Introduction
Infantile hemangiomas are mostly superficial and
often exist in the infant patient's skin and subcutaneous tissues.
If the infant patient has ulcers and recurrent hemorrhage, scar and
functional damage, it can increase the difficulty of the treatment
of infantile hemangiomas and endanger the health and safety of the
infant patient (1). At present,
clinical treatment methods of infantile hemangiomas are mainly
steroid drugs, laser, surgery, interferon, bleomycin and
vincristine. The systematic application of steroid drugs is a
first-line method in the clinical treatment of hemangiomas, and it
can effectively control the progress of the disease. However, the
long-term application is prone to irritability, Cushing's syndrome,
immune dysfunction, retarded growth and other side-effects.
Moreover, the clinical curative effects of steroid drugs are
controversial in clinical medicine (2,3). As a
non-selective β-receptor blocker, propranolol can effectively slow
down or control the proliferation of hemangiomas and accelerate the
regression of hemangiomas. However, its mechanism for the treatment
of hemangiomas is not yet completely clear and needs further study
(4). Laser, as a commonly used method
for clinical treatment of hemangiomas, has remarkable clinical
effects. Laser therapy for minor hemangiomas can obtain
satisfactory results. Based on the above, propranolol combined with
laser was adopted in the treatment of infantile hemangiomas and the
application value was analyzed in this study, so as to improve the
short-term curative effect and safety of the treatment of infantile
hemangiomas.
Materials and methods
Patients
Inclusion criteria (5,6)
i) All the selected patients were clinically
diagnosed with infantile hemangiomas and all of them were single
hemangiomas. ⅱ) Patients without drug allergy history or allergic
constitution. ⅲ) Patients who had not received special treatment
prior to the study, such as freezing, hormone, injection of
sclerosing agent and nuclide application. ⅳ) Patients with no
neurological diseases, blood system diseases and other benign and
malignant tumors. ⅴ) The present study was approved by the Ethics
Committee of the Affiliated Hospital of Jining Medical University
(Jining, China); and ⅵ) The parents of the infant patients were
informed of the study and signed the informed consent.
Exclusion criteria (7)
i) Patients with light allergy. ⅱ) Patients with
cicatricial diathesis. ⅲ) Patients with bleeding or coagulation
disorders. ⅳ) Patients with vascular malformations, visceral
hemangiomas, thrombocytopenic purpura and other related syndromes.
ⅴ) Involuntary participants; and ⅵ) Those with low treatment
cooperation.
General data
A total of 100 cases of infantile hemangiomas
admitted to the Affiliated Hospital of Jining Medical University
from October 2014 to June 2016 were selected into this study, and
all of them met the inclusion criteria. According to the random
number table method, they were divided into the control and
observation groups, with 50 cases in each group. There were 22
males and 28 females in the control group, at the age of 1–7
months, with an average age of 3.6±1.2 months, including 19 cases
of superficial hemangiomas, 31 cases of mixed hemangiomas. There
were 20 males and 30 females in the observation group, at the age
of 1–6 months, with an average age of 3.4±1.5 months, including 21
cases of superficial hemangiomas and 29 cases of mixed hemangiomas.
There were no statistically significant differences in basic data
between the two groups of infant patients, and the data were
comparable (p>0.05).
Methods
The infant patients in the observation group were
treated with propranolol combined with laser, while the infant
patients in the control group were treated with laser alone (laser
method was the same as that in the observation group). The two
groups of infant patients were followed up for 3–6 months.
Propranolol
Before treatment, the infant patients underwent
related auxiliary examinations such as blood routine, electrolytes,
chest radiograph, liver and kidney function, cardiac enzymes,
electrocardiogram and B-ultrasound of tumor location, and computed
tomography angiography (CTA) and magnetic resonance angiography
(MRA) were performed if necessary. The infant patients with normal
blood routine, electrolytes, chest radiograph, liver and kidney
function, cardiac enzymes and electrocardiogram were guided and
helped to take propranolol (NMPN H61020344; Shaanxi Yongshou
Pharmaceutical Co., Ltd., Shaanxi, China) orally, at 1.0 mg/(kg·d)
divided into 3 doses on the first day. The infant patients were
observed, and if no special discomfort occurred, propranolol was
given at 1.5 mg/(kg·d) divided into 3 doses on the second day. The
infant patients were observed, and if no special discomfort
occurred, propranolol was given at 2.0 mg/(kg·d) divided into 3
doses on the third day. The infant patients' vital signs were
monitored closely during treatment, and the normal group continued
to take the dose according to the dose of the third day. The infant
patients were given blood routine, electrolytes, liver and kidney
function, cardiac enzymes and electrocardiogram reexamination every
2–3 weeks during initial medication time. If there was no abnormal
occurrence after oral medication for 2 months, the reexamination
interval was changed to 1 month. B-ultrasound reexamination of
tumor location was conducted if necessary.
Laser
The long-pulse 1,064 nm Nd:YAG laser was used for
the therapy, with light spot of 9 mm, by the application of contact
synchronous cooling technology. The laser parameters were set as
follows: the pulse width was 12 msec, single pulse, and the energy
density was 40–70 J/cm2. The low energy density was used
in the first treatment of the infant patients, and the energy
density was gradually increased according to the changes of the
lesions in the infant patients, so as to ensure that the overlap of
each spot was <10%. The endpoint of treatment was as follows:
the local temperature of the tumor was slightly increased; the
volume of the tumor was slightly increased or decreased; the
original skin color (red) of the tumor was darkened or shallower,
and the texture of the tumor became soft. After treatment, the
infant patients were treated with intermittent cold compress with
soft ice bags, 20 min each time, with continuous ice compress for 3
days. The next treatment was performed every 3 weeks until the skin
lesions subsided. If there was obvious edema in the treatment site
of the infant patients, appropriate amount of prednisone could be
given for 3–5 days.
Observation indexes
The healing time, the number of times of laser
therapy, the short-term curative effect, the changes in serum
inflammatory factors before and after treatment and the incidence
of adverse reactions were observed and analyzed between the two
groups of infant patients. i) Short-term curative effect: the
curative effects were evaluated according to the grading standards
formulated by a previous report (8)
as follows: cured: the tumor body in the infant patients was
decreased by ≥76.0% after treatment. Markedly effective: the tumor
body in the infant patients was decreased by 51.0–75.0% after
treatment. Effective: the tumor body in the infant patients was
decreased by 26.0–50.0% after treatment. Ineffective: the tumor
body in the infant patients was decreased by <25.0% after
treatment. ⅱ) Serum inflammatory factors (9): including interleukin (IL)-2, IL-6 and
IL-10. In the two groups of infant patients, 3 ml elbow blood was
extracted in fasting state before and after treatment, and
centrifuged at 1,750 × g for 15 min at 4°C. The serum was separated
and the supernatant was collected. Serum inflammatory factors were
detected by enzyme-linked immunosorbent assay. ⅲ) Adverse reactions
(10): including ulcers, decreased
heart rate, decreased appetite, mild high potassium, nausea and
vomiting.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 statistical software (IBM Corp., Armonk, NY, USA) was adopted
for data analysis. The enumeration data were expressed as
percentages and cases, and χ2 test was used for
comparisons between the groups. The measurement data are expressed
as (means±SD), and t-test was used for comparisons between the
groups. P<0.05 indicates that the difference was statistically
significant.
Results
Healing time and the number of times
of laser therapy
The healing time and the times of laser therapy of
the infant patients in the observation group were less than those
of the infant patients in the control group, and the differences
were statistically significant (p<0.05) (Table I).
 | Table I.Comparisons of the healing time and
the times of laser therapy between the two groups of infant
patients (means±SD). |
Table I.
Comparisons of the healing time and
the times of laser therapy between the two groups of infant
patients (means±SD).
| Groups | Healing time
(month) | Times of laser
therapy (times) |
|---|
| Control (n=50) | 12.6±4.7 | 7.4±1.2 |
| Observation
(n=50) |
8.7±2.5 | 6.9±0.8 |
| t-test | 5.180 | 2.452 |
| P-value | <0.05 | <0.05 |
Short-term curative effect
The short-term curative effect of the observation
group [98.00% (49/50)] was higher than that in the control group
[82.00% (41/50)], and the difference was statistically significant
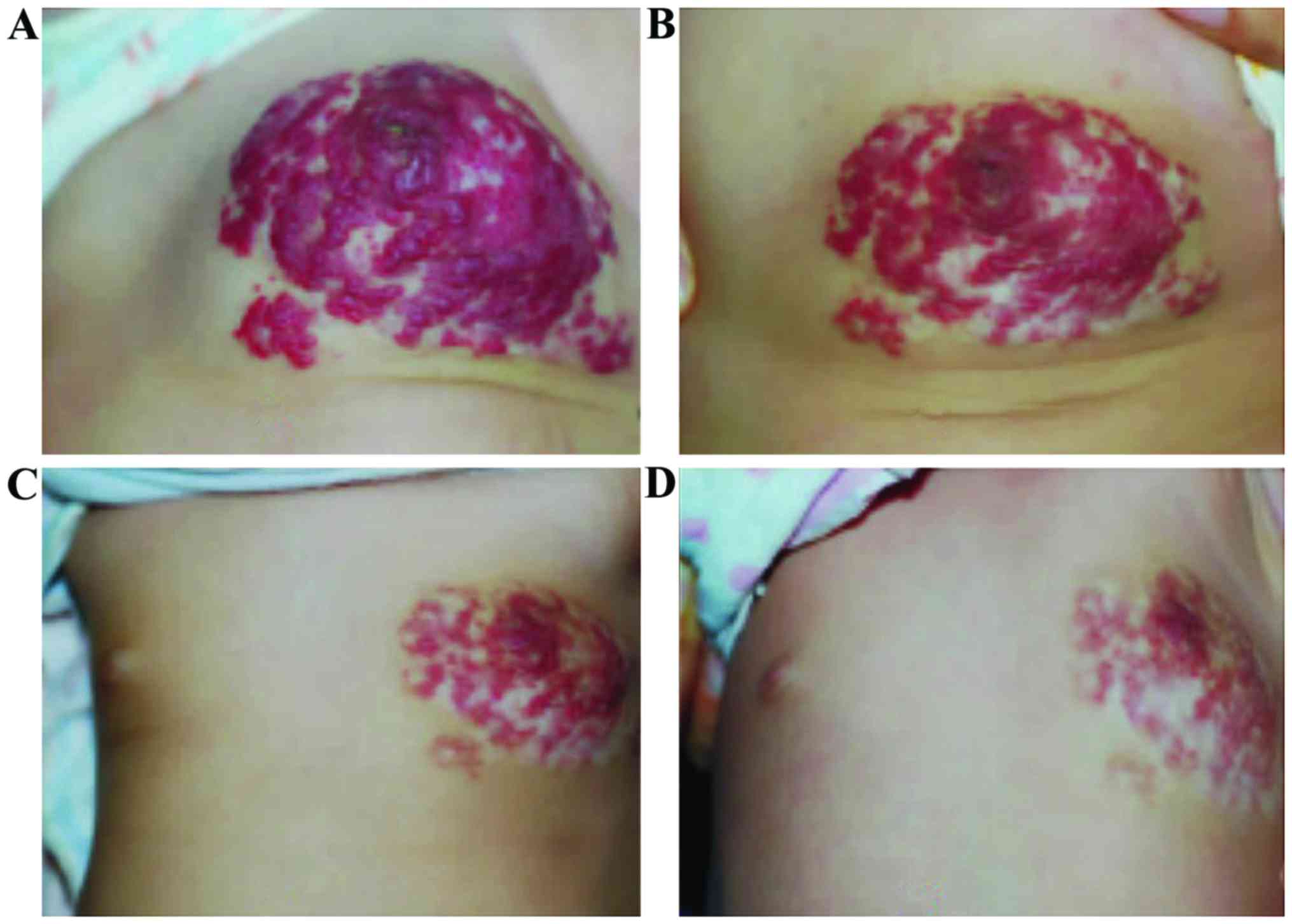
(p<0.05) (Table II, Fig. 1).
 | Table II.Comparison of the short-term curative
effect of the two groups of infant patients (n, %). |
Table II.
Comparison of the short-term curative
effect of the two groups of infant patients (n, %).
| Groups | Cured | Markedly
effective | Effective | Ineffective | Total effective
rate |
|---|
| Control (n=50) | 9
(18.0) | 21 (42.0) | 11 (22.0) | 9 (18.0) | 41 (82.0) |
| Observation
(n=50) | 15 (30.0) | 26 (52.0) | 8
(16.0) | 1 (2.0) | 49 (98.0) |
| χ2 | – | – | – | – | 7.111 |
| P-value | – | – | – | – | <0.05 |
Serum inflammatory factors
The levels of serum inflammatory factors IL-2, IL-6
and IL-10 in the two groups of infant patients after treatment were
lower than those before treatment, and they were lower in the
observation group than those in the control group, and the
differences were statistically significant (p<0.05) (Table III).
 | Table III.Changes in levels of serum
inflammatory factors before and after treatment in the two groups
of infant patients (means±SD). |
Table III.
Changes in levels of serum
inflammatory factors before and after treatment in the two groups
of infant patients (means±SD).
| Groups | Control (n=50) | Observation
(n=50) | t-test | P-value |
|---|
| IL-2/β-actin |
| Before
treatment | 0.89±0.17 | 0.91±0.14 | 0.642 | >0.05 |
| After
treatment | 0.63±0.21 | 0.36±0.08 | 16.875 | <0.05 |
|
t-test | 6.805 | 24.119 |
|
|
|
P-value | <0.05 | <0.05 |
|
|
| IL-6/β-actin |
| Before
treatment | 0.55±0.11 | 0.56±0.08 | 0.520 | >0.05 |
| After
treatment | 0.39±0.07 | 0.25±0.05 | 11.508 | <0.05 |
|
t-test | 8.677 | 23.23 |
|
|
|
P-value | <0.05 | <0.05 |
|
|
| IL-10/β-actin |
| Before
treatment | 0.77±0.24 | 0.79±0.16 | 0.490 | >0.05 |
| After
treatment | 0.56±0.12 | 0.40±0.09 | 7.543 | <0.05 |
|
t-test | 5.534 | 15.022 |
|
|
|
P-value | <0.05 | <0.05 |
|
|
Adverse reactions
There was no statistically significant difference in
the incidence of adverse reactions between the two groups of infant
patients (p>0.05) (Table IV).
 | Table IV.Comparison of the incidence of adverse
reactions between the two groups of infant patients (n, %). |
Table IV.
Comparison of the incidence of adverse
reactions between the two groups of infant patients (n, %).
| Groups | Ulcer | Decreased heart
rate | Decreased
appetite | Mild high
potassium | Nausea and
vomiting | Total incidence |
|---|
| Control (n=50) | 1 (2.0) | 2 (4.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) | 4 (8.0) |
| Observation
(n=50) | 1 (2.0) | 2 (4.0) | 2 (4.0) | 0 (0.0) | 0 (0.0) | 5
(10.0) |
| χ2 | – | – | – | – | – | – |
| P-value | – | – | – | – | – | >0.05 |
Discussion
Infantile hemangiomas are common benign tumors in
infants and children. Under normal circumstances, it does not exist
when the infant is born. Most of infantile hemangiomas grow fast
within 3–12 months and disappear spontaneously after 3–7 years. The
incidence rate is 10–12% in the newborns and >20% in the
underweight premature infants, mostly in female infants (11,12). It
has been found that hemangiomas easily affect the esthetics of the
body, and can cause ulcers and other related reactions, even the
risk of death in serious cases. Currently, propranolol and laser
are important methods for the clinical treatment of infantile
hemangiomas, and they can effectively control the progression of
the disease and reduce the risk of adverse reactions. However, the
specific mechanisms for treatment are not yet completely clear and
remain to be further studied (13).
Propranolol, as a commonly used non-selective
β-receptor blocker, is commonly used in the treatment of
hypertension and supraventricular tachycardia, hyperthyroidism,
angina pectoris and other cardiovascular diseases (14). The peak plasma concentration can be
reached in patient at 1–3 h after oral administration, and its
half-life is approximately 3.0–6.0 h. The relevant mechanisms are
mainly that it can promote vasoconstriction, inhibit angiogenesis
and induce apoptosis of related endothelial cells (15). At the same time, propranolol can
reduce the release of nitric oxide to contract peripheral blood
vessels at the early stage of treatment. With the prolongation of
treatment, Raf mitogen is gradually downregulated, and protein
kinase pathway is activated, and the expression of basic fibroblast
growth factor (bFGF) and vascular endothelial growth factor (VEGF)
is decreased, and the hemangiomas further subside. Moreover, by
antagonizing the glut receptor, or acting on other related unknown
pathways, propranolol can accelerate the apoptosis of vascular
endothelial cells, thereby promoting the regression of the tumor
(16,17).
Laser is a commonly used method for clinical
treatment of hemangiomas, mainly based on the selective
photothermolysis in the treatment of vascular diseases. Hemangioma
endothelial cells are the targets, and oxyhemoglobin and
deoxyhemoglobin are the color bases, both of which have many
absorption peaks (8). A study has
shown that the pulsed dye laser wavelengths are 585 and 595 nm,
which are close to oxyhemoglobin absorption peak. They can induce
specific thermal damage to the abnormal dilated blood vessels,
cause no damage to the adjacent skin tissues, and can further
promote the regression of hemangiomas. However, the penetration
depth is shallow at 1–2 mm and the epidermal melanin is absorbed
completely, combined with the impact of purpura, all of which can
limit the application of pulsed dye laser in the treatment of some
hemangiomas with deeper location and thicker tumor body (18). The wavelength of 1,064 nm is at
another absorption peak with a deeper penetrating power and has no
significant effect on the epidermal melanin barrier. Therefore, the
long-pulse 1,064 nm Nd:YAG laser therapy has a better curative
effect in the treatment of hemangiomas with a certain depth and
thickness, and has no side-effects such as purpura, and the
side-effect of epidermis is smaller (19). Therefore, propranolol combined with
laser in the treatment of hemangiomas can effectively improve the
curative effects. The results in this study showed that, after
treatment, the healing time and the number of times of laser
therapy of the infant patients in the observation group were less
than those in the control group. The short-term curative effect was
higher than that in the control group. The levels of inflammatory
factors were lower than those in the control group. However, there
was no significant difference in the incidence of adverse reactions
between the two groups of infant patients. The results suggested
that propranolol combined with laser in the treatment of infantile
hemangiomas has remarkable curative effects and high safety, and it
can effectively shorten the healing time, reduce the number of
times of laser therapy and the expression of inflammatory factors,
and promote the rehabilitation of infant patients. However, the
sample size in this study was small. Consequently, the observation
time was short, and the long-term curative effect and safety of the
infant patients were not analyzed. At the same time, random
grouping may result in a certain bias on the results. In-depth
study should be carried out in the future by increasing the sample
size.
In summary, the application of propranolol combined
with laser in the treatment of infantile hemangiomas has remarkable
short-term curative effects, high safety and fewer side-effects. It
can effectively reduce the levels of inflammatory factors,
accelerate the regression of hemangiomas, shorten the healing time
of the infant patients, reduce the number of times of laser
therapy, and promote the rehabilitation of the infant patients.
Therefore, it is worthy of clinical promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and XL wrote and finalized this manuscript. XS
and XL were also involved in the conception and design of the
study. NL helped with the patient data collection. SY performed the
laser treatment. XX and LN recorded and analyzed the observation
indexes. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Jining Medical University (Jining,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
parents of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah S and Frieden IJ: Treatment of
infantile hemangiomas with beta-blockers: A review. Skin Therapy
Lett. 18:5–7. 2013.PubMed/NCBI
|
|
2
|
Williams EF III, Hochman M, Rodgers BJ,
Brockbank D, Shannon L and Lam SM: A psychological profile of
children with hemangiomas and their families. Arch Facial Plast
Surg. 5:229–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manunza F, Syed S, Laguda B, Linward J,
Kennedy H, Gholam K, Glover M, Giardini A and Harper JI:
Propranolol for complicated infantile haemangiomas: A case series
of 30 infants. Br J Dermatol. 162:466–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Léauté-Labrèze C, de la Roque Dumas E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haggstrom AN, Drolet BA, Baselga E,
Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
Newell B, et al: Prospective study of infantile hemangiomas:
Clinical characteristics predicting complications and treatment.
Pediatrics. 118:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frieden IJ, Haggstrom AN, Drolet BA,
Mancini AJ, Friedlander SF, Boon L, Chamlin SL, Baselga E, Garzon
MC, Nopper AJ, et al: Infantile hemangiomas: Current knowledge,
future directions. Proceedings of a research workshop on infantile
hemangiomas, April 7–9, 2005, Bethesda, MA. Pediatr Dermatol.
22:383–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chim H, Armijo BS, Miller E, Gliniak C,
Serret MA and Gosain AK: Propranolol induces regression of
hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann
Surg. 256:146–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taraboletti G, Sonzogni L, Vergani V,
Hosseini G, Ceruti R, Ghilardi C, Bastone A, Toschi E, Borsotti P,
Scanziani E, et al: Posttranscriptional stimulation of endothelial
cell matrix metalloproteinases 2 and 1 by endothelioma cells. Exp
Cell Res. 258:384–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arevalo JF, Shields CL, Shields JA, Hykin
PG and De Potter P: Circumscribed choroidal hemangioma:
Characteristic features with indocyanine green videoangiography.
Ophthalmology. 107:344–350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vergani V, Garofalo A, Bani MR, Borsotti
P, Parker MP, Drudis T, Mazzarol G, Viale G, Giavazzi R,
Stetler-Stevenson WG, et al: Inhibition of matrix
metalloproteinases by over-expression of tissue inhibitor of
metalloproteinase-2 inhibits the growth of experimental
hemangiomas. Int J Cancer. 91:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chai Q, Chen WL, Huang ZQ, Zhang DM, Fan S
and Wang L: Preliminary experiences in treating infantile
hemangioma with propranolol. Ann Plast Surg. 72:169–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selmin A, Foltran F, Chiarelli S, Ciullo R
and Gregori D: An epidemiological study investigating the
relationship between chorangioma and infantile hemangioma. Pathol
Res Pract. 210:548–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adler AP, Daly C, Parveen AA, Nevins T,
Shan J, Fairhurst J, Huang T, Martin J, Papadopoulos N, Yancopoulos
GD, et al: Blockade of angiopoietin-2 or Tie2 is equally effective
at inhibiting tumor growth and reducing tumor vessel density in
most human tumor xenograft models. Cancer Res (AACR Annual Meeting
2014). 74 19 Suppl:Abst 4492. 2014.
|
|
15
|
Lomas-Neira JL, Heffernan DS, Ayala A and
Monaghan SF: Blockade of endothelial growth factor, angiopoietin-2,
reduces indices of ARDS and mortality in mice resulting from the
dual-insults of hemorrhagic shock and sepsis. Shock. 45:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tal R, Seifer DB, Grazi RV and Malter HE:
Angiopoietin-1 and angiopoietin-2 are altered in polycystic ovarian
syndrome (PCOS) during controlled ovarian stimulation. Vasc Cell.
5:182013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue CH, Shu H, Feng JH and Zhang DY: The
efficacy and safety of oral propranolol combination with topical
application of timolol maleate eye drops on infant hemangioma. J
Dermatol Venereol. 27:179–184. 2015.
|
|
18
|
Lee D, Boscolo E, Durham JT, Mulliken JB,
Herman IM and Bischoff J: Propranolol targets the contractility of
infantile haemangioma-derived pericytes. Br J Dermatol.
171:1129–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emiralioğlu N, Oğuz B, Akyüz C, Yalçın B,
Kiper N, Ersöz DD, Yalçın E and Özçelik U: Successful treatment of
pulmonary hemangioma with propranolol. Pediatr Pulmonol.
49:829–833. 2014. View Article : Google Scholar : PubMed/NCBI
|