Introduction
Mitochondria are pivotal organelles responsible for
cellular energy metabolism, free radical formation, calcium
homeostasis and the intrinsic apoptotic pathway (1). Each mitochondrion has its own
extra-chromosomal genome, termed mitochondrial DNA (mtDNA). The
number of mtDNA copies is tightly controlled and remains relatively
stable, which is important for maintaining homeostasis (2). However, mutations and alterations of
mtDNA can lead to abnormalities in energy metabolism and oxidative
stress, which may be involved in carcinogenesis (3–5).
Alterations of mtDNA content have been reported in
human malignancies, but with contradictory findings. Increased
mtDNA copy numbers have been identified in endometrial
adenocarcinoma cells (6) and
esophageal squamous cell carcinoma (7). By contrast, decreased mtDNA copy numbers
have been identified in lung cancer (8,9),
colorectal cancer (10), breast
cancer (11) and renal cell carcinoma
(12). Increased circulating mtDNA
could be a compensatory response to decreased respiratory function
(13) and may be associated with
increased tumor burden, increased mitochondrial damage, or release
from apoptotic and/or necrotic cancer cells in cancerous lesions
(14,15). Conversely, decreased mtDNA content may
be a consequence of exposure to excessive reactive oxygen species
(16).
Compared with nuclear DNA, the quantification of
mtDNA has several advantages, including short length, simple
molecular structure and abundance. However, assessing mtDNA in
tumor tissues requires invasive techniques. For dynamic patient
management, a non-invasive diagnostic and prognostic biomarker is
required (14). The monitoring of
circulating mtDNA is non-invasive, convenient and suitable for
dynamic observation, which makes it an ideal candidate for clinical
application. Circulating mtDNA has been considered a biomarker in
numerous types of tumor. As with tissue mtDNA, serum mtDNA values
vary among malignancies and are increased in patients with urologic
malignancies (17), epithelial
ovarian cancer (18), gastric cancer
(19) and testicular cancer (20), but are decreased in patients with
breast cancer (21) and Ewing's
sarcoma (22).
The high morbidity and mortality of lung cancer
(23) has prompted interest in the
study of circulating mtDNA. Hou et al (24) have reported that serum mtDNA is
increased in patients with lung cancer. However, associations
between mtDNA content and tumor size, lymph node metastases,
distant metastases, driver gene mutation status, chemo-sensitivity
and prognosis are still largely unknown. Given the numerous
potential interactions between mtDNA and carcinogenesis, the
current study hypothesized that alterations of plasma mtDNA content
may be a biomarker for the presence and development of lung cancer.
Thus, using quantitative polymerase chain reaction (qPCR) assays,
the current study measured the copy number of plasma mtDNA in
patients with lung cancer. Understanding the associations between
plasma mtDNA content and the clinicopathological characteristics
and prognosis of lung cancer may allow for a non-invasive and
dynamic means of evaluating the disease.
Materials and methods
Patients, study design and sample
collection
A total of 128 patients with lung cancer and 107
well-matched healthy controls from Medical Examination Center were
included in the current study. In the lung cancer group, there are
72 males and 56 female patients with a median age of 55.2±7.8
years. In the healthy control group, there are 57 male and 50
female individuals with a median age of 53.5±8.8 years. The
patients with lung cancer were diagnosed from January to December
2015 at the Hunan Cancer Hospital of China (Changsha, China). The
present study was approved by the Ethics Committee of Hunan Cancer
Hospital. Informed consent was obtained from healthy controls and
each patient according to protocols approved by the hospital's
Ethics Committees. None of the patients had received pre-operative
radiotherapy or chemotherapy. The exclusion criteria included
autoimmune diseases, inherited diseases, mitochondrial-related
diseases, or patients who refused to provide informed consent.
Whole blood was collected in EDTA anticoagulant tubes and samples
were centrifuged at 400 × g at 4°C for 2 h. The plasma samples were
collected and stored at −80°C until use. A case-control study was
conducted to evaluate the associations between mtDNA copy number
and clinical characteristics, and to retrospectively explore the
diagnostic and prognostic value of mtDNA content. The central
laboratory of Hunan Cancer Hospital measured serum tumor
biomarkers. The TNM classification was according to National
Comprehensive Cancer Network Classification Standard; Seventh
Edition (25). The following
categories of patients were enrolled for analysis of
progression-free survival (PFS): Advanced lung cancer (stage III
and IV); Eastern Cooperative Oncology Group performance-status
score of 0 or 1 (on a 5-point scale) (26); and measurable disease according to the
Response Evaluation Criteria in Solid Tumors, version 1.1 (27). Enrolled patients had received no
primary systemic chemotherapy for advanced or metastatic disease.
Epidermal growth factor receptor (EGFR) mutation was detected by
direct sequencing and anaplastic lymphoma kinase (ALK) mutation was
detected by immunohistochemistry as described previously (28). Patients with driver gene mutations who
had received EGFR-TKI or crizotinib were excluded from analysis of
PFS. PFS was defined as the length of time during and following
primary treatment of lung cancer when a patient lived with the
disease but without progression, as demonstrated by radiological
and clinical examinations.
DNA isolation and qPCR
Total plasma DNA was isolated with a QIAamp DNA
Blood Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol. The mtDNA content was measured using qPCR
as described previously (29). The
mtDNA plasmid was constructed by YRBIO (Changsha, China) and a
standard curve was generated using six dilutions of DNA
(101−106 copies/µl). The ratio of
mitochondrial ND1 to human 36B4 was used. Forward ND1 primer:
5′-CCCTAAAACCCGCCACATCT-3′; reverse ND1 primer:
5′-GAGCGATGGTGAGAGCTAAGGT-3′; forward human 36B4 primer:
5′-CAGCAAGTGGGAAGGTGTAATCC-3′; reverse human 36B4 primer:
5′-CCCATTCTATCATCAACGGGTACAA-3′. qPCR was applied to 20-µl reaction
volumes containing 2 µl DNA, 10 µl PCR Master Mix (Toyobo Life
Science, Osaka, Japan), 4 µl primers, 3.6 µl double-distilled water
and 0.4 µl ROX dye (Qiagen, Inc., Valencia, CA, USA). PCR was
performed as follows: 95°C for 60 sec, followed by 95°C for 15 sec
and 60°C for 45 sec, repeated for 40 cycles. Relative gene
expression was calculated using the comparative 2=ΔΔCq
method (30).
Statistical analysis
The SPSS statistical package was used for all
analyses (version 22.0, IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation. The Kolomogorov-Smirnov
test was used to check whether the samples independent exhibited a
normal distribution. For normal distributions, a paired Student's
t-test was used for two groups, and one-way analysis of variance
followed by Tukey's post-hoc test was used for comparison of
multiple groups. For variables not in a normal distribution,
unpaired samples were compared by use of the Mann-Whitney U test,
and multiple independent samples were compared with the
Kruskall-Wallis H test with Tukey's post-hoc test. A receiver
operating characteristic (ROC) curve was used to analyze the
diagnostic applicability of plasma mtDNA with the Youden index for
identification of the optimal cut-off point. The Kaplan-Meier
method was used for prognostic analysis. The log-rank test was
used. Spearman's correlation coefficient was used to calculate the
correlation between carcinoembryonic antigen (CEA) and mtDNA copy
number. It also was used to analyze the correlation with age.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the study
population
The characteristics of the study population are
listed in Table I. A total of 128
patients with lung cancer and 107 healthy controls were enrolled,
and they were well matched in terms of sex, age, and smoking
history. The distributions of tumor size, lymph node metastases and
distant metastases are listed. Of the cancer cases, 32 were TNM
stage I, 20 were stage II, 34 were stage III and 42 were stage IV.
Of the cancer cases, 54 were adenocarcinoma, 44 were squamous cell
carcinoma and 30 were small-cell lung cancer. A total of 29
patients had epidermal growth factor receptor (EGFR) mutations and
9 had an anaplastic lymphoma kinase (ALK) fusion.
 | Table I.Characteristics of study
population. |
Table I.
Characteristics of study
population.
|
Characteristics | Lung cancer
(n=128) | Healthy control
(n=107) | P-value |
|---|
| Sex, n |
|
| 0.694 |
|
Male | 72 | 57 |
|
|
Female | 56 | 50 |
|
| Age, years (mean ±
SD) | 55.2±7.8 | 53.5±8.8 | 0.125 |
| Smoking history,
n |
|
| 0.703 |
|
Yes | 69 | 55 |
|
| No | 59 | 52 |
|
| Tumor size, n |
|
|
|
| T1 | 32 |
|
|
| T2 | 44 |
|
|
| T3 | 22 |
|
|
| T4 | 30 |
|
|
| Lymph node
metastasis, n |
|
|
|
| N0 | 38 |
|
|
| N1 | 22 |
|
|
| N2 | 20 |
|
|
| N3 | 48 |
|
|
| TNM stage, n |
|
|
|
| I | 32 |
|
|
| II | 20 |
|
|
|
III | 34 |
|
|
| IV | 42 |
|
|
| Pathology type,
n |
|
|
|
|
Adenocarcinoma | 54 |
|
|
|
Squamous cell carcinoma | 44 |
|
|
| Small
cell lung cancer | 30 |
|
|
| Main driver gene
mutation |
|
|
|
|
EGFR | 29 |
|
|
|
ALK | 9 |
|
|
Distribution of plasma mtDNA content
in patients with lung cancer and healthy controls
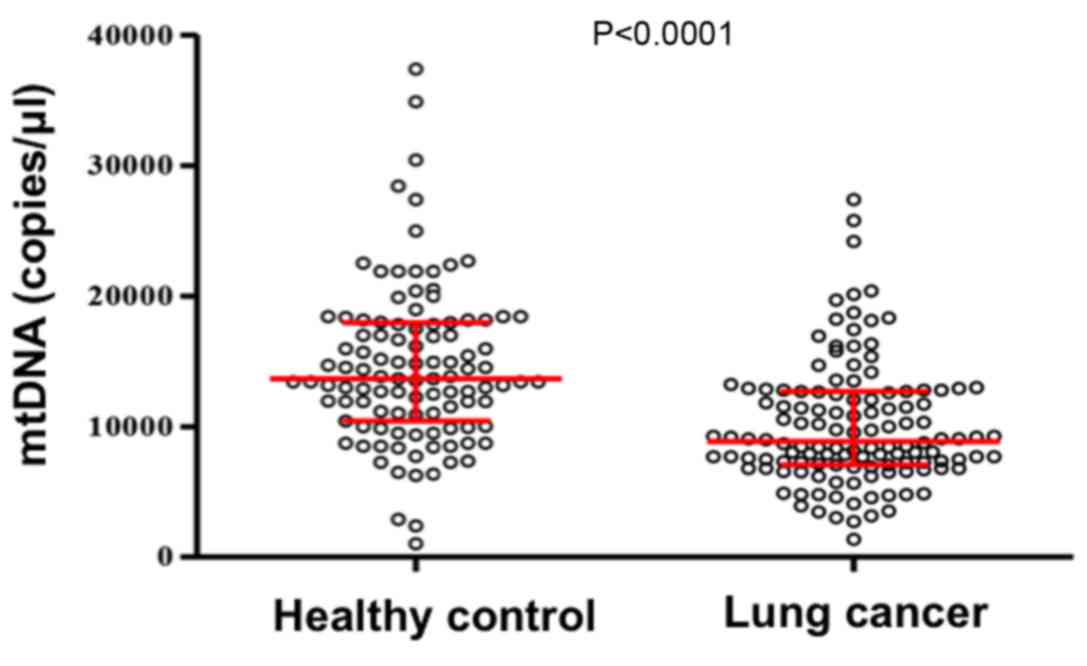
A qPCR assay was performed to explore the
distribution of plasma mtDNA content in patients with lung cancer
and healthy control subjects. As illustrated in Fig. 1, plasma mtDNA content in patients with
lung cancer was significantly lower compared with that in healthy
controls (0.89×104 and 1.37×104 copies/µl,
respectively; P<0.0001).
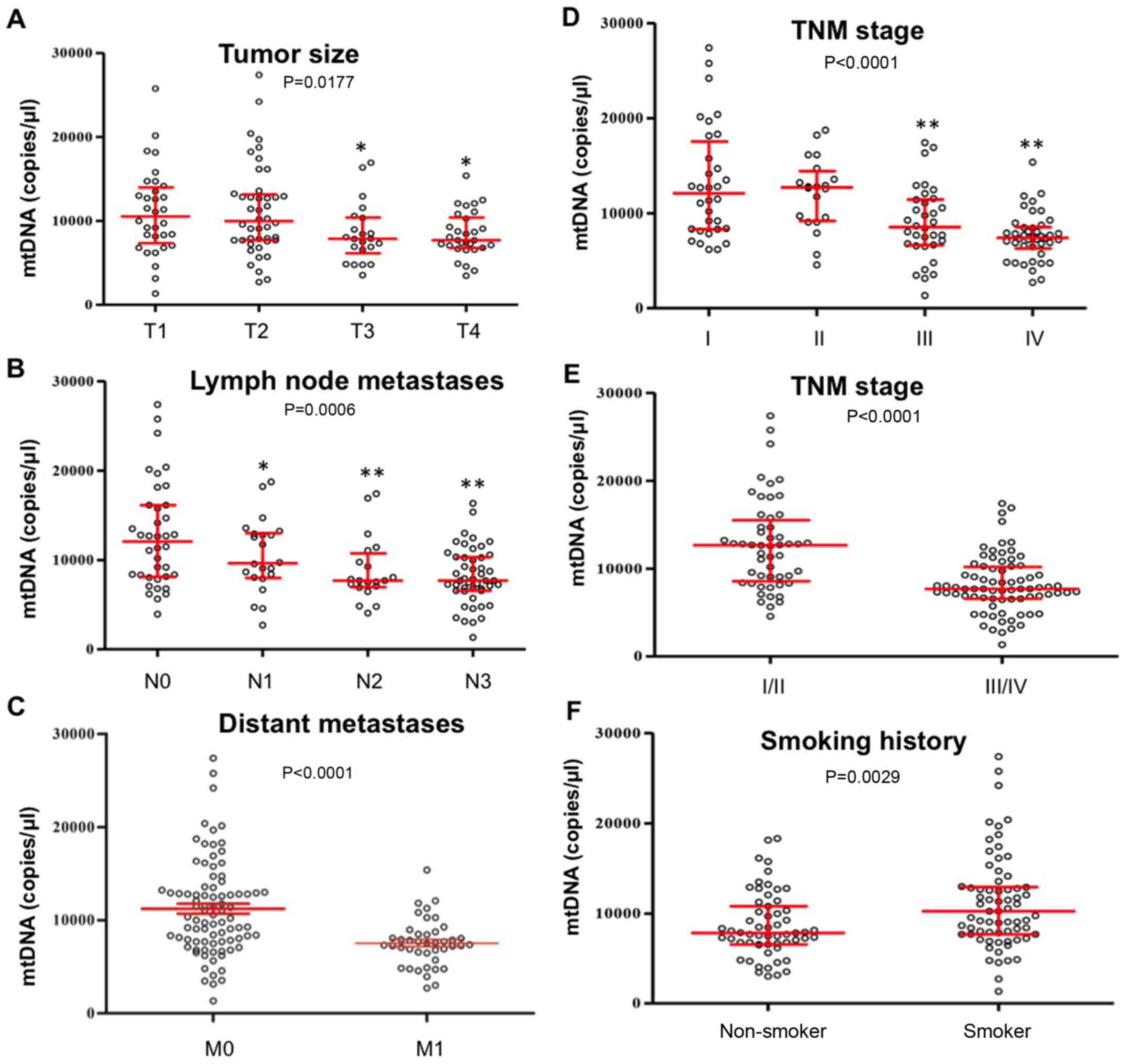
Associations between plasma mtDNA
content and clinicopathological characteristics of lung cancer
The associations between plasma mtDNA content and
clinicopathological characteristics of lung cancers were also
evaluated. As illustrated in Fig.
2A-E, plasma mtDNA content was negatively associated with tumor
size (T3 and T4 vs. T1, P<0.05), lymph node metastases (N1-3 vs.
N0, P<0.05), distant metastases (M1 vs. M0, P<0.0001) and TNM
stage (III and IV vs. I, P<0.01; III/IV vs. I/II, P<00001).
As illustrated in Fig. 2F, mtDNA
contents were significantly higher in patients with smoking history
compared with in non-smokers (P=0.0029). However, mtDNA contents
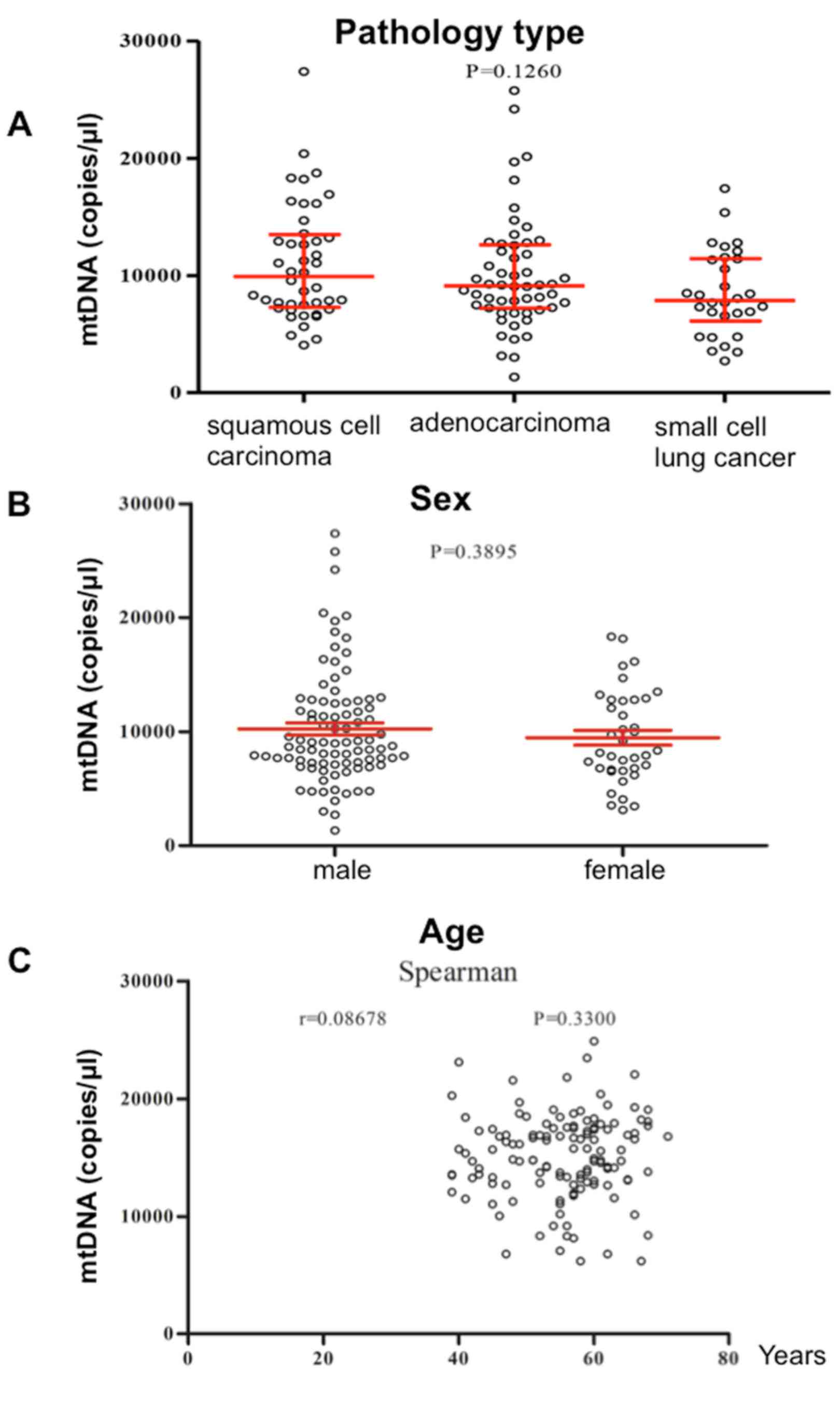
were not associated with pathological type (Fig. 3A) or sex (Fig. 3B), and were not correlated with age
(Fig. 3C) (P>0.05).
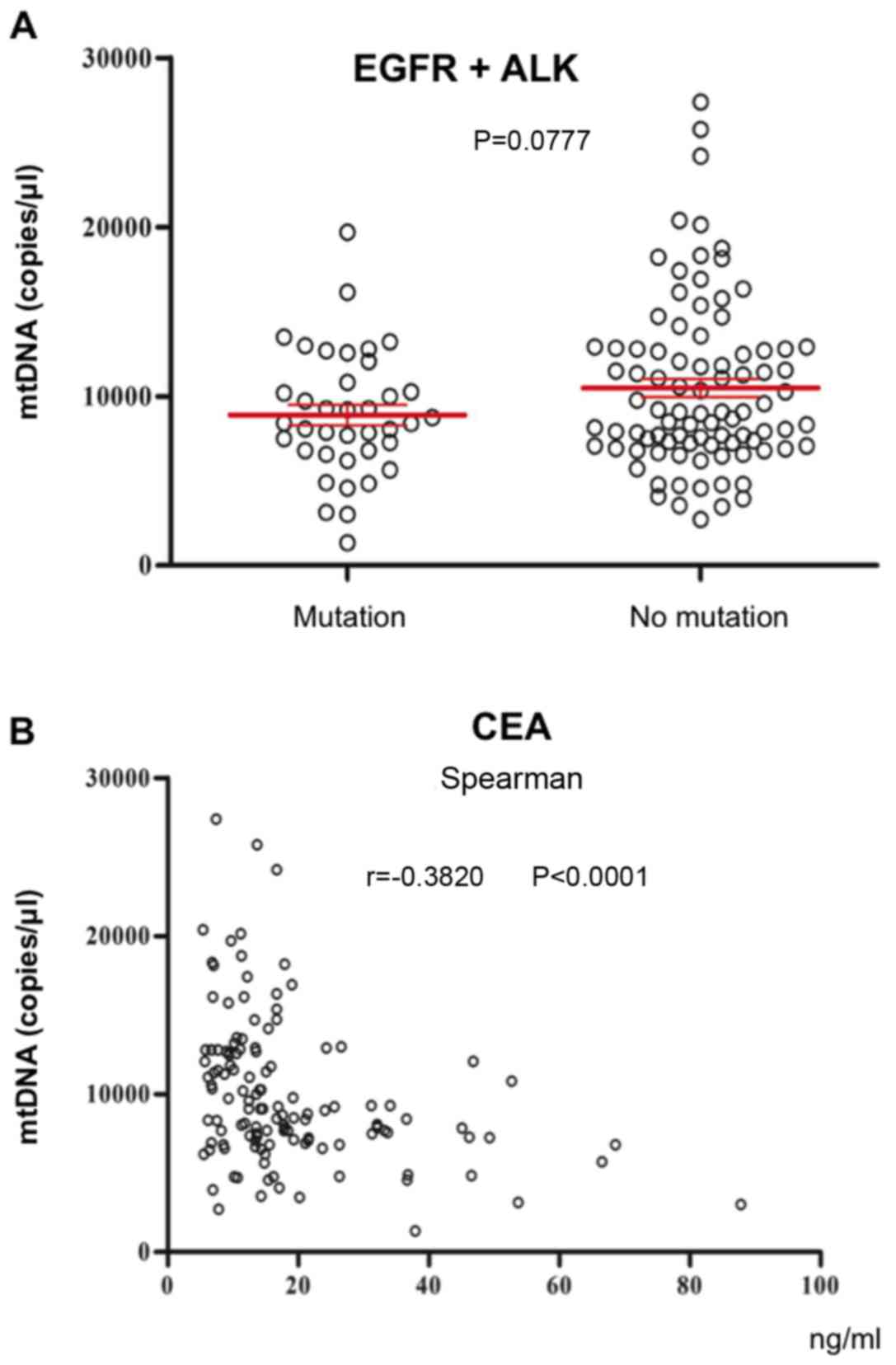
Associations between plasma mtDNA
content and driver gene mutation status and serum tumor
biomarkers
There is a strong association between driver gene
mutations and the progression of lung cancer (31). The current study analyzed plasma mtDNA
content in patients with or without epidermal growth factor
receptor/anaplastic lymphoma kinase (EGFR/ALK) mutations. The
results revealed that plasma mtDNA content in patients with
EGFR/ALK mutations was not significantly different compared with
patients without EGFR/ALK mutations (P=0.0777). As demonstrated in
Fig. 4B, CEA levels were negatively
correlated with plasma mtDNA copy number (r=−0.3820, P<0.0001).
Serum tumor biomarkers, neuron-specific enolase, carbohydrate
antigen-125 and carbohydrate antigen-199, were not correlated with
plasma mtDNA content (data not shown).
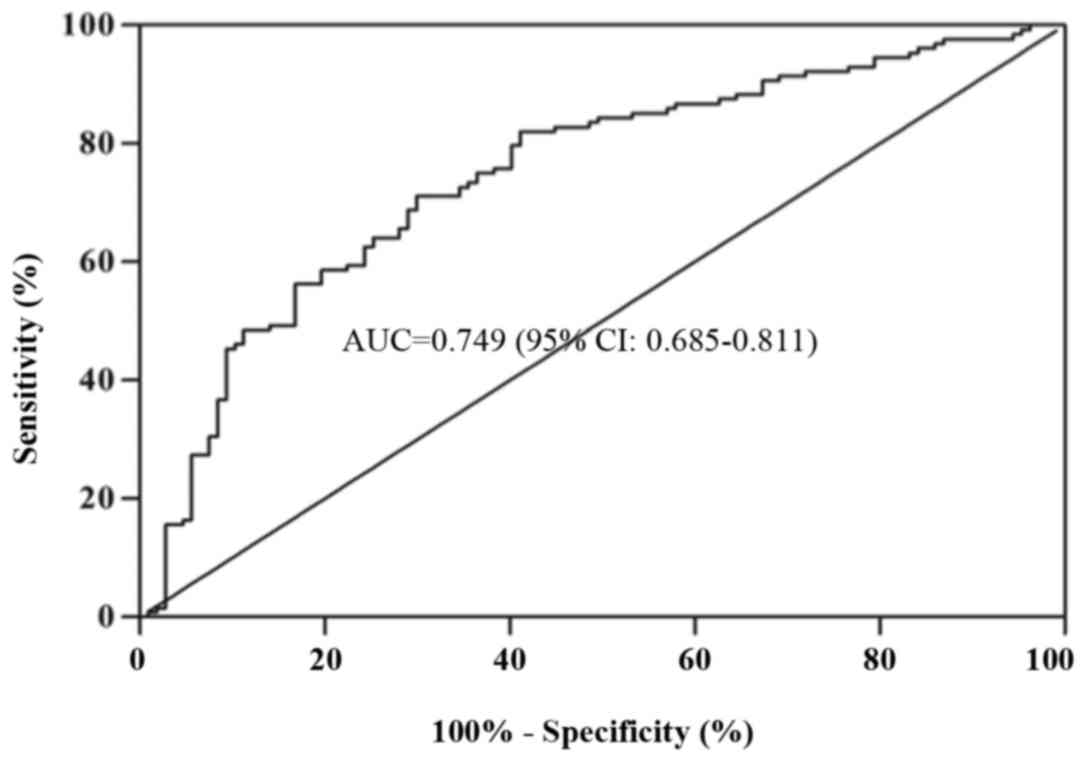
Diagnostic value of mtDNA in patients
with lung cancer
An ROC curve was used to analyze the diagnostic
applicability of plasma mtDNA. As demonstrated in Fig. 5, plasma mtDNA facilitated the
detection of lung cancer at a cutoff value of 1.19×104
copies/µl with a sensitivity of 71.1% and specificity of 70.1%.
With this cutoff value, the diagnostic accuracy of plasma mtDNA is
71.1%, the positive predictive value is 74.2%, and the negative
predictive value is 67.6%. This finding suggests promise for mtDNA
as a reliable test for lung cancer.
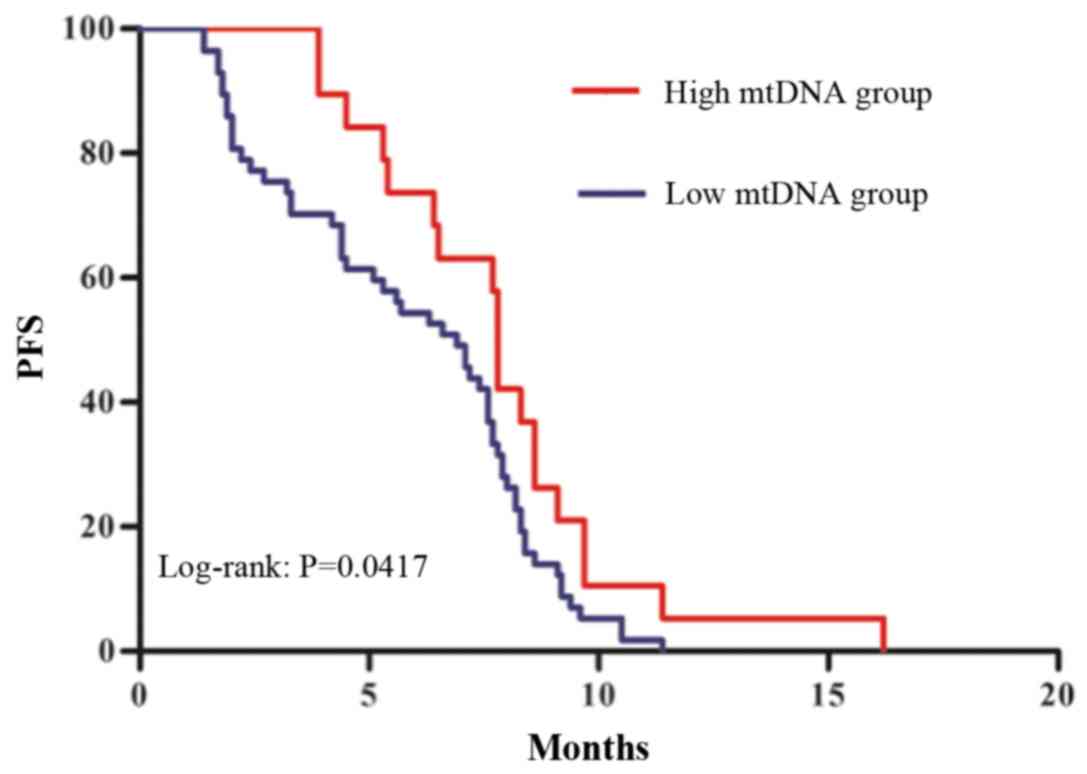
Prognostic value of mtDNA in advanced
lung cancer patients
mtDNA content has previously been associated with
prognosis in patients with colon cancer and gastric cancer at an
advanced stage (32,33). To explore the prognostic value of
mtDNA in patients with lung cancer, the current study determined
the PFS in patients with advanced stage (III and IV) cancer divided
into two groups based on mtDNA cutoff point of 1.02×104
copies/µl. As illustrated in Fig. 6,
PFS was longer in patients with mtDNA content higher than the
cutoff point compared with patients with mtDNA content lower than
the cutoff point, as assessed by Kaplan-Meier curve analysis (7.8
vs. 6.9 months; P<0.05).
Discussion
The morbidity and mortality of lung cancer are still
extremely high for patients with malignant tumors. There were an
estimated 222,500 new cases and 155,870 deaths in the United States
in 2017 (34). Lack of methods for
early diagnosis and effective treatment are at least partly
responsible for the low 5-year survival rate (35). Although quantitative analyses have
focused on the identification of biomarkers for lung cancer, there
is still a need for novel, specific biomarkers for early detection,
prognosis and dynamic observation.
Energy metabolism reprogramming is an important
characteristic of cancer (36). One
of the underlying mechanisms is mitochondrial dysfunction caused by
disorders of structure and of the mitochondrial genome (36). Abnormalities of mtDNA may lead to
increased reactive oxygen species, disrupted calcium homeostasis,
reprogramed metabolism and resistance to apoptosis (37). Changes in mtDNA content have been
reported in malignancies, with both down- and upregulation of mtDNA
content in solid tumors. Decreased mtDNA content has been
associated with renal cell carcinoma (38), hepatocellular carcinoma (39) and esophageal adenocarcinoma (40). By contrast, increased mtDNA content in
pancreatic cancer (41), colorectal
cancer (42) and breast cancer
(43) has been reported. Since mtDNA
may be involved in carcinogenesis, oncologists and pulmonologists
have been motivated to monitor its dynamic alterations in patients
with lung cancer. Hou et al (24) reported increased serum mtDNA content,
while others have revealed significantly reduced mtDNA content in
lung cancer tissues (8,9). In the current study, it was identified
that circulating mtDNA content was significantly lower in patients
with lung cancer compared with healthy subjects. The lower mtDNA
content may reflect the reduced capacity of compensatory responses
to oxidative stress damage (44). The
decrease in mtDNA may also be a consequence of mutation or
depletion in the mtDNA D-loop caused by reactive oxygen species
(45).
Alterations in somatic mtDNA copy number have been
revealed to strongly correlate with clinicopathological
characteristics, early diagnosis, progression, and radiotherapy and
chemotherapy efficacy in malignancies (14). The current study identified that lower
plasma mtDNA contents were negatively associated with tumor size,
lymph node metastases, distant metastases and serum CEA level, but
were not associated with age, sex, pathological type or main driver
gene mutation status. The incidence of D-loop mutations of mtDNA in
advanced cancer stages has been revealed to be higher when compared
with patients in the early stage of disease (8), which may lead to lower mtDNA copy
numbers in patients with late-stage cancer. These observations may
account for the current findings that plasma mtDNA content was
negatively associated with tumor size, lymph node metastases and
distant metastases.
Serum tumor biomarkers including CEA have been
widely used to monitor the progression of tumors (46). In the current study, a negative
correlation between plasma mtDNA content and serum CEA level was
revealed, which to the best of our knowledge, is the first time
this correlation has been recorded. Both serum CEA levels and
plasma mtDNA can be dynamically monitored for chemotherapeutic
effects and disease progression (46). Thus, the combined determination of CEA
and mtDNA content may improve the efficacy and prognostic ability
of these tests in lung cancer. In related work, no relationship
between mtDNA content and serum prostate-specific antigen levels
has been identified in prostate cancer and epithelial ovarian
cancer (18,47). Further studies should be performed to
explore the predictive value of the combination of dynamic
monitoring of CEA level and circulating mtDNA alteration during
chemotherapy. However, the efficacy of chemotherapy in multiple
solid tumors is affected by a number of factors (48). A perspective study, with these
relevant factors well matched, is required to explore the
predictive value of dynamic circulating mtDNA content.
The quantification of plasma mtDNA may be used to
recognize patients with poor prognosis, similar to the lower mtDNA
copy number in patients who have advanced breast cancer, glioma and
prostate cancer (32,33,49,50). Lower
mtDNA content has been identified to be associated with tumor
progression in lung cancer tissues following chemotherapy (51). Variations in mtDNA have been reported
to be associated with radiation-induced toxicity (52). The change of mtDNA content can also
improve the cytotoxicity of chemotherapeutic agents. DNA damage in
mitochondria alters the mitochondrial apoptotic signaling pathway,
thereby promoting the survival of cancer cells and even changing
their resistance to anticancer drugs (53). Radiotherapy and chemotherapy are the
most important treatments for advanced lung cancer. Clinically,
sensitivity to radiotherapy and chemotherapy are determining
factors for prognosis. Given that mtDNA depletion affects
radiotherapy and chemotherapy sensitivity (5), the current study further explored the
prognostic value of lower plasma mtDNA content in advanced lung
cancer patients. As previously reported (54), the current study revealed that a lower
mtDNA copy number was associated with poor prognosis in patients
with advanced stage cancer, which suggests that plasma mtDNA copy
number is a promising prognostic candidate for lung cancer.
Further studies are required to elucidate the
mechanisms and consequences of decreased mtDNA content in lung
cancer carcinogenesis. The lower mtDNA copy number may alter
mitochondrial gene expression and lead to decreased mitochondrial
function and energy metabolism and cause increased generation of
reactive oxygen species (55,56), enhanced glycolysis, and even
alterations in mitochondrial bioenergetic and biosynthetic states
(57). Decreased mtDNA may also
promote epithelial-mesenchymal transition (58–60) and
apoptosis-resistant cancer cells through the phosphoinositide
3-kinase/Akt signaling pathway (61).
Changes in mtDNA copy number may explain the persistent deficiency
in mitochondrial activity. Thus, the mtDNA-depleted ρ0 cell line
may be used to further explore the consequences and mechanism of
mtDNA deficiency in lung cancer carcinogenesis.
To the best of our knowledge, the current study is
the largest that has explored the diagnostic, predictive and
prognostic clinical application of plasma mtDNA content in patients
with lung cancer. The current study is also the most comprehensive
study of plasma mtDNA alteration in patients with lung cancer.
However, further studies of specific subgroups with large sample
sizes are still required to further confirm the subgroup
conclusions made by the current study.
In conclusion, decreased plasma mtDNA content was
associated with tumor metastatic potential and unfavorable
prognosis in patients with lung cancer. Monitoring circulating
mtDNA content is a promising approach for diagnosis and prognosis
of lung cancer. Further studies of the epigenetic alterations of
mtDNA are required to understand its downstream effectors and role
in lung cancer pathogenesis. Although translating plasma mtDNA
quantification into routine clinical practice may take several
steps, knowledge regarding the potential applicability of
circulating mtDNA quantification in the diagnosis and prognosis of
lung cancer has progressed considerably.
Acknowledgements
Not applicable.
Funding
The present study was supported by grant from the
National Natural Science Foundation of China (grant no. 81401631)
and by grant from Key Research and Develop Program of Hunan
Province, China (grant no. 2017WK2061).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JC and LZ conceived and designed the experiments;
LZ, XY, HZ and YL performed the experiments and contributed to
molecular analysis; WW and LW analyzed the data and LZ wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by Department of
Ethics Committee of Hunan Cancer Hospital (Changsha, China), and
written informed consent was obtained from all participants.
Patient consent for publication
Informed consent for the publication or any
associated images was obtained from healthy controls and patients
according to protocols approved by the hospital's Ethics
Committee.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallace DC: Mitochondria and cancer. Nat
Rev Cancer. 12:685–698. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Erchia AM, Atlante A, Gadaleta G, Pavesi
G, Chiara M, De Virgilio C, Manzari C, Mastropasqua F, Prazzoli GM,
Picardi E, et al: Tissue-specific mtDNA abundance from exome data
and its correlation with mitochondrial transcription, mass and
respiratory activity. Mitochondrion. 20:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akhmedov AT and Marin-Garcia J:
Mitochondrial DNA maintenance: An appraisal. Mol Cell Biochem.
409:283–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu CC, Tseng LM and Lee HC: Role of
mitochondrial dysfunction in cancer progression. Exp Biol Med
(Maywood). 241:1281–1295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Gisbergen MW, Voets AM, Starmans MH,
de Coo IF, Yadak R, Hoffmann RF, Boutros PC, Smeets HJ, Dubois L
and Lambin P: How do changes in the mtDNA and mitochondrial
dysfunction influence cancer and cancer therapy? Challenges,
opportunities and models. Mutat Res Rev Mutat Res. 764:16–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Liu VW, Xue WC, Tsang PC, Cheung
AN and Ngan HY: The increase of mitochondrial DNA content in
endometrial adenocarcinoma cells: A quantitative study using
laser-captured microdissected tissues. Gynecol Oncol. 98:104–110.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin CS, Lee HT, Lee SY, Shen YA, Wang LS,
Chen YJ and Wei YH: High mitochondrial DNA copy number and
bioenergetic function are associated with tumor invasion of
esophageal squamous cell carcinoma cell lines. Int J Mol Sci.
13:11228–11246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HC, Yin PH, Lin JC, Wu CC, Chen CY, Wu
CW, Chi CW, Tam TN and Wei YH: Mitochondrial genome instability and
mtDNA depletion in human cancers. Ann N Y Acad Sci. 1042:109–122.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai JG, Zhang ZY, Liu QX and Min JX:
Mitochondrial genome microsatellite instability and copy number
alteration in lung carcinomas. Asian Pac J Cancer Prev.
14:2393–2399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin PC, Lin JK, Yang SH, Wang HS, Li AF
and Chang SC: Expression of beta-F1-ATPase and mitochondrial
transcription factor A and the change in mitochondrial DNA content
in colorectal cancer: Clinical data analysis and evidence from an
in vitro study. Int J Colorectal Dis. 23:1223–1232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan AX, Radpour R, Haghighi MM, Kohler C,
Xia P, Hahn S, Holzgreve W and Zhong X: Mitochondrial DNA content
in paired normal and cancerous breast tissue samples from patients
with breast cancer. J Cancer Res Clin Oncol. 135:983–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meierhofer D, Mayr JA, Foetschl U, Berger
A, Fink K, Schmeller N, Hacker GW, Hauser-Kronberger C, Kofler B
and Sperl W: Decrease of mitochondrial DNA content and energy
metabolism in renal cell carcinoma. Carcinogenesis. 25:1005–1010.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosgood HD III, Liu CS, Rothman N,
Weinstein SJ, Bonner MR, Shen M, Lim U, Virtamo J, Cheng WL,
Albanes D and Lan Q: Mitochondrial DNA copy number and lung cancer
risk in a prospective cohort study. Carcinogenesis. 31:847–849.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu M: Circulating cell-free mitochondrial
DNA as a novel cancer biomarker: Opportunities and challenges.
Mitochondrial DNA. 23:329–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strelkova I, Abdullaev SA, Snigireva GP,
Bezlepkin VG and Gaziev AI: Share of extracellular mutated
mitochondrial DNA increases in plasma of lung cancer patients
following radiotherapy. Biomed Khim. 56:517–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimura T and Kunugita N: Mitochondrial
reactive oxygen species-mediated genomic instability in low-dose
irradiated human cells through nuclear retention of cyclin D1. Cell
Cycle. 15:1410–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ellinger J, Muller DC, Muller SC, Hauser
S, Heukamp LC, von Ruecker A, Bastian PJ and Walgenbach-Brunagel G:
Circulating mitochondrial DNA in serum: A universal diagnostic
biomarker for patients with urological malignancies. Urol Oncol.
30:509–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zachariah RR, Schmid S, Buerki N, Radpour
R, Holzgreve W and Zhong X: Levels of circulating cell-free nuclear
and mitochondrial DNA in benign and malignant ovarian tumors.
Obstet Gynecol. 112:843–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandes J, Michel V, Camorlinga-Ponce M,
Gomez A, Maldonado C, De Reuse H, Torres J and Touati E:
Circulating mitochondrial DNA level, a noninvasive biomarker for
the early detection of gastric cancer. Cancer Epidemiol Biomarkers
Prev. 23:2430–2438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ellinger J, Albers P, Muller SC, von
Ruecker A and Bastian PJ: Circulating mitochondrial DNA in the
serum of patients with testicular germ cell cancer as a novel
noninvasive diagnostic biomarker. BJU Int. 104:48–52. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kohler C, Radpour R, Barekati Z,
Asadollahi R, Bitzer J, Wight E, Bürki N, Diesch C, Holzgreve W and
Zhong XY: Levels of plasma circulating cell free nuclear and
mitochondrial DNA as potential biomarkers for breast tumors. Mol
Cancer. 8:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu M, Wan YF and Zou QH: Cell-free
circulating mitochondrial DNA in the serum: A potential
non-invasive biomarker for Ewing's sarcoma. Arch Med Res.
43:389–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou YL, Chen JJ, Wu YF, Xue CJ, Li FZ,
Zheng Q and Chen H: Clinical significance of serum mitochondrial
DNA in lung cancer. Clin Biochem. 46:1474–1477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rusch VW, Asamura H, Watanabe H, Giroux
DJ, Rami-Porta R and Goldstraw P; and Members of IASLC Staging
Committee, : The IASLC lung cancer staging project: A proposal for
a new international lymph node map in the forthcoming seventh
edition of the TNM classification for lung cancer. J Thorac Oncol.
4:568–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prigerson HG, Bao Y, Shah MA, Paulk ME,
LeBlanc TW, Schneider BJ, Garrido MM, Reid MC, Berlin DA, Adelson
KB, et al: Chemotherapy use, performance status, and quality of
life at the end of life. JAMA Oncol. 1:778–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Jaime Corral J, Gray JE, Powderly J,
Chouaid C, et al: Durvalumab as third-line or later treatment for
advanced non-small-cell lung cancer (ATLANTIC): An open-label,
single-arm, phase 2 study. Lancet Oncol. 19:521–536. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, Hann HW, Wan S, Hann RS, Wang C, Lai
Y, Ye X, Evans A, Myers RE, Ye Z, et al: Cell-free circulating
mitochondrial DNA content and risk of hepatocellular carcinoma in
patients with chronic HBV infection. Sci Rep. 6:239922016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Campbell JD, Alexandrov A, Kim J, Wala J,
Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et
al: Distinct patterns of somatic genome alterations in lung
adenocarcinomas and squamous cell carcinomas. Nat Genet.
48:607–616. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, He S, Zhu X, Qiao W and Zhang J:
High copy number of mitochondrial DNA predicts poor prognosis in
patients with advanced stage colon cancer. Int J Biol Markers.
31:e382–e388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang G, Qu Y, Dang S, Yang Q, Shi B and
Hou P: Variable copy number of mitochondrial DNA (mtDNA) predicts
worse prognosis in advanced gastric cancer patients. Diagn Pathol.
8:1732013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hofmann JN, Hosgood HD III, Liu CS, Chow
WH, Shuch B, Cheng WL, Lin TT, Moore LE, Lan Q, Rothman N and
Purdue MP: A nested case-control study of leukocyte mitochondrial
DNA copy number and renal cell carcinoma in the prostate, lung,
colorectal and ovarian cancer screening trial. Carcinogenesis.
35:1028–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao S, Yang Y, Liu J, Liu H, Ge N, Yang
H, Zhang H and Xing J: Association of mitochondrial DNA content in
peripheral blood leukocyte with hepatitis B virus-related
hepatocellular carcinoma in a Chinese Han population. Cancer
science. 102:1553–1558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu E, Sun W, Gu J, Chow WH, Ajani JA and
Wu X: Association of mitochondrial DNA copy number in peripheral
blood leukocytes with risk of esophageal adenocarcinoma.
Carcinogenesis. 34:2521–2524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lynch SM, Weinstein SJ, Virtamo J, Lan Q,
Liu CS, Cheng WL, Rothman N, Albanes D and Stolzenberg-Solomon RZ:
Mitochondrial DNA copy number and pancreatic cancer in the
alpha-tocopherol beta-carotene cancer prevention study. Cancer Prev
Res (Phila). 4:1912–1919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qu F, Liu X, Zhou F, Yang H, Bao G, He X
and Xing J: Association between mitochondrial DNA content in
leukocytes and colorectal cancer risk: A case-control analysis.
Cancer. 117:3148–3155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen J, Platek M, Mahasneh A, Ambrosone CB
and Zhao H: Mitochondrial copy number and risk of breast cancer: A
pilot study. Mitochondrion. 10:62–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
von Kleist-Retzow JC, Hornig-Do HT,
Schauen M, Eckertz S, Dinh TA, Stassen F, Lottmann N, Bust M,
Galunska B, Wielckens K, et al: Impaired mitochondrial Ca2+
homeostasis in respiratory chain-deficient cells but efficient
compensation of energetic disadvantage by enhanced anaerobic
glycolysis due to low ATP steady state levels. Exp Cell Res.
313:3076–3089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HC, Hsu LS, Yin PH, Lee LM and Chi CW:
Heteroplasmic mutation of mitochondrial DNA D-loop and 4977-bp
deletion in human cancer cells during mitochondrial DNA depletion.
Mitochondrion. 7:157–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou W, Zhu M, Gui M, Huang L, Long Z,
Wang L, Chen H, Yin Y, Jiang X, Dai Y, et al: Peripheral blood
mitochondrial DNA copy number is associated with prostate cancer
risk and tumor burden. PLoS One. 9:e1094702014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lissoni P, Brivio F, Fumagalli L, Messina
G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A and Gardani
GS: Efficacy of cancer chemotherapy in relation to the pretreatment
number of lymphocytes in patients with metastatic solid tumors. Int
J Biol Markers. 19:135–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weerts MJ, Sieuwerts AM, Smid M, Look MP,
Foekens JA, Sleijfer S and Martens JW: Mitochondrial DNA content in
breast cancer: Impact on in vitro and in vivo phenotype and patient
prognosis. Oncotarget. 7:29166–29176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tu H, Gu J, Meng QH, Kim J, Davis JW, He
Y, Wagar EA, Thompson TC, Logothetis CJ and Wu X: Mitochondrial DNA
copy number in peripheral blood leukocytes and the aggressiveness
of localized prostate cancer. Oncotarget. 6:41988–41996. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin CS, Wang LS, Tsai CM and Wei YH: Low
copy number and low oxidative damage of mitochondrial DNA are
associated with tumor progression in lung cancer tissues after
neoadjuvant chemotherapy. Interact Cardiovasc Thorac Surg.
7:954–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fachal L, Mosquera-Miguel A, Gomez-Caamano
A, Sánchez-García M, Calvo P, Lobato-Busto R, Salas A and Vega A:
Evaluating the role of mitochondrial DNA variation to the genetic
predisposition to radiation-induced toxicity. Radiother Oncol.
111:199–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yeung M, Hurren R, Nemr C, Wang X,
Hershenfeld S, Gronda M, Liyanage S, Wu Y, Augustine J, Lee EA, et
al: Mitochondrial DNA damage by bleomycin induces AML cell death.
Apoptosis. 20:811–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dang S, Qu Y, Wei J, Shao Y, Yang Q, Ji M,
Shi B and Hou P: Low copy number of mitochondrial DNA (mtDNA)
predicts worse prognosis in early-stage laryngeal cancer patients.
Diagn Pathol. 9:282014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cook CC, Kim A, Terao S, Gotoh A and
Higuchi M: Consumption of oxygen: A mitochondrial-generated
progression signal of advanced cancer. Cell death Dis. 3:e2582012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vermulst M, Bielas JH and Loeb LA:
Quantification of random mutations in the mitochondrial genome.
Methods. 46:263–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin CS, Lee HT, Lee MH, Pan SC, Ke CY,
Chiu AW and Wei YH: Role of mitochondrial DNA copy number
alteration in human renal cell carcinoma. Int J Mol Sci. 17:2016.
View Article : Google Scholar
|
|
58
|
Guha M, Srinivasan S, Ruthel G, Kashina
AK, Carstens RP, Mendoza A, Khanna C, Van Winkle T and Avadhani NG:
Mitochondrial retrograde signaling induces epithelial-mesenchymal
transition and generates breast cancer stem cells. Oncogene.
33:5238–5250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Naito A, Cook CC, Mizumachi T, Wang M, Xie
CH, Evans TT, Kelly T and Higuchi M: Progressive tumor features
accompany epithelial-mesenchymal transition induced in
mitochondrial DNA-depleted cells. Cancer Sci. 99:1584–1588. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Adib-Conquy M and Cavaillon JM:
Compensatory anti-inflammatory response syndrome. Thromb Haemost.
101:36–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Moro L, Arbini AA, Yao JL, di San t'Agnese
PA, Marra E and Greco M: Mitochondrial DNA depletion in prostate
epithelial cells promotes anoikis resistance and invasion through
activation of PI3K/Akt2. Cell Death Differ. 16:571–583. 2009.
View Article : Google Scholar : PubMed/NCBI
|