Introduction
Cervical cancer (CC) is the second most common type
of cancer affecting the female population. The World Health
Organization (WHO) and GLOBOCAN 2012 have recorded ~528,000 new
cases of CC globally (1). The
development of CC takes place over several years, and involves a
precancerous stage known as cervical intraepithelial neoplasia
(CIN), which is divided into three main stages (CIN1, CIN2, CIN3).
The primary cause of this disease is infection by human
papillomavirus (HPV). At present, over 100 types of HPV have been
identified, of which the most prevalent types are HPV16/18, which
are responsible for cervical carcinogenesis (2). According to the latest data the most
common HPV virus identified in high-grade lesions is HPV16 (47.4%)
followed by HPV31 (12.4%), HPV33 (7.1%) and HPV18 (7.1%) (3). The HPV genome is divided into three main
regions; the long control region (LCR), which is composed of six
open reading frames (ORFs); and the ‘late region’, with two ORFs
coding for the viral structural proteins L1 and L2 (4). The major mechanism that engages HPV16/18
in cervical carcinogenesis is the manifestation of two early viral
genes: E6 and E7 (known as viral oncogenes). E6 protein binds to
the tumor suppressor gene p53 and causes its degradation while E7
inactivates the pRb gene. These mechanisms cause disruptions of
cell cycle regulation (5).
Cytological screening has reduced the number of
CC-associated mortalities; however, CC is still among the most
prevalent oncological diseases, as many women underestimate the
importance of regular gynecological examinations.
At present, classical cytology-based screening is
being replaced in favor of diagnosing patients with high-risk HPV
types, studies are also focused on additional co-factors, such as
epigenetics and the search for suitable bio-markers identified via
a triage test, PAP smear or HPV test. Epigenetic changes are stable
alterations of gene expression without alteration in the DNA
sequence itself, and can cause disease even in the absence of a
mutation in the gene (6). These
changes also include DNA methylation, which is characterized as a
covalent chemical modification by the addition of a methylated
group to the fifth carbon of the cytosine ring (5mC), thereby
preventing access to proteins. DNA methylation is a typical
mammalian cellular process and is one of the most well-established
markers that defines a molecular landscape that is altered in
cancer. Cytosine methylation in vertebrates is found
primarily at CG dinucleotides (CpGs). CpGs are usually
unmethylated in normal cells, while sporadic CpG sites in the other
parts of the genome are methylated. This is associated with gene
silencing. Aberrant DNA methylation occurs in the majority of types
of cancer, and cause the silencing of some tumor-suppressor genes
(TSG) leading to tumor cell growth (7). CpGs hypermethylation is not randomly
distributed in carcinogenesis, and therefore may provide a useful
signature for tumor diagnosis and prognosis (8).
Following this, several biomarkers (CADM1, DAPK1,
CDH1, EPB41L3, FAM19A4, MAL, PAX1, TERT, PRDM14) have been
studied which describes the hypermethylation that affect the
expression of these genes, thus inducing or accelerating the
carcinogenic mechanism, e.g., by deregulation of TSG (9). The present study focused on determining
the mean intron region methylation of the two TSGs. They are known
as T-lymphocyte maturation associated protein (MAL) and cell
adhesion molecule 1 (CADM1).
CADM1 was first observed in patients with
non-small cell lung carcinoma (NSCLC) and mapped on
chromosome 11q23 (10). This gene was
proven to suppress tumor growth through anti-proliferative and
pro-apoptotic activity, and the loss of CADM1 expression
leads to tumor formation and metastasis (11,12).
Hypermethylation of CADM1 is one of the principal causes of
gene silencing (13) and was also
detected in 40% cases of lung cancer, 32% of prostate cancer cases,
27% of pancreatic adenocarcinoma cases and 83% of cervical
carcinoma cases (14,15). The MAL gene is located in the
2q11.1 region and the hypermethylation of its promoter region
diminishes its tumor suppressor activity. In 90% of samples from
spinocellular carcinoma patients and 93% of samples from patients
with diagnosed adenocarcinoma, hypermethylation of certain areas of
this TSG has been demonstrated (16).
In several studies, DNA methylation of the CADM1/MAL
gene promoter has been shown to increase with the severity of
cervical disorder (17–19) and that these epigenetic changes point
to the presence of more severe high-grade dysplasias (CIN2+). The
levels of average methylation in TSG is extremely high in cervical
carcinomas and significantly increases in CIN3 lesions in women
with high risk (HR)-HPV infection (20).
The aim of the present study was to investigate the
methylation levels of CADM1 and MAL using cytological
smears by quantitative pyrosequencing analysis. The focus was on
specific intronic regions have not been described in much detail
previously, in order to examine the significance of the methylation
levels of individual CpGs in CADM1 and MAL in
HPV16/18 positive patients and also in samples of patients with
cervical inflammation.
Materials and methods
Specimens and study population
Cervical specimens were obtained from 91 female
patients in collaboration with the Department of Obstetrics and
Gynecology at Jesenius Faculty of Medicine in Martin (Martin,
Slovakia) and the Department of Molecular Biology and Division of
Oncology (Biomedical Center Martin JFM CU, Slovakia). Cytological
smears from the uterine cervix were collected and stored in a
LBC/APTIMA transport fluid medium. The present study was approved
by the Ethical Committee of Jessenius Faculty of Medicine in Martin
and all patients provided written informed consent. The patient's
clinical protocols were reviewed for clinical data, diagnosis and
age of patients. The smears were divided into two groups (1st
group: divided into 4 subgroups according to dysplasia, 2nd group:
controls) according to the diagnosis of the patients. 1st group
included patients who had been operated or diagnosed with cervical
carcinoma or had visited an outpatient clinic for due to the
presence or deterioration of a cervical lesion. Based on the
condition and progression of cervical neoplasia, the following four
subgroups were established diagnosis group (Dg).1: cervical
inflammation without dysplasia, 20 samples, Dg.2: CIN1, 14 samples,
Dg.3: 19 samples of CIN2+ [CIN2, CIN3, carcinoma in situ
(CIS) included] and Dg.4, 7 patients with squamous cell carcinoma
(SCC). Clinical diagnosis of individual samples were confirmed by
histological examination. As the 2nd group: controls (Dg. 0), 31
samples were collected from patients without uterine cervical
lesions, with normal onco-cytology outcome and who had not
previously received cervical surgery. A total of 20 inflammation
samples, 40 CIN1-CIN3/CIS or carcinomas and 31 control samples were
collected. The highest median age (48.7 years) was in the SCC group
(range, 32–68 years) and 41.5 years in patients with cervical
inflammation (range, 22–64 years). The lowest median age was in the
CIN2+ group (32 years) with range 21–65 years, followed by CIN1 (33
years) with a range of 19–67 years. The control group only has a
slightly higher median age (38 years) than the CINs (range 23–75
years (Table I). In this case we did
not confirm any statistical significance and therefore the
differences in the median age between groups did not affect the
results.
 | Table I.Median age of the patients and
positivity rates for HPV16/18 in the cervical scrapes in regard to
their most severe underlying histological diagnosis. |
Table I.
Median age of the patients and
positivity rates for HPV16/18 in the cervical scrapes in regard to
their most severe underlying histological diagnosis.
| Dg. | Histology | Total n | Median age (range),
years | HPV 16/18 positive,
n (%) |
|---|
| 0 | Control | 31 | 38 (23–75) | 6 (19) |
| 1 | Inflammation | 20 | 41.5 (22–64) | 7 (35) |
| 2 | CIN 1 | 14 | 33 (19–67) | 6 (42.8) |
| 3 | CIN 2+ | 19 | 32 (21–65) | 13 (68.4) |
| 4 | SCC | 7 | 48.7 (32–68) | 7 (100) |
DNA extraction and bisulfite
conversion
Cervical cells (from all groups) yielded from swab
smears were stored in LBC/APTIMA vials, and nucleic acids were
extracted using a kit, subject to the type of medium in which the
samples were stored. The first type contained dissolved cervical
cells in the LBC medium, and cells were isolated by the DNase Blood
and Tissue kit (Qiagen GmbH, Hilden, Germany). For the second type
of DNA extraction a commercially available MasterPure™
Complete DNA and RNA Purification kit (Epicentre; Illumina, Inc.,
San Diego, CA, USA) was used, for the cells contained in 500 µl of
APTIMA transport medium. The concentration and quality of the
sample was determined by measuring the sample purity in a UV
spectrophotometer, and loaded onto 1.5% agarose gel. Subsequently,
1–2 µg of DNA was bisulfite-treated using an EpiTect Bisulfite kit
(Qiagen, Inc., Valencia, CA, USA). Up to 2 µg of DNA were used in a
total reaction volume of 20 µl; the total amount of bisulfite
reaction was 140 µl (also containing 85 µl bisulfite mix and 35 µl
of DNA Protect Buffer). The exact protocol for this reaction was
described previously (21). Following
bisulfite treatment genomic DNA was stored at −20°C until
polymerase chain reaction (PCR) analysis.
HPV DNA and detection
A PCR reaction was used to diagnose the most common
HPV genotypes (HPV16 and 18) by using primers that have been
described and published in previous literature (22). The primers were designed according to
the sequences from the PGMY09/11 primer set (23). The resulting sequence and the
optimization of the PCR conditions were described previously
(23).
CpG assays and analysis of selected
regions of CADM1 and MAL
For the amplification of bisulfite-converted DNA a
PyroMark PCR kit (Qiagen, Inc.) was used. The total PCR reaction
volume was 25 µl. For analysis of selected regions of CADM1
(3 CpG) and MAL (4 CpG) genes, commercially available CpG
assays [PyroMark CpG Assay (200) Hs_CADM1_01_PM (978746,
PM00049686), PyroMark CpG Assay (200) Hs_MAL_01_PM (978746,
PM00011935, Qiagen GmbH)] (21) were
used and with the exact sequence region for analysis (Figs. 1 and 2).
Briefly, the obtained PCR products were stored overnight in the
refrigerator (+4°C), and 5 µl of the product was removed for
electrophoretic analysis (1.75% agarose gel with ethidium bromide
staining). Subsequently, 10–20 µl of PCR products were subjected to
pyrosequencing by PyroMark Q96 ID (Qiagen, Inc). Completely
methylated and unmethylated DNA were used as control samples
(EpiTect Control DNA, methylated/EpiTect Control DNA, unmethylated;
Qiagen GmbH). For the immobilization of the PCR product to the
beads, a mixture of 10 µl optimized biotinylated PCR product, 1.5
µl streptavidin-coated sepharose beads, 40 µl Binding buffer and
28.5 µl deionized water was prepared. Bisulfite modified DNA was
placed into a thermal cycler with the following program:
Denaturation (95°C, 5 min), incubation (60°C, 25 min), denaturation
(95°C, 5 min), incubation (60°C, 85 min), denaturation (95°C, 175
min), incubation (60°C, 25 min) and incubation (20°C). The total
volume of a capture was pipetted into each well of the Pyromark
plate low. Analysis was conducted according to the manufacturer's
protocol, which was described previously (21).
Statistical data analysis
The two-sample test for equality of proportions with
a continuity correction was used to examine the hypothesis of the
equality of proportions of HPV+ and HPV- in patients and controls.
Robust one-way analysis of variance was used to test the hypothesis
of equality of medians in the patients groups for each CpG island.
The test was followed by the Tukey honest significant difference
post-hoc test. The effect size was quantified by the 95% CI.
Univariate and multivariate logistic regression models were used to
analyze the dependence between a response and predictor(s). The
predictive ability was visualized by the receiver operating curve
(ROC) and quantified by the AUC, the area under ROC. The cut-off on
the class probability was determined by the Youden method, and
translated into a cutoff on the median methylation. All analyses
were performed using R version 3.2.3 (24). P<0.05 was considered to indicate a
statistically significant difference.
Results
HPV 16/18 detection and
genotyping
The present study confirmed the presence of one or
two high-risk genotypes of HPV in 39 cases (42.85%); however, the
number of positive subtypes of HPV DNA per patient were not
discerned. HPV DNA was present in 19% (6/31) of controls, in 35%
(7/20) of patients with inflammation, in 42.8% (6/14) of CIN1
cases, in 68.4% (13/19) of CIN2+ cases and in 100% (7/7) of SCC
(Table I). Logistic regression was
used to analyze the dependence between the status and age in the
patient and control groups. However, the association was not
statistically significant (P=0.636). The two-sample test for
equality of proportions with a continuity correction was used to
examine the hypothesis of the equality of proportions of HPV+ and
HPV- in patients and controls. HPV infection has been significantly
present in CIN2+ cases (P=0.001528), with a 95% CI of −0.78 and
−0.19, and in SCC cases (P=0.0002933), 95% CI: (−1, −0.58).
Pyrosequencing
Samples modified with sodium bisulfite were
subsequently used in the PCR reaction. Using methylate-specific
primers, we converted cytosine to uracil, respectively thymine in
the PCR product while methylated cytosine remained unchanged. The
present study focused on the detection of methylated regions in the
sequence of two TSGs (MAL and CADM1) by
pyrosequencing. By logistic regression it was identified that the
MAL/CADM1 methylation status in the control group was
not associated with age of patients (P-values are not presented).
By the Welch two-sample test with the one-side alternative the
median methylation in HPV+/− DNA in all patient groups were
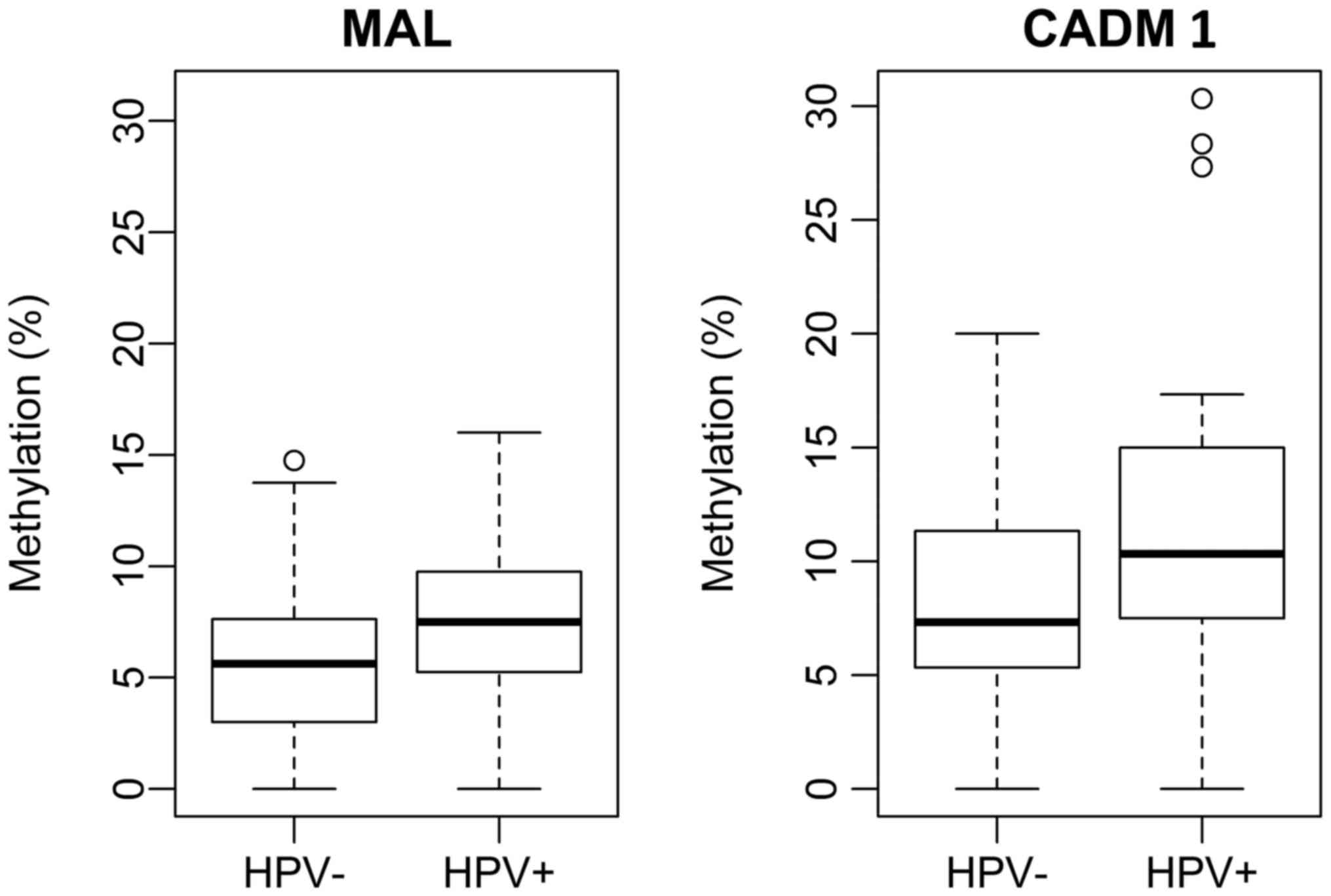
compared. For MAL and CADM1 the median methylation
was identified as significantly higher in HPV positive patients
[P=0.0097, 95% CI: (−0.030, −0.003) for MAL/P=0.0024, 95%
CI: (−0.06, −0.01) for CADM1] than in the patients negative
for HPV DNA (Fig. 3).
Average methylation and determining
the degree of methylation of individual CpG islands
By the Robust One-way ANOVA the hypothesis of
equality of medians in the patients groups for each CpG island were
examined. The ANOVA P-values were P=0.0002 for MAL (1st
CpG), P=0.0066 for MAL (2nd CpG), P=0.1099 for MAL
(3rd CpG), P=0.0207 for MAL (4th CpG)/P=0.0074 for
CADM1 (1st CpG), P=0.0063 for CADM1 (2nd CpG),
P=0.0007 for CADM1 (3rd CpG). Tukey's post-hoc HSD test was
used to perform the multiple comparisons testing. Pairs (CpG
islands) with significantly different methylations are presented in
Table II.
 | Table II.Multiple comparisons testing of each
CpG island in the two tumor-suppressor genes. |
Table II.
Multiple comparisons testing of each
CpG island in the two tumor-suppressor genes.
| Dg. | MAL 1. | MAL 2. | MAL 3. | MAL 4. | CADM1
1. | CADM1
2. | CADM1
3. |
|---|
| 0 vs. 1 |
0.00000c |
0.00000c |
0.01914a |
0.00031c |
0.00010c |
0.00006c |
0.00002c |
| 0 vs. 2 |
0.00632b |
0.01790a | 0.11492 | 0.46309 | 0.57563 | 0.46030 |
0.03467a |
| 0 vs. 3 |
0.00692b |
0.00179b |
0.02417a |
0.01624a |
0.00147b |
0.02081a |
0.00170b |
| 0 vs. 4 |
0.01825a | 0.06606 | 0.95381 | 0.12388 |
0.02095a |
0.04557a |
0.01732a |
| 1 vs. 2 | 0.36317 | 0.06729 | 0.60254 |
0.03027a |
0.02851a | 0.05680 | 0.17161 |
| 2 vs. 3 | 0.57294 | 0.83631 | 0.88263 | 0.24036 |
0.02276a | 0.21970 | 0.80962 |
| 2 vs. 4 | 0.41027 | 0.59283 | 0.18941 | 0.25177 |
0.03047a | 0.06590 | 0.30761 |
An ANOVA with Tukey's post hoc test, was used to
test the hypothesis of equality of medians in the patient groups,
for averaged 4 resp. 3 CpG islands. The ANOVA P-values were:
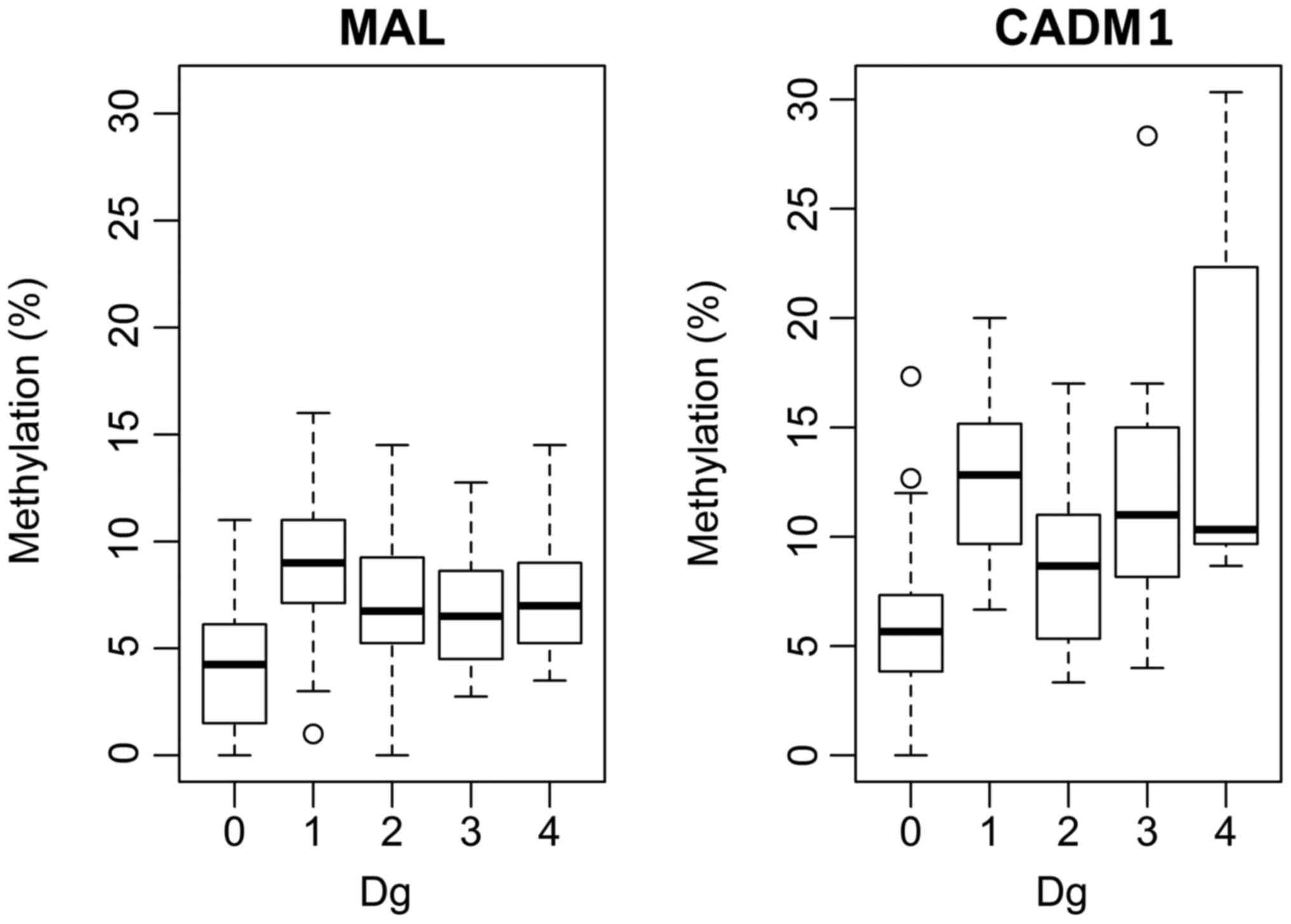
P=0.0026 for MAL and P=0.0001 for CADM1. Fig. 4 illustrated the pairs with
significantly different methylations. MAL was demonstrated
to have a significantly lower median methylation compared with
CADM1 for each group (Dg.0: P=0.010145, 95% CI: −0.036,
−0.003; Dg.1: P=0.0001447, 95% CI: −0.050, −0.0180; Dg.2:
P=0.001147, 95% CI: −0.0378, −0.003; Dg.3: P=0.0003808, 95% CI:
−0.0745, −0.0235; Dg.4: P=0.04694).
Logistic regression models using the most promising
markers were investigated to see whether marker combinations could
be used to improve the discrimination between cases and the control
group. Multinomial logistic regression was used to examine whether
HPV status impacted DNA methylation at specific CpG islands or if
there is any dependence between age of patient and increasing risk
of neoplasia or HPV. For CADM1 the present study confirmed
that the last CpG (3rd) was significantly more methylated in
samples with inflammation (P=0.001), CIN1 (P=0.086), CIN2+
(P=0.038) compared with the other examined CpGs (1st, 2nd). In
MAL it was also discovered that the first CpG was more
significantly methylated than other CpGs (P=0.043 for inflammation,
P=0.017 for CIN1, P=0.155 for CIN2+). For SCC (Dg.4) the second and
third CpGs for the gene MAL were significantly more
methylated (P=0 for 2nd CpG, P=0.012 for 3rd CpG) than in other
groups. For CADM1 no statistically significant differences
were identified in the SCC (Dg.4) group (Table III).
 | Table III.Multinomial logit model of diagnosis
as a function of age, HPV, MAL 1–4 and CADM1 1–3. |
Table III.
Multinomial logit model of diagnosis
as a function of age, HPV, MAL 1–4 and CADM1 1–3.
| Dg. no. | Age | HPV | MAL 1 | MAL 2 | MAL 3 | MAL 4 | CADM1 1 | CADM1 2 | CADM1 3 |
|---|
| 1 | 0.054 | 0.622 | 0.043 | 0.697 | 0.111 | 0.315 | 0.989 | 0.678 | 0.001 |
| 2 | 0.321 | 0.093 | 0.017 | 0.195 | 0.875 | 0.043 | 0.091 | 0.584 | 0.086 |
| 3 | 0.423 | 0.028 | 0.155 | 0.721 | 0.867 | 0.451 | 0.183 | 0.261 | 0.038 |
| 4 | 0.584 | 0.000 | 0.909 | 0.000 | 0.012 | 0.227 | 0.346 | 0.233 | 0.316 |
Determination of the methylation
cut-off using logistic regression (MAL, CADM1)
Logistic regression was used to examine to the
dependence between the case/control statuses (control group vs. Dg.
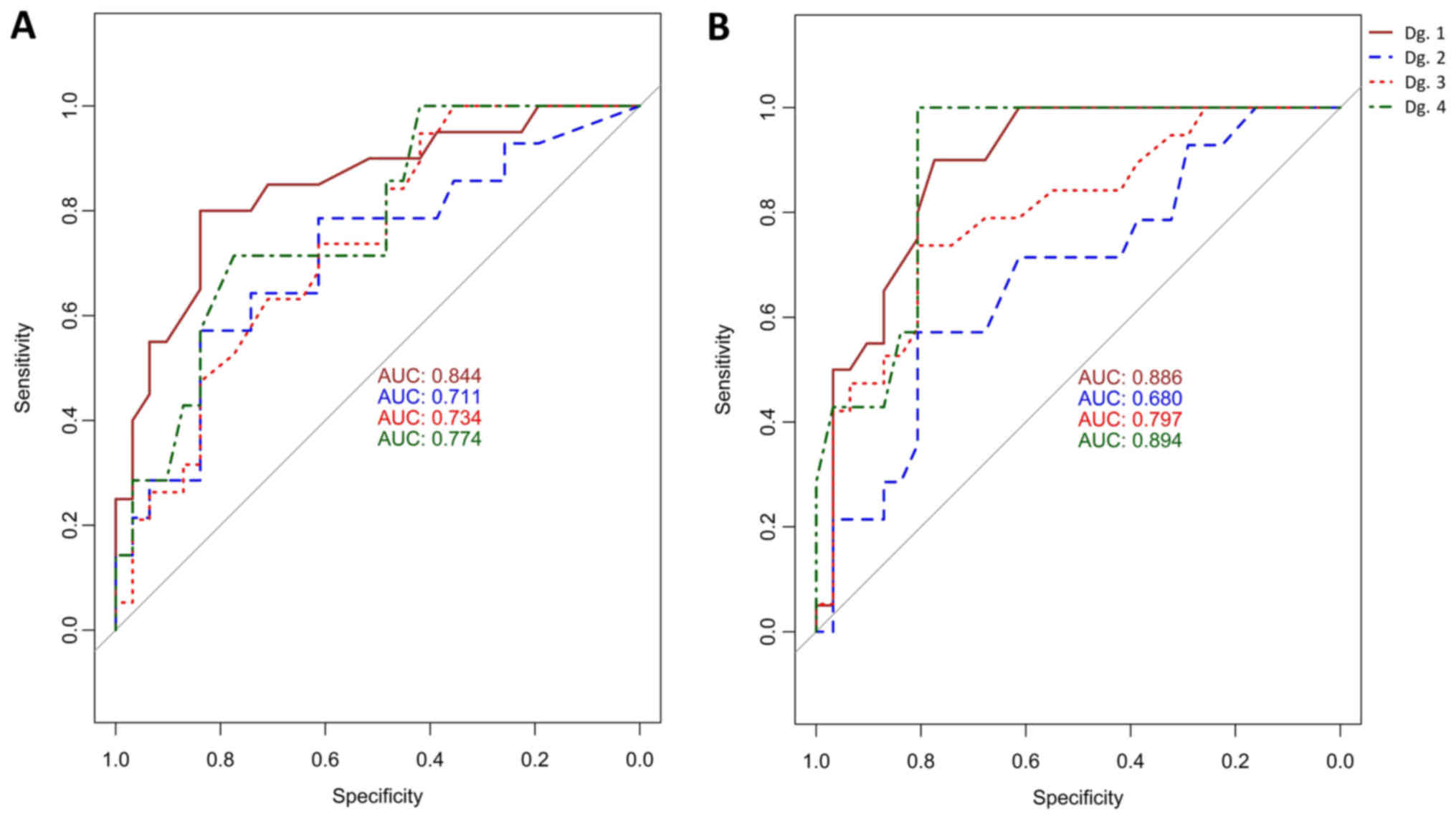
1–4). The AUC values for MAL were: 84% for cervical
inflammation, 71% for CIN1, 73.4% for CIN2+, 77% for SCC; and for
CADM1 were: 88.6% for cervical inflammation, 68% for CIN1,
80% for CIN2+ and 89% for SCC. The ROC graph is shown in Fig. 5 (left) for MAL and Fig. 5 (right) for CADM1. The cut-off
on the class probability was determined by the Youden method. The
cutoff for MAL and CADM1 on the median methylation
corresponding to the cutoff on the class probabilities was 0.09.
Using this cutoff value, MAL had the highest sensitivity and
specificity in inflammation samples (80 and 83.9% respectively)
followed by SCC samples (71.4 and 77.4% respectively). CIN1 was
less sensitive (57.1%) but still had high specificity (83.9%).
However, for the MAL gene CIN2+ group had the highest
sensitivity (94.7%) but the lowest specificity (41.9%).
CADM1 had the highest sensitivity (100%) and also
specificity (80.6%) in SCC and also in CIN2+ where the sensitivity
was 73.7% with an even higher specificity (80.6%). In patients with
inflammation a high sensitivity (90%) and specificity (77.4%) were
observed. The lowest sensitivity (57.1%) but still high specificity
(80.6%) for the median methylation was found in CIN1. The
dependence between women with inflammation and CIN1 was also
calculated (AUC=68% for MAL, AUC=74.4% for CADM1),
CIN1 and CIN2+ (AUC=50.5% for MAL, AUC=66.5% for
CADM1), CIN1 and SCC (AUC=56% for MAL, AUC=78.5% for
CADM1).
Discussion
Epidemiological and molecular studies have
demonstrated that persistent infection of HR-HPV is one of the
causes of cervical carcinogenesis (25,26) and
the major mechanism that engages HPV16/18 in cervical
carcinogenesis is the manifestation of two early viral genes E6 and
E7 (4). Only a minority of all
persistent HR-HPV infections alters the expression of viral genes
E6 and E7, consequently resulting in an increased expression of
oncoproteins (26). This process is
histologically known as CIN2/3 and, without treatment can
ultimately lead to CC. Recent studies have demonstrated that the
inclusion of testing to detect the presence of HR-HPV DNA in
cytology after 6 months of patient treatment has significantly
increased the sensitivity of CIN2+ detection (27,28).
HR-HPV testing is an attractive modality for primary screening due
to its extremely high sensitivity for CIN3+ lesions. However, the
specificity of HR-HPV testing is lower compared with cytology
(approximately 4–6%) (29). In order
to manage the increased number of colposcopy referrals, HR-HPV
positive women should be further stratified by secondary triage
tests such as p16/Ki67 immunostaining and methylation markers
(29). In the present study, HPV DNA
was detected in 42.8% CIN1, 68.4% CIN2+ and 100% of SCC samples.
From these results, an increase in the incidence of HPV DNA
positive samples was observed as the severity of the lesion
increased.
Recent results suggest that other factors, such as
genetic and epigenetic changes (CpG DNA methylation), are required
for the onset of carcinogenesis (30). Approximately ten human genes have
consistently elevated methylation in cervical pre-cancers including
CADM1, EPB41L3, FAM19A4, MAL, miR-124, PAX1 and SOX1.
Methylation testing is still in the early stages of development;
however, it is exhibiting promise as an accurate molecular
classifier (31).
Methylation of CpG regions of multiple TSGs occurs
at different stages of development of CIN and in the process of
transition to invasive cervical carcinoma, of which CADM1
and MAL are the most commonly methylated genes (17,32,33). The
principal effect of DNA methylation in promotor/intronic regions of
CADM1 and MAL is their downregulation. MAL was
identified to be an essential component of the glycolipid-enriched
membrane (GEM) rafts, which have been implicated in the polarized
sorting of apical proteins (34,35). This
down-regulation may disturb regular apical sorting, thereby
disrupting cellular polarity, which is a phenomenon observed in
epithelial cells infected with HPV. The down-regulation of
CADM1 may result in the loss of the Rb tumor suppressor
pathway signaling, which represents a relatively common event in
cervical carcinogenesis (36).
However, the molecular mechanism of CADM1/MAL
involvement in cervical carcinogenesis has not yet been thoroughly
investigated and the definitive confirmation that the
down-regulation of these genes in primary CCs will require further
study.
A previous study reported that there was a
correlation between methylation and the progression of neoplasia to
a higher stage. There were no difference in the median methylation
between the control group and CIN1 or between CIN1 and CIN2+. Based
on these results they considered that the methylation degree of
individual CpG islands in the promoter region of the CADM1
gene was lower in normal squamous epithelium than in CINs (37). However, when CIN passed to the
invasive cervical cancer (ICC), the average methylation of specific
CpG regions increased significantly, which could lead to increased
or complete inhibition of gene expression and result in a loss of
cell adhesion and tumor-suppressor function of the gene and thereby
inducing malignant transformation cells (37). The authors in the above-mentioned
study also confronted the results of the median methylation in TSG
and progression of disease with results reported by other study
(17). These publications
demonstrated that the frequency of average methylation in the
promoter region of the gene increases with the severity of disease
and that the presence of methylation in the ICC was significantly
different from the degree of methylation occurring in other types
of lesions.
Based on these results the present study
hypothesized that progression of disease was dependent on the
degree of the median methylation or HPV infection and not on the
age of the patient. The same analysis was also applied in an
additional study (38) and confirmed
statistical significance (P=0.05). A previous study monitored the
degree of methylation of the CADM1/MAL genes from the
uterine cervix specimens using the quantitative
methylation-specific PCR method and demonstrated that the median
methylation of these genes is a significant predictor of the CIN1
status: positive patients (35%), CIN2: positive patients (32.8%),
CIN3: positive patients (59%). They also identified a statistically
significant association between the HR-HPV infection positivity and
the transition of the disease to a more advanced stage (39).
The MassARRAY technique EpiTYPER DNA was used for
analysis of the 15 methylated regions of the CADM1 in ICC,
CIN1, CIN2/3 tissue samples and a control group. In 9 CpGs they
exhibited an increased methylation status in ICC compared with the
control group, and the average degree of methylation (2, 3, 4. and
5. CpG) in the selected promoter region was higher than in the
remaining CpG islands. However, CpGs 9 and 10 were less methylated.
This provides evidence for varying degrees of methylation in the
individual CpG regions in the CADM1 gene (37). In our selected area the final CpG
located behind the first exon was significantly more methylated in
each group than the first two CpGs (P-values: 0.001 for
inflammation samples, 0.086 for CIN1, 0.038 for CIN2+). The present
study also identified that one CpG (1st CpG) was significantly more
methylated in gene MAL than other 3 CpGs (P-values: 0.043
for inflammation samples, 0.017 for CIN1, 0.155 for CIN2+).
In several studies, DNA methylation of the
CADM1/MAL gene promoter region has been demonstrated
to increase with the severity of cervical disorders (17–19) and
that these epigenetic changes suggest the presence of CINs. These
findings are also supported by the results of an additional study,
which demonstrated that levels of the median methylation in tumor
suppressor genes are extremely high in cervical carcinomas and
significantly increase in CIN3 lesions in women with HR-HPV
infections that persists over a period of time longer than 5 years
(20). The present study confirmed in
both genes (CADM1/MAL) that the median methylation is
significantly higher in HPV positive patients than in HPV negative
patients. A higher median methylation and more HPV positive
patients were identified in SCC than in CIN1 cases.
In a previous study, by analysis (pyrosequencing) of
the promoter regions of the genes on tumor cell lines (C33A HPV
negative, HeLa HPV18 positive, SiHa and CaSki HPV16 positive) the
hypermethylation of the CADM1 gene in the CaSki, SiHa and
HeLa cell lines were detected; however, not in the C33A cell line.
The methylation status of the MAL was positive for the CaSki
and SiHa cell lines but not for the HeLa and C33A cell lines. Based
on these results, hypermethylation of the CADM1 gene was
demonstrated to be associated with HR-HPV infection.
Hypermethylation of the MAL gene was also associated with
infection with high-risk HPV types. Although the methylation stage
indicates that the HeLa and C33A cell lines are not statistically
significant (P=0.148) (40). In an
additional study the methylation state of the promoter region of
the CADM1 gene (nucleotides −444 to −305) were analyzed by
pyrosequencing. They demonstrated that methylation was
significantly increased in HPV positive HeLa cell lines (71.7%),
SiHa (84.8%), CaSki (95.2%) and significantly decreased in HPV
negative cell lines C33A (2.6%) (41). For further analysis cell lines were
not used; however, tissue taken from patients with L-SIL and H-SIL
as well as from patients with cervical carcinoma were analyzed. The
degree of average methylation for the CADM1 was 3.5 (95% CI,
3.0–4.0) in the L-SIL group, 5.6 (95% CI, 4.0–4.7) in the H-SIL
group and 17.7 (95% CI, 10.8–29.1) in carcinomas. For the
MAL gene, the average methylation rate was 2.7 (95% CI,
2.5–3.0) in the L-SIL group, 3.7 (95% CI, 3.0–4.6) in the H-SIL and
13 (95% CI, 7.6–22) in carcinomas. For both genes P<0.001
(40). The degree of average
methylation increased significantly in carcinoma samples as well as
in pre-invasive cervical lesion samples (39). The present study also confirmed that
the median methylation significantly increased from CIN1 cases
trough CIN2+ cases to carcinomas in both genes, while the median
methylation of CADM1 was higher trough each group than the
median methylation of the gene MAL.
In the present study, statistically significant
differences were identified in the inflammation samples when
compared with the control or CIN1 group, which may suggest that
hypermethylation of the TSGs in inflammatory samples is not random
and that may be indicative of the early stages of carcinogenesis.
Previous studies generally did not contain an inflammatory group,
which could lead to an underestimation of at-risk patients. It is
well-established that inflammation is a possible precursor of CIN1,
and may culminate in CC (38),
therefore it would be appropriate to include a cervical
inflammation group in further methylation studies.
In conclusion, the present study focused on the
detection of methylation in the region of two TSGs (MAL and
CADM1). Several major studies have confirmed a significant
increase of the median methylation in the specific promoter region
of these genes tested on cell lines, while the percentage of
methylation increased with the progression of neoplasia to cervical
carcinoma (39,40). However, in the majority of studies,
pyrosequencing was not used as a sensitive tool for quantitative
analysis of CpG methylation. Since many patients underestimate the
importance of regular medical check-ups, it is important during
examinations to provide them with the necessary comfort, not to
exacerbate their stress and try to capture the dysplasia at an
early stage. The specimens used in the present study therefore
constituted of cytological smears from the cervix. CADM1 was
confirmed to be more methylated in almost every study group
compared with MAL, and that third CpG in CADM1
exhibited higher methylation levels during the transition of CIN
and has the potential to serve as a promising biomarker for future
study. There was also a significantly higher median methylation in
HPV+ patients than in HPV-patients which supports the suitability
of this combination (methylation levels/HPV positivity) as an early
detection tool for this severe oncological disease.
Acknowledgements
Not applicable.
Funding
The present study was funded by the project
implementation Biomedical Center Martin (grant no. 26220220187),
and was supported by the Operational Program Research and
Innovation funded by the ERDF, the project ‘Molecular Diagnostics
of Cervical Cancer’ (grant no. 26220220113) and by VEGA (grant no.
1/0380/18).
Availability of data and materials
The datasets generated/analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM and ZL designed the study. SM and VH performed
the experiments. MG conducted the statistical analysis. JV, MN, EK
and TB obtained and handled cervical specimens and clinical data.
SM analyzed the data and was a major contributor in writing the
manuscript. MK performed the histological examination of the
samples and clinical data. ZL, PZ and JD supervised the entire
study, and participated in study design and coordination. All
authors read and revised the manuscript, and approved the final
version to be published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jessenius Faculty of Medicine in Martin. All patients
provided written informed consent prior to their inclusion in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:359–386. 2015.
View Article : Google Scholar
|
|
2
|
Resende LS, Rabelo-Santos SH, Sarian LO,
Alves Figueiredo RR, Ribeiro AA, Zeferino LC and Derchain S: A
porsingle and multiple HPV type infections in Brazilian women of
differentrait of t age strata with squamous or glandular cervical
lesions. BMC Infect Dis. 22:2142014. View Article : Google Scholar
|
|
3
|
Bruni 1, Barrionuevo-Rosas L, Albero G, et
al: ICO information centre on HPV and cancer (HPV Information
Centre). Human Papillomavirus Relat Dis Eur. 2017.
|
|
4
|
Zheng ZM and Baker CC: Papillomavirus
genome structure, expression, and post-transcriptional regulation.
Front Biosci. 11:2286–2302. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piyathilake CJ, Mascaluso M, Alvares RD,
Chen M, Badiga S, Edberg JC, Partridge EE and Johanning GL: A
higher degree of methylation of the HPV 16 E6 gene is associated
with a lower likelihood of being diagnosed with cervical
intraepithelial neoplasia. Cancer. 117:957–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balch C, Fang F, Matei DE, Huang TH and
Nephew KP: Minireview: Epigenetic changes in ovarian cancer.
Endocrinology. 150:4003–4011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qureshi SA, Bashir MU and Yaqinuddin A:
Utility of DNA methylation markers for diagnosing cancer. Int J
Surg. 8:194–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mersakova S, Nachajova M, Szepe P,
Kasajova PS and Halasova E: DNA methylation and detection of
cervical cancer and precancerous lesions using molecular methods.
Tumour Biol. 37:23–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murakami Y, Nobukuni T, Tamura K, Maruyama
T, Sekiya T, Arai Y, Gomyou H, Tanigami A, Ohki M, Cabin D, et al:
Localization of tumor suppressor activity important in nonsmall
cell lung carcinoma on chromosome 11q. Proc Natl Acad Sci USA.
95:8153–8158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ando K, Ohira M, Ozaki T, Nakagawa A,
Akazawa K, Suenaga Y, Nakamura Y, Koda T, Kamijo T, Murakami Y and
Nakagawara A: Expression of TSLC1, a candidate tumor suppressor
gene mapped to chromosome 11q23, is downregulated in unfavorable
neuroblastoma without promoter hypermethylation. Int J Cancer.
123:2087–2094. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito T, Shimada Y, Hashimoto Y, Kaganoi J,
Kan T, Watanabe G, Murakami Y and Imamura M: Involvement of TSLC1
in progression of esophageal squamous cell carcinoma. Cancer Res.
63:6320–6326. 2003.PubMed/NCBI
|
|
13
|
Mao X, Seidlitz E, Truant R, Hitt M and
Ghosh HP: Re-expression of TSLC1 in a non-small-cell lung cancer
cell line induces apoptosis and inhibits tumor growth. Oncogene.
23:5632–5642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steenbergen RD, Kramer D, Braakhuis BJ,
Stern PL, Verheijen RH, Meijer CJ and Snijders PJ: TSLC1 gene
silencing in cervical cancer cell lines and cervical neoplasia. J
Natl Cancer Inst. 96:294–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overmeer RM, Henken FE, Snijders PJ,
Claassen-Kramer D, Berkhof J, Helmerhorst TJ, Heideman DA, Wilting
SM, Murakami Y, Ito A, et al: Association between dense CADM1
promoter methylation and reduced protein expression in high-grade
CIN and cervical SCC. J Pathol. 215:388–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Momparler RL and Bovenzi V: DNA
methylation and cancer. J Cell Physiol. 183:145–154. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steenbergen RD, Snijders PJ, Heideman DA
and Meijer CH: Clinical implications of (epi) genetic changes in
HPV-induced cervical precancerous lesions. Nat Rev Cancer.
14:395–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Overmeer RM, Louwers JA, Meijer CJ, van
Kemenade FJ, Hesselink AT, Daalmeijer NF, Wilting SM, Heideman DA,
Verheijen RH, Zaal A, et al: Combined CADM1 and MAL promoter
methylation analysis to detect (pre-)malignant cervical lesions in
high-risk HPV-positive women. Int J Cancer. 129:2218–2225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Strooper LM, van Zummeren M,
Steenbergen RD, Bleeker MC, Hesselink AT, Wisman GB, Snijders PJ,
Heideman DA and Meijer CJ: CADM1, MAL and miR124-2methylation
analysis in cervical scrapes to detect cervical and endometrial
cancer. J Clin Pathol. 67:1067–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bierkens M, Hesselink AT, Meijer CJ,
Heideman DA, Wisman GB, van der Zee AG, Snijders PJ and Steenbergen
RD: CADM1 and MAL promoter methylation levels in hrHPV-positive
cervical scrapes increase proportional to degree and duration of
underlying cervical disease. Int J Cancer. 133:1293–1299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meršaková S, Visnovsky J, Holubekova V,
Nachajova M, Kudela E, Danko J and Lasabova Z: Detection of
methylation of the promoter region of the MAL and CADM1 genes by
pyrosequencing in cervical carcinoma. Neuro Endocrinol Lett.
35:619–623. 2014.PubMed/NCBI
|
|
22
|
Gravitt PE, Peyton CL, Alessi TQ, Wheeler
CM, Coutlée F, Hildesheim A, Schiffman MH, Scott DR and Apple RJ:
Improved amplification of genital human papillomaviruses. J Clin
Microiol. 38:357–361. 2000.
|
|
23
|
Janusicova V, Mendelova A, Zubor P,
Kapustova I, Svecova I, Kudela E, Burjanivova T, Lasabova Z and
Danko J: mRNA expression in cervical specimens for determination of
severe dysplasia or worse in HPV-16/18-positive squamous lesions. J
Low Genit Tract Dis. 18:273–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
RC Team: R: A language and environment for
statistical computing, . R foundation for statistical computing.
Vienna, Austria: URL. https://www.R-project.org/2015
|
|
25
|
Lazo PA: The molecular genetics of
cervical carcinoma. Br J Cancer. 80:2008–2018. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 Suppl 1:S2–S23. 2015. View Article : Google Scholar
|
|
27
|
Kocken M, Helmerhorst TJ, Berkhof J,
Louwers JA, Nobbenhuis MA, Bais AG, Hogewoning CJ, Zaal A,
Verheijen RH, Snijders PJ and Meijer CJ: Risk of recurrent
high-grade cervical intraepithelial neoplasia after successful
treatment: A long-term multi-cohort study. Lancet Oncol.
12:441–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kocken M, Uijterwaal MH, de Vries AL,
Berkhof J, Ket JC, Helmerhorst TJ and Meijer CJ: High-risk human
papillomavirus testing versus cytology in predicting post-treatment
disease inwomen treated for high-grade cervical disease: A
systematic review and meta-analysis. Gynecol Oncol. 125:500–507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hesselink AT, Heideman DA, Steenbergen RD,
Coupé VM, Overmeer RM, Rijkaart D, Berkhof J, Meijer CJ and
Snijders PJ: Combined promoter methylation analysis o CADM1 and
MAL: An obkective triage tool for high-risk human papillomavirus
DNA-positive women. Clin Cancer Res. 17:2459–2465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang HJ: Aberrant DNA methylation in
cervical carcinogenesis. Chin J Cancer. 32:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cushieri K, Ronco G, Lorincz A, Smith L,
Ogilvie G, Mirabello L, Carozzi F, Cubie H, Wentzensen N, Snijders
P, et al: Eurogin roadmap 2017: Triage strategies for the
management of HPV positivie women in cervical screening programs.
Int J Cancer. 143:735–745. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wentzensen N, Sherman ME, Schiffman M and
Wang SS: Utility of methylation markers in cervical cancer early
detection: Appraisal of the state-of-the-science. Gynecol Oncol.
112:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tornesello ML, Buonaguro L, Giorgi-Rossi P
and Buonaguro FM: Viral and cellular biomarkers in the diagnosis of
cervical intraepithelial neoplasia and cancer. Biomed Res Int.
2013:5196192013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin-Belmonte F, Arvan P and Alonso MA:
MAL mediates apical transport of secretory proteins in polarized
epithelial Madin-Darby canine kidney cells. J Biol Chem.
276:49337–49342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hatta M, Nagai H, Okino K, Onda M,
Yoneyama K, Ohta Y, Nakayama H, Araki T and Emi M: Down-regulation
of members of glycolipid-enriched membrane raft gene family, MAL
and BENE, in cervical squamous cell cancers. J Obstet Gynaecol Res.
30:53–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Xie HY, Ding SM, Xing CY, Chen A,
Lai MC, Zhou L and Zheng SS: CADM1 regulates the G1/S transition
and represses tumorigenicity through the Rb-E2F pathway in
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
15:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Tao L, Tan Q, Dong Y, Pan X, Pang L,
Qi Y, Zou H, Liang W, Liu W, et al: CpG island methylation of the
CADM1 gene correlates with cervical carcinogenesis in the Uighur
and Han populations of Xinjiang, China. Int J Clin Exp Pathol.
9:6977–6987. 2016.
|
|
38
|
van Baars R, van der Marel J, Snijders PJ,
Rodriquez-Manfredi A, ter Harmsel B, van den Munckhof HA, Ordi J,
del Pino M, van de Sandt MM, Wentzensen N, et al: CADM1 and MAL
methylation status in cervical scrapes is representative of the
most severe underlying lesion in women with multiple cervical
biopsies. Int J Cancer. 138:463–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uijterwaal MH, van Zummeren M, Kocken M,
Luttmer R, Berkhof J, Witte BI, van Baal WM, Graziosi GCM,
Verheijen RHM, Helmerhorst TJM, et al: Performance of
CADM1/MAL-methylation analysis for monitoring of women treated for
high-grade CIN. Gynecol Oncol. 143:135–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ki EY, Lee KH, Hur SY, Rhee JE, Kee MK,
Kang C and Park JS: Methylation of cervical neoplastic cells
infected with human papillomavirus 16. Int J Gynecol Cancer.
26:176–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Woo HJ, Kim SJ, Song KJ, Kim SS, Yoon CH,
Choi BS and Rhee JE: Hypermethylation of the tumor-suppressor cell
adhesion molecule 1 in human papillomavirus-transformed cervical
carcinoma cells. Int J Oncol. 46:2656–2662. 2015. View Article : Google Scholar : PubMed/NCBI
|