Introduction
Cancer is one of the most common causes of mortality
worldwide. Although various therapies for cancer have been
developed, further research is required to decrease the mortality
caused by cancer.
Phytochemicals are present in dietary plant-based
products, and several phytochemicals have been identified to
inhibit tumorigenesis in experimental animals and/or in
vitro assays (1). Therefore,
chemoprevention using phytochemicals may be a way to decrease
cancer-associated mortality. The phytochemical damnacanthal
(3-hydroxy-1-methoxyanthraquinone-2-aldehyde; DAM) is an
anthraquinone compound, primarily present in plants of the
Rubiaceae family. It has been reported to have anticancer and
cancer-preventive activities, acting via various molecular targets,
including induction of non-steroidal anti-inflammatory
drugs-activated gene 1 (NAG-1) expression (2), cyclin D1 downregulation (3), activation of p38 mitogen-activated
protein kinase signaling pathway (4)
and inhibition of tyrosine kinase (5–7).
Chitosan, a cationic natural polysaccharide present
in the exoskeleton of crustaceans, has been widely used as a drug
delivery system. The advantages of using chitosan in nanoparticles
(NPs) are its biocompatibility, biodegradability, non-toxicity,
non-immunogenicity and abundance of functional groups.
Encapsulation of DAM improved the mode of action of the NPs and
decreased their toxicity, indicating that there may be multiple
potential applications of phytochemical delivery by chitosan
(8).
One of the key proteins that regulates tumor
suppressors in cancer cells is the chromosome maintenance protein 1
(CRM1, also known as exportin 1), which serves a pivotal function
in tumorigenesis (9) and may be a
target for anticancer drugs. CRM1 is a nuclear export receptor
involved in the export of large macromolecules including RNA and
protein from the nuclear membrane to the cytoplasm. Excessive
nuclear export may be one of the factors contributing to resistance
to chemotherapy and cancer development (10). It has been identified that CRM1 is
highly expressed in cancer, and a number of nuclear tumor
suppressor proteins, including p53, p21 and NAG-1 (11,12), are
translocated to the cytoplasm and are degraded. Overexpression of
CRM1 has been associated with poor prognosis in patients with
several types of cancer (13). Thus,
CRM1 may be considered a promising therapeutic target for
anticancer drug development.
The aims of the present study were to evaluate the
effect of DAM and its nanoformulation on CRM1 expression and to
elucidate the underlying molecular mechanisms of DAM-mediated
anticancer activity.
Materials and methods
Materials
DAM was isolated from the roots of Morinda
citrifolia and was purified as described previously (14). Chitosan with 90% deacetylation
(Mv, 150,000) was purchased from Seafresh
Chitosan (Lab) Co. Ltd. (Chumphon, Thailand). Cancer cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA). HCT-116 and U2OS cells were cultured in McCoy's 5A medium
(Welgene Inc., Gyeongsan, Korea) supplemented with 10% fetal bovine
serum (Hyclone; GE Healthcare Life Sciences, Logan UT, USA), 100
µg/ml penicillin and 100 µg/ml streptomycin. The cells were
incubated at 37°C under a humidified atmosphere containing 5%
CO2. Antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), except for the anti-NAG-1
antibody, which was obtained as previously described (15).
Preparation of DAM-NPs
DAM-NPs were prepared using deoxycholic and
poly(ethylene glycol) methyl ether-grafted chitosan as a drug
carrier. DAM was incorporated into the NPs using the dialysis
method described previously (8).
Briefly, the polymer was dissolved in water and mixed with DAM
solution in dimethyl sulfoxide (DMSO). The mixture was
ultrasonicated and dialyzed in 0.9% NaCl at 4°C. DAM-NPs were
freeze-dried for further use.
Cell proliferation assay
The effect of DAM and DAM-NPs on the proliferation
of HCT-116 and U2OS cells was investigated using the CellTiter 96
Aqueous One Solution Cell Proliferation Assay (Promega Corporation,
Madison, WI, USA). The cells were seeded at a concentration of
3,000 cells/well in 96-well tissue culture plates. The cells were
then treated with 1 or 10 µM DAM or DMSO as a control, and DAM-NPs
(equivalent concentration of 50 µM DAM) or DAM-free NPs as
controls. At 0, 1 and 3 days after treatment (for DAM-NPs and NPs)
or 0, 1 and 4 days after treatment (for DAM and DMSO), 20 µl
CellTiter 96 Aqueous One Solution was added to each well. The plate
was then incubated at 37°C for 1 h. The absorbance at 490 nm was
determined using an iMARK™ microplate absorbance reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Apoptosis assay
Apoptotic cells were detected using the Annexin
V-Fluorescein Isothiocyanate Apoptosis Detection kit (BioVision,
Inc., Milpitas, CA, USA). Briefly, cells were plated in 6-well
culture dishes and treated with vehicle, DAM, NPs and DAM-NPs
followed by incubation at 37°C for 24 h. Samples were prepared
according to the manufacturer's protocol. Apoptosis was detected
using a Cell Lab™ Quanta SC flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA) with excitation and emission settings of 488 and 530
nm, respectively. The images were captured with a Quanta SC flow
cytometer (Beckman Coulter, Inc.) and processed with Flowing
Software 2.5.1 (University of Turku, Turku, Finland).
Reverse
transcription-semi-quantitative polymerase chain reaction
(RT-PCR)
HCT-116 and U2OS cells were grown to between 80 and
90% confluence in a 6-cm plate followed by treatment with 50 µM DAM
or DAM-NPs (equivalent concentration of 50 µM DAM), and DMSO or NPs
as vehicle controls. After 24 h, total RNA was extracted using
TRIzol® LS reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and then reverse-transcribed
into cDNA using a Verso cDNA synthesis kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. PCR
was performed for 30 cycles of 94°C for 30 sec, 60°C for 30 sec and
72°C for 1 min with specific primers for human (h)CRM1 and hGAPDH
(hCRM1 forward, 5′-AATGTGAGAGCCTGCAAAGC-3′; hCRM1 reverse,
5′-CGGCTCACCCAACCAGATAT-3′; hGAPDH forward,
5′-GACCACAGTCCATGCCATCACT-3′; hGAPDH reverse,
5′-TCCACCCTGTTGCTGTAG-3′). Each PCR product was electrophoresed on
a 1.4% agarose gel with NEOgreen (NeoScience, Co., Ltd., Suwon,
Korea) staining. Each value was normalized to the expression of
hGAPDH.
Western blot analysis
HCT-116 and U2OS cells were grown to between 80 and
90% confluence in a 6-cm plate and treated with 50 µM DAM or
DAM-NPs (equivalent concentration of 50 µM DAM), and DMSO or NPs as
vehicle controls. Total cell lysates were isolated using
radioimmunoprecipitation assay cell lysis buffer (1X) with EDTA
supplemented with protease inhibitor (0.5 mM phenylmethylsulfonyl
fluoride) and phosphatase inhibitor (1 mM
Na3VO4). Nuclear and cytoplasmic extracts
were purified by using NE-PER Nuclear and Cytoplasmic Extraction
Reagents (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Protein concentration was determined by
the bicinchoninic acid protein assay (Pierce; Thermo Fisher
Scientific, Inc.) using bovine serum albumin (Pierce; Thermo Fisher
Scientific, Inc.) as the standard. 10% SDS-PAGE was used to
separate 60 or 30 µg of proteins from the cell lysates. These
proteins were then transferred onto a nitrocellulose membrane. The
blot was blocked with 5% skimmed milk in Tris-buffered saline (TBS)
containing 0.1% Tween-20 (TBS-T), applied overnight. The membranes
were incubated at room temperature with primary antibodies against
NAG-1, CRM1 (sc-74454), β-actin (sc-47778), α-tubulin (sc-398103)
and lamin A/C (sc-376248), diluted in 5% skimmed milk in TBS-T
solution (1:1,000) for 1 h. Following washing with TBS-T four
times, the membrane was incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (Thermo Fisher Scientific, Inc.;
cat. no. 31460) or HRP-conjugated goat anti-mouse (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 62-6520) for 1 h and
washed with TBS-T six times. The proteins were detected by
chemiluminescence using Enhanced Chemiluminescence Western Blotting
Detection Reagent (GE Healthcare, Little Chalfont, UK) and
visualized using MicroChemi (software 4.2; DNR Bio-Imaging Systems,
Ltd., Neve Yamin, Israel).
Statistics
Statistical analysis was performed with SSPS
software (version 25; SPSS, Chicago, IL, USA). Statistical
significance was determined by analysis of variance and Scheffe's
test. P<0.05 was considered to indicate a statistically
significant difference. Results are expressed as the mean ±
standard deviation.
Results
DAM-NPs inhibit cell
proliferation
It has been identified previously that treatment
with DAM inhibits cancer cell proliferation (2). In the present study, the
anti-proliferative effect of DAM and DAM-NPs on human colorectal
and osteosarcoma cancer cells, HCT-116 and U2OS, respectively, was
identified. The two cell lines, which each express wild-type p53,
were used in this assay because it is known that DAM induces p53
expression and its induction leads to cell proliferation inhibition
in cancer cells (16). The cells were
treated with 1 or 10 µM DAM for 1, 2 and 4 days. The cells were
also treated with DAM-NPs at the equivalent DAM concentration (50
µM) for 1 and 3 days. The results indicated that DAM and DAM-NPs
significantly decreased the proliferation of HCT-116 (P<0.001
and P<0.01, respectively) and U2OS (P<0.01 and P<0.001,
respectively) cells after 1 day of treatment, compared with control
cell proliferation (Fig. 1A-D).
Furthermore, cell proliferation continuously decreased after 4 days
of treatment. It was also identified that cell proliferation arrest
by DAM and DAM-NPs resulted from induction of early apoptosis, as
assessed using an Annexin V assay (Fig.
1E and F). These results indicated that DAM-NPs exhibit a
similar activity to that of DAM in that they inhibit the
proliferation of cancerous cells (2).
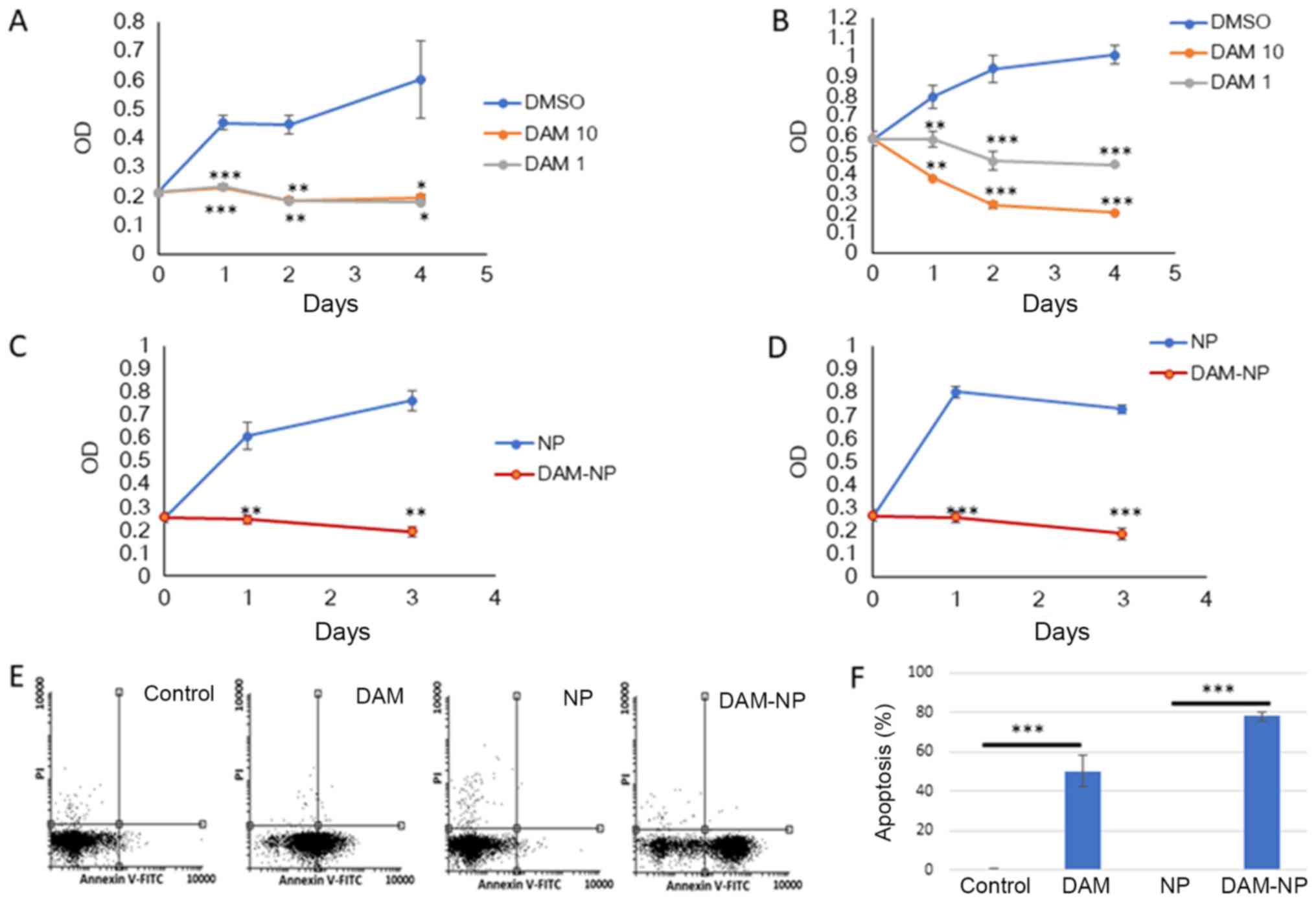 | Figure 1.Effect of DAM and DAM-NPs on HCT-116
and U2OS cell proliferation. (A) HCT-116 and (B) U2OS cells were
treated with DAM or DMSO for 4 days, and (C) HCT-116 and (D) U2OS
cells were also treated with NPs or DAM-NPs (50 µM final DAM
concentration) for 3 days. Results are expressed as the mean ±
standard deviation of four independent experiments. *P<0.05,
**P<0.01, ***P<0.001 vs. DMSO or NP-treated cells. U2OS cells
were plated at 1×106 cells/well in 6-well plates,
incubated with vehicle or 50 µM DAM or DAM-NPs for 24 h and
analyzed for apoptosis. (E) Representative flow cytometric
profiles. (F) Early apoptosis rate (Annexin V-positive and
PI-negative). Results are expressed as the mean ± standard
deviation of three independent experiments. ***P<0.001 vs.
DMSO-treated cells. DAM, damnacanthal; DAM-NPs, DAM nanoparticles;
DMSO, dimethyl sulfoxide; PI, propidium iodide; FITC, fluorescein
isothiocyanate; NPs, nanoparticles; OD, optical density. |
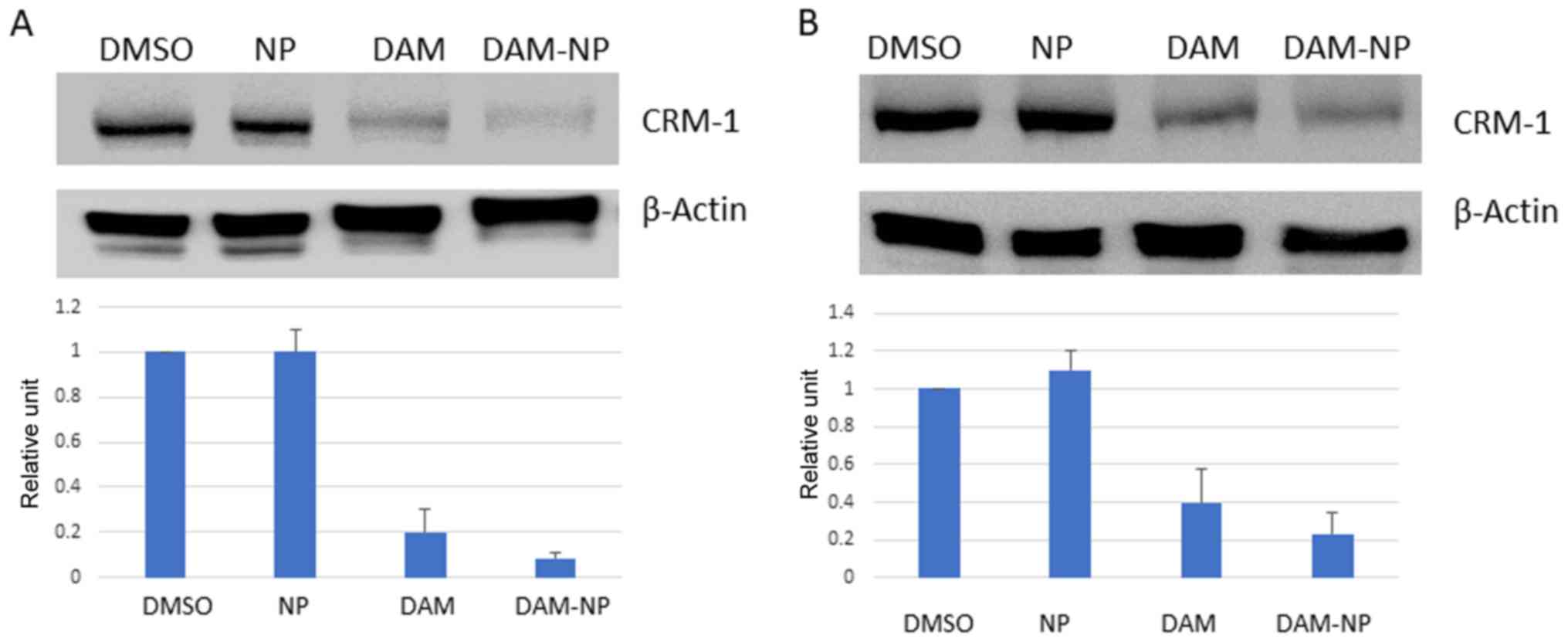
DAM and DAM-NPs inhibit CRM1
expression
To investigate the effect of DAM and DAM-NPs on CRM1
expression, HCT-116 and U2OS cells were treated with 50 µM DAM and
DAM-NPs at the equivalent DAM concentration (50 µM). Western blots
from total cell lysates with 60 µg protein were performed to
determine CRM1 expression. As presented in Fig. 2, CRM1 was downregulated in HCT-116 and
U2OS cells treated with DAM and DAM-NPs compared with that in
control cells. This result indicated that DAM and DMA-NPs decrease
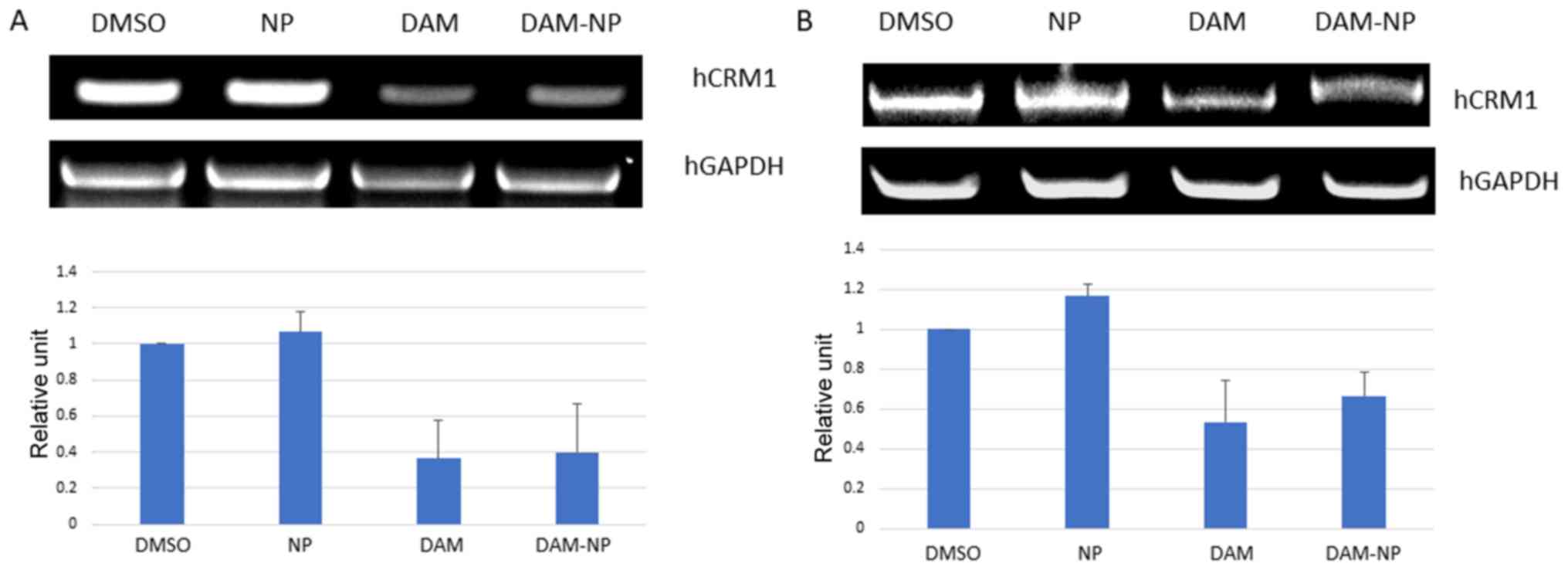
CRM1 expression at the protein level. To further verify the CRM1
downregulation at the transcriptional level, mRNA expression from
HCT-116 and U2OS cells following treatment with DAM and DAM-NPs for
24 h was analyzed. As presented in Fig.
3A and B, the level of CRM1 mRNA decreased in the two cell
lines following treatment. These results indicated that DAM and
DAM-NPs also downregulate CRM1 expression at the transcriptional
level.
CRM1 downregulation results in the
accumulation of NAG-1
It has been identified that NAG-1 is a tumor
suppressor protein translocated from the nucleus to the cytoplasm
by CRM1 (12). To confirm that the
decrease in CRM1 expression led to the nuclear accumulation of
NAG-1, western blot analyses for NAG-1 in nuclear as well as in
cytoplasmic extracts were performed. As presented in Fig. 4, an increase in the NAG-1 level in the
nuclear fraction was observed in DAM- and DAM-NP-treated cells.
This may have resulted from the inhibition of CRM1 and may be
mediated by DAM or DAM-NPs.
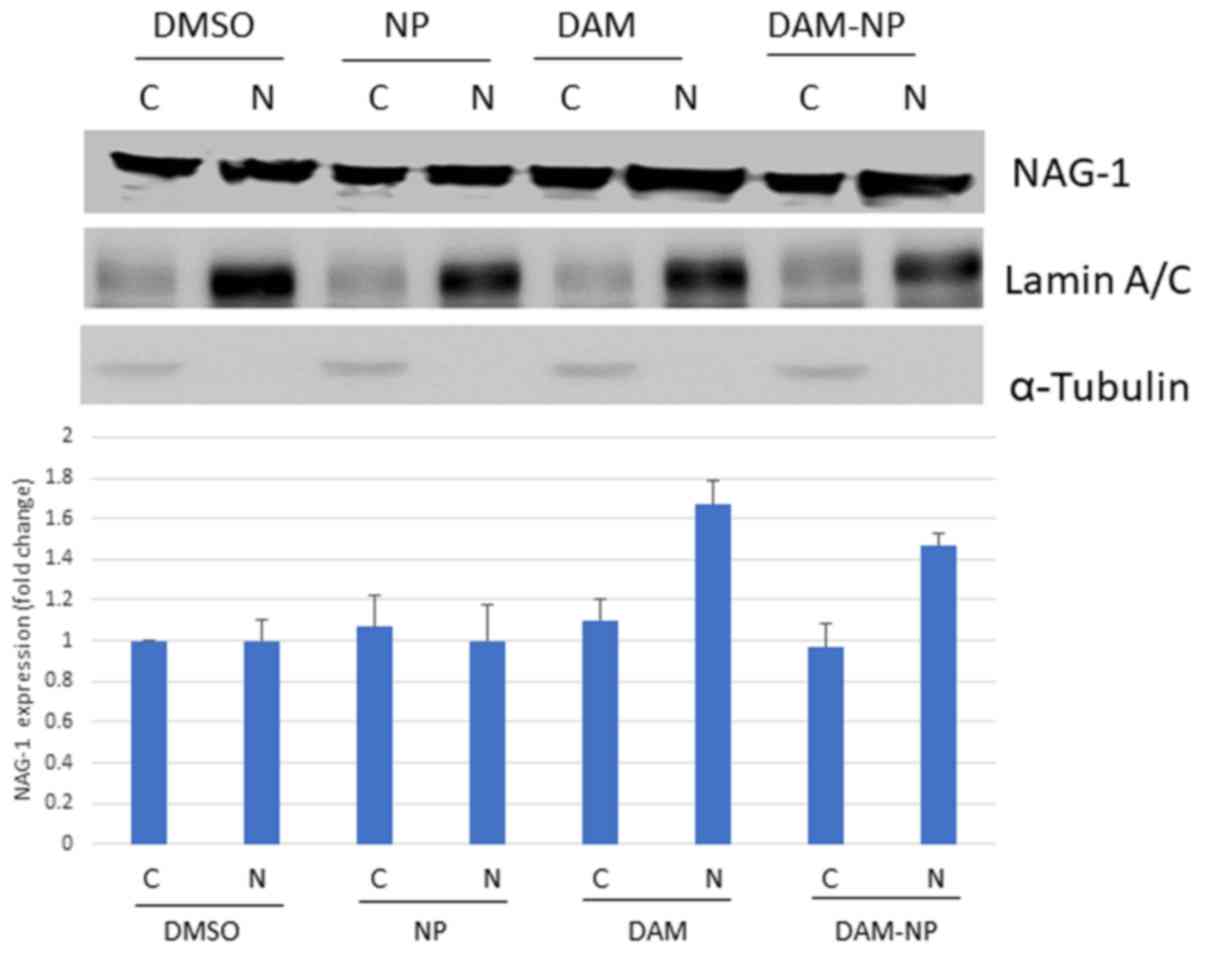 | Figure 4.DAM and DAM-NPs increase NAG-1
expression in the nucleus of HCT-116 cells. HCT-116 cells were
treated with control (0.1% DMSO or NPs), DAM (50 µM) or DAM-NPs (50
µM final DAM concentration) for 24 h. Nuclear and cytoplasmic
extracts were subjected to western blot analysis using anti-NAG-1,
anti-lamin A/C and anti-α-tubulin antibodies. Results are
representative of three independent experiments. The densitometry
represents NAG-1 fold induction, compared with DMSO-treated
cytoplasmic NAG-1 expression. NAG-1, non-steroidal
anti-inflammatory drugs-activated gene 1; DAM, damnacanthal;
DAM-NPs, DAM nanoparticles; DMSO, dimethyl sulfoxide; NPs,
nanoparticles; C, cytoplasmic; N, nuclear. |
Discussion
Cancer is the primary cause of mortality in
Thailand, and the second most common cause of mortality worldwide
(17,18). In the last few decades, potential
therapeutics for cancer have been investigated in a number of ways;
however, extensive efforts are required to decrease the incidence
of cancer and associated mortality. It has been indicated that
plant phytochemicals serve an important function in
anti-carcinogenesis (1). DAM,
extracted from M. citrifolia, commonly called noni (19), has potential anticancer effects. In
the present study, it was identified that DAM inhibits the
proliferation of HCT-116 and U2OS cells. DAM and encapsulated DAM
inhibited CRM1 expression in the two cell lines, which may be key
to its anticancer activity.
CRM1 is an export protein that facilitates the
transport of large molecules including tumor suppressor proteins
from the nucleus to the cytoplasm (20). Previous studies have identified that
CRM1 overexpression occurs in various types of cancer such as
osteosarcoma, ovarian cancer, pancreatic cancer, glioma, cervical
cancer and renal cell carcinoma (21–26),
making CRM1 a focal target for anticancer drugs. In the present
study, it was identified that DAM and DAM-NPs downregulate CRM1
expression in HCT-116 and U2OS cells. This result was corroborated
by analyzing the expression of NAG-1 protein in the nucleus and
cytoplasm. Following treatment with DAM and DAM-NPs, NAG-1
expression was increased in the nucleus compared with in the
cytoplasm. This may be a consequence of CRM1 downregulation and the
associated decrease in NAG-1 transportation from the nucleus to the
cytoplasm. CRM1 is highly expressed in several types of cancer, and
its inhibition is beneficial. Although it requires further
investigation, DAM may affect CRM1 activity by directly binding to
CRM1, similar to leptomycin B (a synthetic CRM1 inhibitor). These
results are of importance because DAM is a natural compound, which
is associated with fewer side effects compared with synthetic CRM1
inhibitors and may be used to develop potent anticancer drugs.
Acknowledgements
The authors would like to thank Mr Hyun Jik Lee from
Seoul National University (Seoul, Korea) for his technical support
in the present study.
Funding
The present study was supported by the Research
Resettlement Fund for the new faculty, the Research Institute for
Veterinary Science, and BK21 PLUS Program for Creative Veterinary
Science Research Center, Seoul National University, and by a
National Research Foundation of Korea grant funded by the Korea
government (2018R1A2B2002923). The present study was also supported
by the Royal Golden Jubilee PhD Program (PHD/0235/2549),
Thailand.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NC, PR, KS and SJB designed and conceived the
present study. NC, YY and TN performed the experiments. NC and SJB
wrote the manuscript. WG, SC, JKS and SJB analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DAM
|
damnacanthal
|
|
DAM-NPs
|
DAM nanoparticles
|
|
CRM1
|
chromosome maintenance protein 1
|
References
|
1
|
Wenzel U, Kuntz S, Brendel MD and Daniel
H: Dietary flavone is a potent apoptosis inducer in human colon
carcinoma cells. Cancer Res. 60:3823–3831. 2000.PubMed/NCBI
|
|
2
|
Nualsanit T, Rojanapanthu P, Gritsanapan
W, Lee SH, Lawson D and Baek SJ: Damnacanthal, a noni component,
exhibits antitumorigenic activity in human colorectal cancer cells.
J Nutr Biochem. 23:915–923. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sukamporn P, Rojanapanthu P, Silva G,
Zhang X, Gritsanapan W and Baek SJ: Damnacanthal and its
nanoformulation exhibit anti-cancer activity via cyclin D1
down-regulation. Life Sci. 152:60–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI
and Liu HJ: Activation of p38 MAPK by damnacanthal mediates
apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-α
and p53 pathways. Eur J Pharmacol. 650:120–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faltynek CR, Schroeder J, Mauvais P,
Miller D, Wang S, Murphy D, Lehr R, Kelley M, Maycock A, Michne W,
et al: Damnacanthal is a highly potent, selective inhibitor of
p56lck tyrosine kinase activity. Biochemistry. 34:12404–12410.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Vilas JA, Quesada AR and Medina MA:
Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent
antitumor compound against Hep G2 human hepatocellular carcinoma
cells. Sci Rep. 5:80212015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohashi K, Sampei K, Nakagawa M, Uchiumi N,
Amanuma T, Aiba S, Oikawa M and Mizuno K: Damnacanthal, an
effective inhibitor of LIM-kinase, inhibits cell migration and
invasion. Mol Biol Cell. 25:828–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sukamporn P, Baek SJ, Gritsanapan W,
Chirachanchai S, Nualsanit T and Rojanapanthu P: Self-assembled
nanomicelles of damnacanthal-loaded amphiphilic modified chitosan:
Preparation, characterization and cytotoxicity study. Mater Sci Eng
C Mater Biol Appl. 77:1068–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanai M, Hanashiro K, Kim SH, Hanai S,
Boulares AH, Miwa M and Fukasawa K: Inhibition of Crm1-p53
interaction and nuclear export of p53 by poly(ADP-ribosyl)ation.
Nat Cell Biol. 9:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Tanani M, Dakir el-H, Raynor B and
Morgan R: Mechanisms of nuclear export in cancer and resistance to
chemotherapy. Cancers (Basel). 8:pii: E352016. View Article : Google Scholar
|
|
11
|
Gravina GL, Senapedis W, McCauley D,
Baloglu E, Shacham S and Festuccia C: Nucleo-cytoplasmic transport
as a therapeutic target of cancer. J Hematol Oncol. 7:852014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min KW, Liggett JL, Silva G, Wu WW, Wang
R, Shen RF, Eling TE and Baek SJ: NAG-1/GDF15 accumulates in the
nucleus and modulates transcriptional regulation of the Smad
pathway. Oncogene. 35:377–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Q, Chen X, Zhou Q, Burstein E, Yang S
and Jia D: Inhibiting cancer cell hallmark features through nuclear
export inhibition. Signal Transduct Target Ther. 1:160102016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nualsanit T, Rojanapanthu P, Gritsanapan
W, Kwankitpraniti T, Min KW and Baek SJ: Damnacanthal-induced
anti-inflammation is associated with inhibition of NF-κB activity.
Inflamm Allergy Drug Targets. 10:455–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek SJ, Okazaki R, Lee SH, Martinez J,
Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF and
Eling TE: Nonsteroidal anti-inflammatory drug-activated gene-1 over
expression in transgenic mice suppresses intestinal neoplasia.
Gastroenterology. 131:1553–1560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aziz MY, Omar AR, Subramani T, Yeap SK, Ho
WY, Ismail NH, Ahmad S and Alitheen NB: Damnacanthal is a potent
inducer of apoptosis with anti-cancer activity by stimulating p53
and p21 genes in MCF-7 breast cancer cells. Oncol Lett.
7:1479–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Registry Unit NCIT: Cancer in
Thailand. VIII. New Thammada Press (Thailand) Co., Ltd.; 2015
|
|
18
|
Potter JD, Slattery ML, Bostick RM and
Gapstur SM: Colon cancer: A review of the epidemiology. Epidemiol
Rev. 15:499–545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan-Blanco Y, Vaillant F, Pérez A, Reynes
R, Brillouet JM and Brat P: The noni fruit (Morinda citrifolia L.):
A review of agricultural research, nutritional and therapeutic
properties. J Food Comp Analy. 19:645–654. 2006. View Article : Google Scholar
|
|
20
|
Ishizawa J, Kojima K, Hail N Jr, Tabe Y
and Andreeff M: Expression, function, and targeting of the nuclear
exporter chromosome region maintenance 1 (CRM1) protein. Pharmacol
Ther. 153:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen
P, Sun YJ, Tang LN and Zheng SE: The expression of CRM1 is
associated with prognosis in human osteosarcoma. Oncol Rep.
21:229–235. 2009.PubMed/NCBI
|
|
22
|
Noske A, Weichert W, Niesporek S, Röske A,
Buckendahl AC, Koch I, Sehouli J, Dietel M and Denkert C:
Expression of the nuclear export protein chromosomal region
maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian
cancer. Cancer. 112:1733–1743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH
and Sun YJ: Prognostic value of CRM1 in pancreas cancer. Clin
Invest Med. 32:E3152009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen A, Wang Y, Zhao Y, Zou L, Sun L and
Cheng C: Expression of CRM1 in human gliomas and its significance
in p27 expression and clinical prognosis. Neurosurgery. 65:153–160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Watt PJ, Maske CP, Hendricks DT,
Parker MI, Denny L, Govender D, Birrer MJ and Leaner VD: The
Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed
in cervical cancer and are critical for cancer cell survival and
proliferation. Int J Cancer. 124:1829–1840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue H, Kauffman M, Shacham S, Landesman
Y, Yang J, Evans CP and Weiss RH: CRM1 blockade by selective
inhibitors of nuclear export attenuates kidney cancer growth. J
Urol. 189:2317–2326. 2013. View Article : Google Scholar : PubMed/NCBI
|