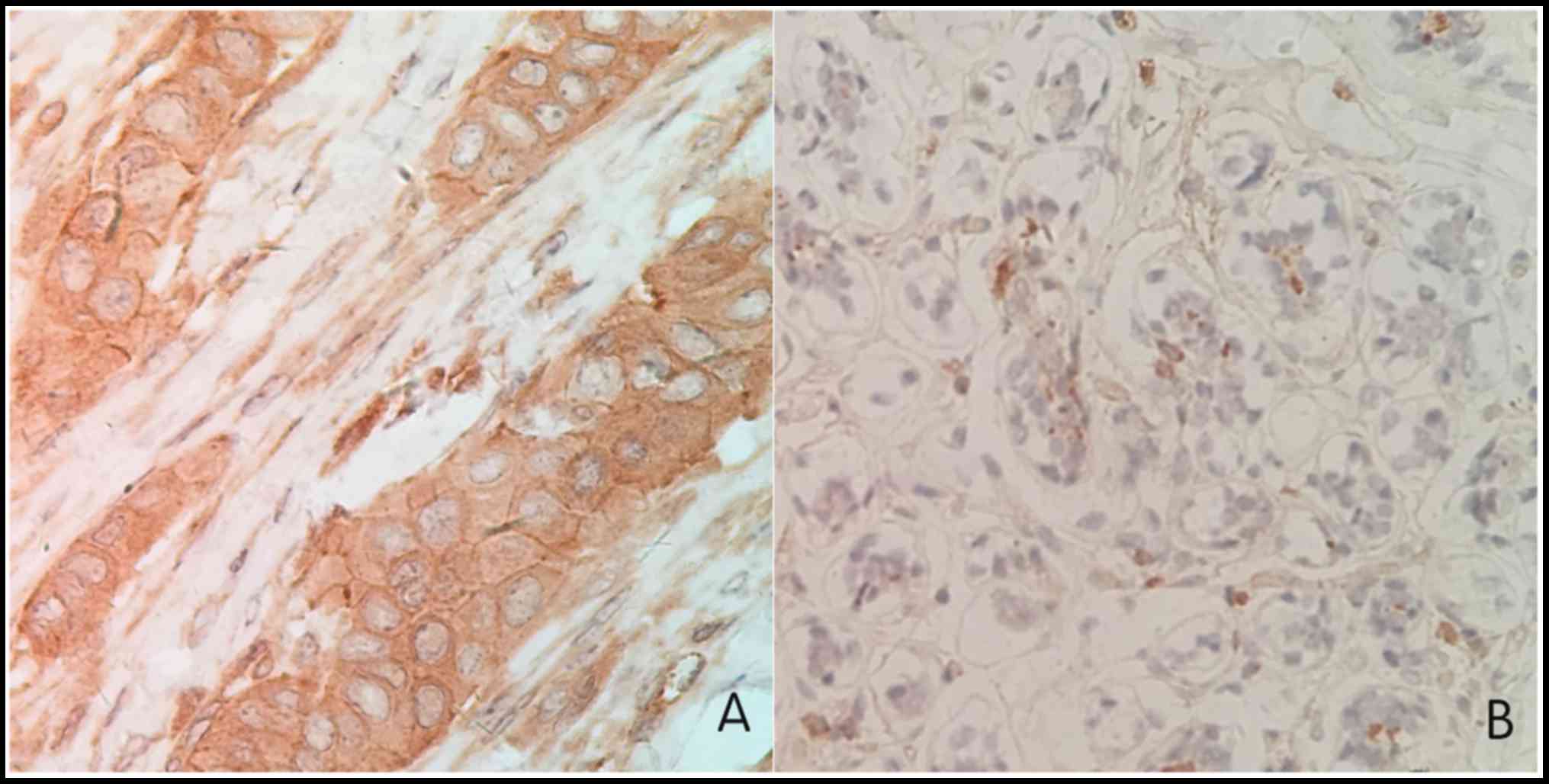
CENPU protein expression was evaluated in 15 paired
tumor and normal breast specimens via immunohistochemistry. The
expression of CENPU was significantly increased in breast cancer
tissues (93.3%) than in adjacent normal tissues (33.3%; Fig. 1; Table
I; P=0.001). Furthermore, a comprehensive analysis of a set of
106 breast cancer samples gene expression data from TCGA database
revealed that CENPU mRNA was often upregulated in breast cancer
tissues relative to non-tumor tissues, and CENPU mRNA expression
was 4.63-fold higher in cancer tissues than in the normal tissues
(P<0.001; Table II). These
results demonstrated that CENPU expression was significantly
upregulated in breast cancer tissues compared with adjacent normal
tissues.
 | Table I.CENPU expression in paired breast
cancer and adjacent normal tissues. |
Table I.
CENPU expression in paired breast
cancer and adjacent normal tissues.
| Samples | N | CENPU expression
(%) | χ2 | P-value |
|---|
| Breast cancer | 15 | 14 (93.30) | 11.627 | 0.001 |
| Normal breast
tissues | 15 | 5
(33.30) |
|
|
 | Table II.Analysis of the expression of CENPU
genes in breast cancer and adjacent normal tissues from The Cancer
Genome Atlas data. |
Table II.
Analysis of the expression of CENPU
genes in breast cancer and adjacent normal tissues from The Cancer
Genome Atlas data.
|
|
| CENPU expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Samples | N | Upregulated | Unchanged | Downregulated | P-value | FC |
|---|
| Paired breast
cancer and adjacent normal tissues | 106 | 89 (84%) | 17 (16%) | 0 (0%) | <0.001 | 4.630 |
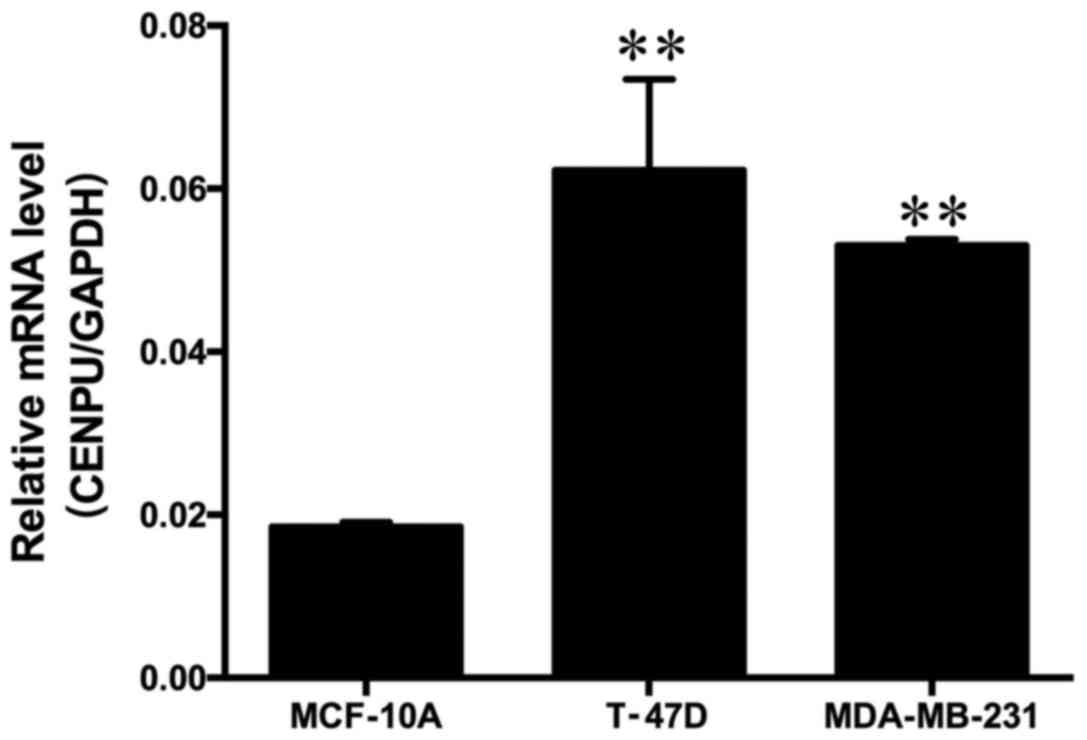
The tissue expression results indicated that high
expression of CENPU may also be present in the breast cancer cell
lines. To test this hypothesis, the CENPU gene expression in two
breast cancer cell lines (T-47D and MDA-MB-231) and the normal
breast MCF-10A cell line was evaluated using RT-qPCR. It was
identified that CENPU mRNA was highly expressed in breast cancer
cell lines compared with the normal breast cell line (Fig. 2).
Knockdown of CENPU by lentivirus
transfection in breast cancer cells
Since the MDA-MB-231 cell line represents a class of
poorly differentiated, highly proliferative and aggressive breast
cancer compared with T-47D, the MDA-MB-231 cell line was selected
for the lentivirus-mediated CENPU-downregulation experiment to
investigate the function of CENPU in cell proliferation and
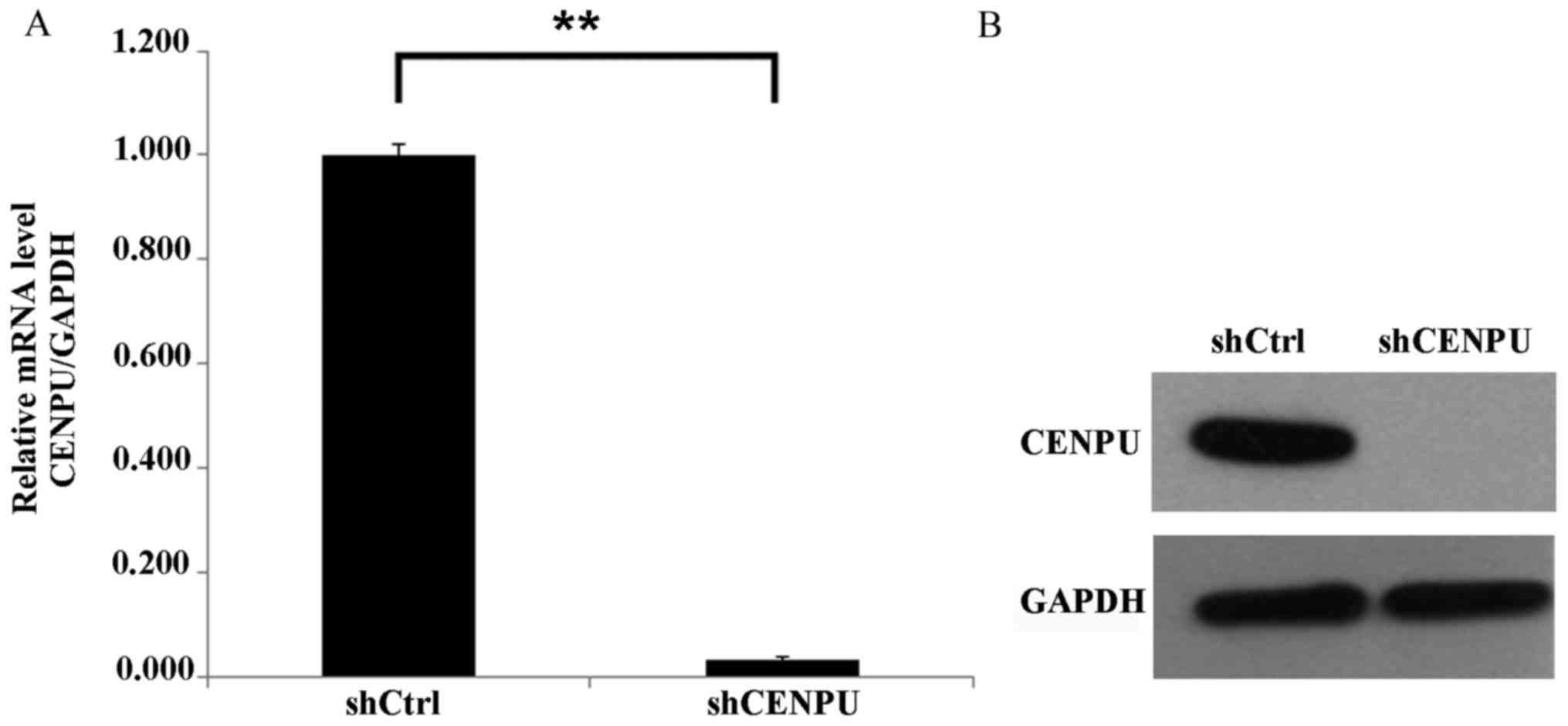
apoptosis. The shCENPU and shCtrl lentiviruses were generated and
transfected into the MDA-MB-231 cell line. The lentivirus
transfection efficiency was measured by GFP expression. After 3
days of transfection, >80% of the cells were transfected.
Post-transfection, the expression of CENPU in MDA-MB-231 cells was
analyzed by RT-qPCR for mRNA expression and western blotting for
protein expression. As presented in Fig.
3, the mRNA and protein expression levels of CENPU were
significantly inhibited in CENPU-shRNA cells compared with the
control cells (P<0.01), indicating successful downregulation of
gene expression by CENPU-shRNA.
Downregulation of CENPU inhibits cell
proliferation
The CENPU expression data presented earlier
indicated that overexpression of the CENPU gene may be associated
with enhanced proliferation of cancer cells. However,
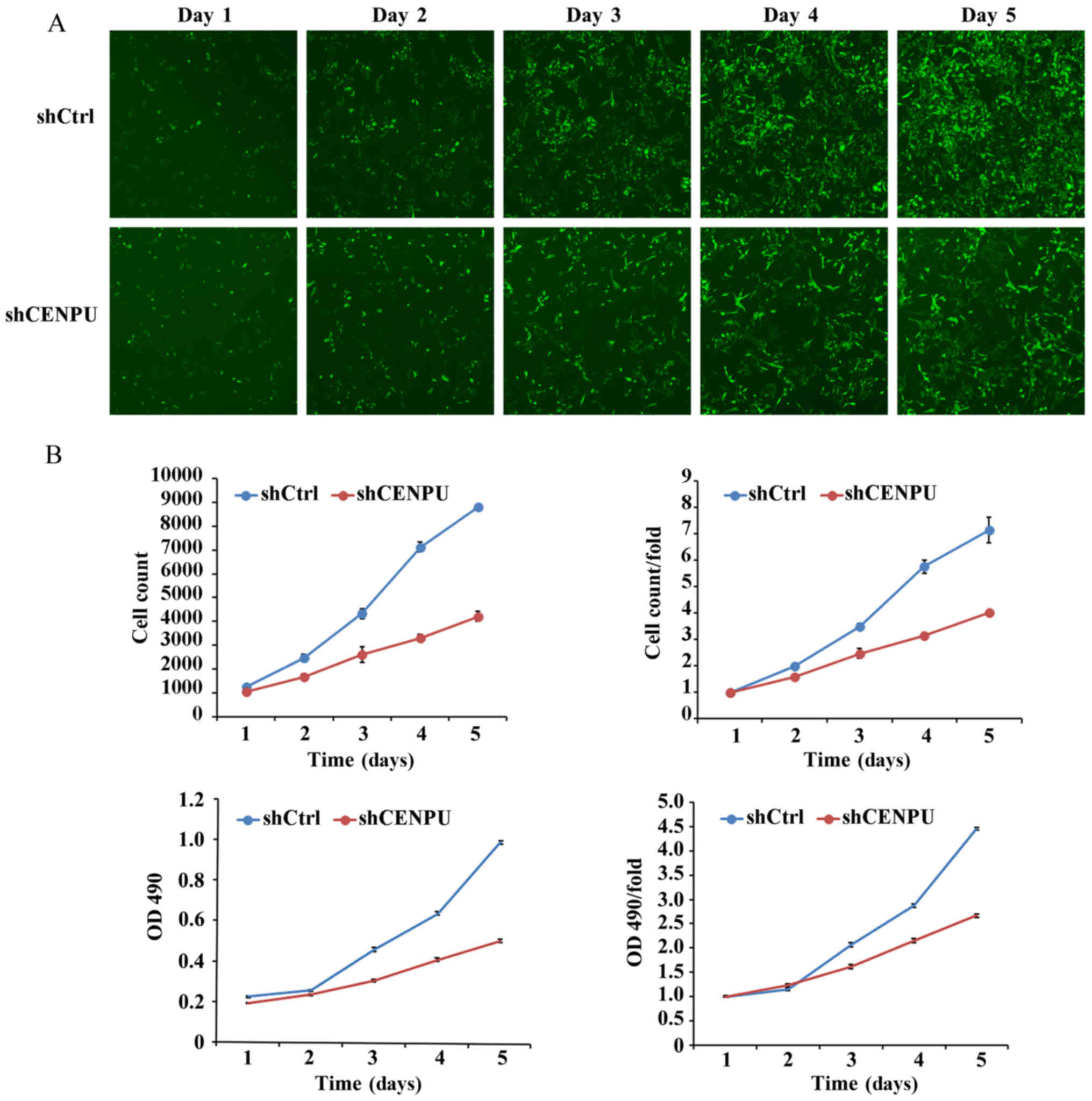
downregulation of CENPU expression inhibited cell proliferation. To
test this hypothesis, the cell growth of the transfected breast
cancer MDA-MB-231 cell line with the shCENPU and shCtrl
lentiviruses was monitored by measuring the GFP expression and
performing MTT assays over a 5 day period. As presented in Fig. 4, the proliferation of MDA-MB-231 cells
was significantly decreased in the CENPU-shRNA cells compared with
that of the control (P<0.01). The cell proliferation difference
between the CENPU-shRNA cells and the control cells could be
observed on the 2nd day of analysis. The difference became more
notable in a time-dependent manner, indicating the inhibitory
effect of CENPU-knockdown on cell proliferation.
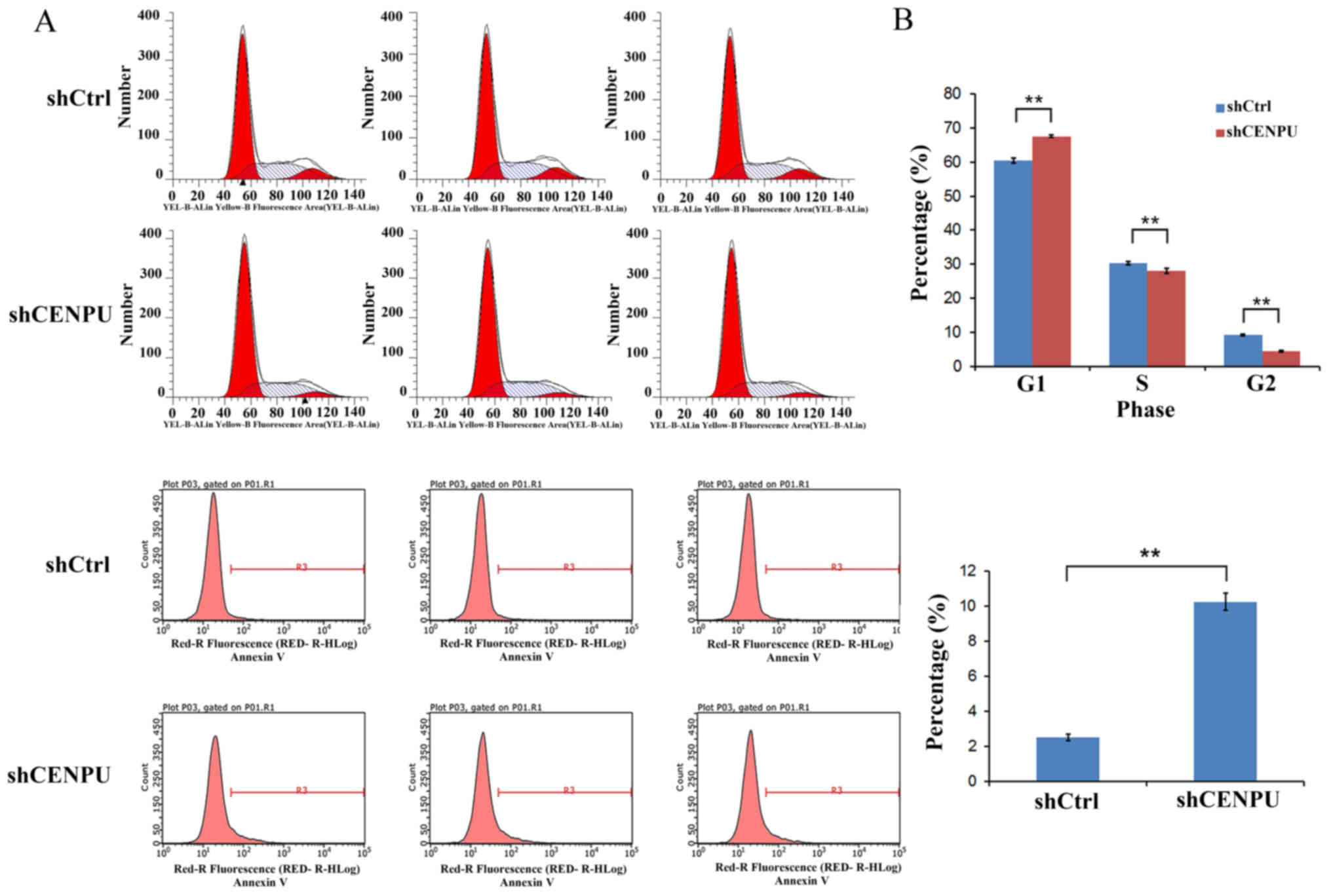
Downregulation of CENPU suppresses
cell cycle progression
The effect of CENPU-downregulation on the cell cycle
progression was further evaluated in breast cancer cells by FACS.
The resulting data revealed that CENPU-downregulation induced a
pronounced alteration in cell cycle distribution. After 5 days of
lentivirus infection, the number of cells in the G2+S phase was
significantly decreased in CENPU-knockdown cells relative to the
control, whereas the number of cells in the G1 phase was
significantly increased (P<0.01; Fig.
5A). These data indicated that cells were arrested in the G1
phase following CENPU-downregulation.
Downregulation of CENPU gene
expression promotes cell apoptosis
To investigate the effect of CENPU on cell
apoptosis, Annexin V-APC staining and flow cytometric analysis were
performed following lentivirus transfection. The data indicated
that downregulation of CENPU gene expression in MDA-MB-231 cells
was able to significantly increase the percentage of apoptotic
cells compared with the percentage of control cells (P<0.01;
Fig. 5B), indicating a marked
apoptotic effect of CENPU-downregulation.
Discussion
CENPU was initially identified as a novel protein
that is specifically associated with Myeloid leukemia factor 1
(MLF1). It encodes a 46-kDa protein that contains two nuclear
localization signals, two nuclear receptor binding motifs, two
leucine zippers and several potential phosphorylation sites
(13). The precise function of CENPU
remains unresolved; however, activity as a suppressor of
transcription has been demonstrated by a study using Kaposi's
sarcoma-associated herpes virus (6).
CENPU was also identified as a constitutive component of the
centromere and served an important function in cell cycle
progression (23,24). It was required for maintenance of
sister chromatid adhesion and stable kinetochore-microtubule
attachment (23,25). Deletion of CENPU may cause a
G2/M delay and abnormal chromosome segregation (23). Previous studies revealed that CENPU
was a phosphorylation substrate of PLK1, and that the
phosphorylation-dependent CENPU-PLK1 interaction is required for
PLK1 recruitment to the interphase and mitosis kinetochores, which
are vital for proper mitotic progression (24,26).
The number of studies on the association between
CENPU expression and human carcinoma is increasing. Hanissian et
al (11,13) have identified that CENPU, which was
co-expressed with MLF1, may serve an important function in
erythroleukemias and glioblastoma pathogenesis by preventing cell
apoptosis and facilitating cell proliferation. Zhang et al
(10) demonstrated that
CENPU-knockdown was able to significantly suppress prostate cancer
cell proliferation and colony formation, and promote cell
apoptosis. In addition to these studies, Chou et al
(27) have identified that CENPU is
one of the genes most commonly associated with breast cancer by
pooled cDNA microarray analysis using logistic regression,
artificial neural networks and decision trees. Huang et al
(12) demonstrated that CENPU is
significantly upregulated in luminal breast cancer tissue in
comparison with adjacent normal tissue in a validated cohort and a
TCGA cohort. In the present study, immunohistochemical and TCGA
analysis suggested that CENPU expression was significantly
upregulated in human breast cancer. In addition, RT-qPCR analysis
revealed that CENPU mRNA was also highly expressed in breast cancer
T-47D and MDA-MB-231 cell lines compared with the normal breast
cell line. These results suggested a possible function of CENPU in
breast cancer pathogenesis.
In order to clarify the cellular mechanism of
CENPU in breast cancer development, the expression of CENPU
was downregulated and the effects on the proliferation, apoptosis
and cell cycle progression of the MDA-MB-231 cells was evaluated.
As was identified by RT-qPCR and western blotting analysis, the
mRNA and protein expressions of CENPU were significantly
decreased in CENPU-shRNA cells relative to the control (P<0.01),
which indicated successful transfection and gene expression
knockdown. Furthermore, the present study demonstrated that the
proliferation activity was significantly suppressed in
CENPU-knockdown cells relative to the control (P<0.01).
The inhibited cell growth may be induced by the alteration of the
cell cycle or cell apoptosis; therefore, FACS was performed for
cell cycle and apoptosis analysis. It was identified that
downregulation of CENPU induced significantly increased cell cycle
arrest at the G1 phase (P<0.01). In addition to dysregulated
cell cycle progression, it was also identified that the percentage
of apoptotic cells was increased following CENPU-downregulation
(P<0.01).
In conclusion, the present study was the first to
demonstrate the effect of CENPU silencing by lentiviral mediated
RNA interference on the proliferation and apoptosis of breast
cancer. Suggesting that downregulation of CENPU gene expression may
suppress cell proliferation, increase the apoptotic rate and alter
cell cycle distribution in human breast cancer cells. These results
implied that CENPU may serve an essential function in breast cancer
pathogenesis and development. Although the present study clearly
illustrated the possible molecular mechanism of the CENPU gene in
breast cancer development promoting cell proliferation and
inhibiting cell apoptosis, further studies are required to
precisely characterize the cellular and molecular mechanisms of
CENPU and its potential relevance to the clinical and pathological
parameters for patients with breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Public Welfare Technology Research Program (grant no.
LGF18H160028), the Zhejiang Provincial Health Bureau Foundation
(grant no. 2013KYA004), the National Natural Science Foundation of
China (grant no. 81771520) and the innovation discipline of
Zhejiang Province to Xujiao Chen.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FP and SL made substantial contributions to study
conception and design, SL, YL, GM and XC were involved in
acquisition of data, analysis and interpretation of data. SL
drafted the manuscript and revised it critically for important
intellectual content.
Ethics approval and consent to
participate
Written informed consent was obtained from each of
the patients, in compliance with the Declaration of Helsinki, and
the present study was approved by the Medical Ethics Committee of
Zhejiang Hospital (approval no. 2017-19K).
Patient consent for publication
All the patients have written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang WT and Zhu XZ: The introduction of
2012 WHO classification of tumours of the breast. Zhonghua Bing Li
Xue Za Zhi. 42:78–80. 2013.(In Chinese). PubMed/NCBI
|
|
2
|
Kreiter E, Richardson A, Potter J and
Yasui Y: Breast cancer: Trends in international incidence in men
and women. Br J Cancer. 110:1891–1897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makki J, Myint O, Wynn AA, Samsudin AT and
John DV: Expression distribution of cancer stem cells, epithelial
to mesenchymal transition, and telomerase activity in breast cancer
and their association with clinicopathologic characteristics. Clin
Med Insights Pathol. 8:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Makki J: Diversity of breast carcinoma:
Histological subtypes and clinical relevance. Clin Med Insights
Pathol. 8:23–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morris LG and Chan TA: Therapeutic
targeting of tumor suppressor genes. Cancer. 121:1357–1368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YT, Tan YJ and Oon CE: Molecular
targeted therapy: Treating cancer with specificity. Eur J
Pharmacol. 834:188–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bode AM, Dong Z and Wang H: Cancer
prevention and control: Alarming challenges in China. Natl Sci Rev.
3:117–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan HY, Zhang YJ, Wang XP, Deng JH, Zhou
FC and Gao SJ: Identification of a novel cellular transcriptional
repressor interacting with the latent nuclear antigen of Kaposi's
sarcoma-associated herpesvirus. J Virol. 77:9758–9768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Ji G, Shao Y, Qiao S, Jing Y, Qin
R, Sun H and Shao C: MLF1 interacting protein: A potential gene
therapy target for human prostate cancer? Med Oncol.
32:4542015.PubMed/NCBI
|
|
11
|
Hanissian SH, Teng B, Akbar U, Janjetovic
Z, Zhou Q, Duntsch C and Robertson JH: Regulation of myeloid
leukemia factor-1 interacting protein (MLF1IP) expression in
glioblastoma. Brain Res. 1047:56–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang DP and Luo RC: MLF1IP is correlated
with progression and prognosis in luminal breast cancer. Biochem
Biophys Res Commun. 477:923–926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanissian SH, Akbar U, Teng B, Janjetovic
Z, Hoffmann A, Hitzler JK, Iscove N, Hamre K, Du X, Tong Y, et al:
cDNA cloning and characterization of a novel gene encoding the
MLF1-interacting protein MLF1IP. Oncogene. 23:3700–3707. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grbesa I, Pajares MJ, Martinez-Terroba E,
Agorreta J, Mikecin AM, Larráyoz M, Idoate MA, Gall-Troselj K, Pio
R and Montuenga LM: Expression of sirtuin 1 and 2 is associated
with poor prognosis in non-small cell lung cancer patients. PLoS
One. 10:e01246702015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH,
Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, et al: Expression of
DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You TK, Kim KM, Noh SJ, Bae JS, Jang KY,
Chung MJ, Moon WS, Kang MJ, Lee DG and Park HS: Expressions of
E-cadherin, Cortactin and MMP-9 in Pseudoepitheliomatous
hyperplasia and squamous cell carcinoma of the head and neck: Their
relationships with clinicopathologic factors and prognostic
implication. Korean J Pathol. 46:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandran UR, Medvedeva OP, Barmada MM,
Blood PD, Chakka A, Luthra S, Ferreira A, Wong KF, Lee AV, Zhang Z,
et al: TCGA expedition: A data acquisition and management system
for TCGA data. PLoS One. 11:e01653952016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burnette WN: ‘Western blotting’:
Electrophoretic transfer of proteins from sodium dodecyl
sulfate-polyacrylamide gels to unmodified nitrocellulose and
radiographic detection with antibody and radioiodinated protein A.
Anal Biochem. 112:195–203. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuentes JM, Lompré AM, Møller JV, Falson P
and le Maire M: Clean Western blots of membrane proteins after
yeast heterologous expression following a shortened version of the
method of Perini et al. Anal Biochem. 285:276–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minoshima Y, Hori T, Okada M, Kimura H,
Haraguchi T, Hiraoka Y, Bao YC, Kawashima T, Kitamura T and
Fukagawa T: The constitutive centromere component CENP-50 is
required for recovery from spindle damage. Mol Cell Biol.
25:10315–10328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee KS, Oh DY, Kang YH and Park JE:
Self-regulated mechanism of Plk1 localization to kinetochores:
Lessons from the Plk1-PBIP1 interaction. Cell Div. 3:42008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hua S, Wang Z, Jiang K, Huang Y, Ward T,
Zhao L, Dou Z and Yao X: CENP-U cooperates with Hec1 to orchestrate
kinetochore-microtubule attachment. J Biol Chem. 286:1627–1638.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hori T, Okada M, Maenaka K and Fukagawa T:
CENP-O class proteins form a stable complex and are required for
proper kinetochore function. Mol Biol Cell. 19:843–854. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou HL, Yao CT, Su SL, Lee CY, Hu KY,
Terng HJ, Shih YW, Chang YT, Lu YF, Chang CW, et al: Gene
expression profiling of breast cancer survivability by pooled cDNA
microarray analysis using logistic regression, artificial neural
networks and decision trees. BMC Bioinformatics. 14:1002013.
View Article : Google Scholar : PubMed/NCBI
|