Introduction
Retinoblastoma (RB) is a primary malignant tumor in
children's eyes, which frequently occurs in the retinal nuclear
layer and has a familial genetic tendency (1,2). RB mostly
occurs in both eyes accompanied by multiple lesions, and
intracranial and systemic metastasis occurs easily if the tumor
grows and breaks through the eyeball, seriously threatening the
life of child patients (3). Early and
effective treatment can make child patients retain partial visual
function and obtain longer survival time, while the optimal
opportunity for treatment will be delayed if there are no timely
diagnosis and treatment leading to an extremely poor prognosis of
child patients. Intracranial invasion and metastasis of tumors
often occur in advanced child patients after treatment, causing
great harm (4,5). Therefore, searching the molecular
biological markers for the early diagnosis of RB, investigating the
molecular signaling regulation mechanism in the occurrence and
development of RB and finding the therapeutic targets for
inhibiting the occurrence and development of RB are of great
significance in improving the early diagnosis and treatment of RB
and the prognosis of the patients.
Micro-ribonucleic acid (miRNA) is a newly-discovered
highly-conserved endogenous non-coding hairpin nucleotide
transcript, ~19–25 bases in length, which widely exists in
eukaryotic cells and is generated by the endogenous pre-miRNA via
Dicer cleavage, with 18–25 nucleotides in size (6,7). miRNA
plays different roles in different physiological processes,
including developmental regulation, nervous system development,
cell proliferation and apoptosis. Moreover, miRNA widely exists in
tissues and cells, and is involved in a series of vital activities,
such as individual development, cell proliferation, apoptosis and
differentiation, which is closely related to the occurrence and
development of a variety of malignant tumors, so it can serve as a
therapeutic target for various malignant tumors (8,9).
Currently, a large number of studies have found that some specific
miRNAs are abnormally expressed in RB and have close associations
with the occurrence and development of RB (10). miR-204 has been studied in a variety
of malignant tumors, including breast, gastric, prostate and
endometrial cancer, and its expression is low in these tumors.
miR-204 can bind to target genes to be involved in the pathway of
tumor cells, affecting the growth of tumor cells and playing a role
as cancer suppressor gene in a variety of malignant tumors
(11,12). Y79 cells are established via the
primary culture of tumors resected from the right eye of RB
patients with a strong RB maternal familial heredity, whose
ultrastructures, such as nuclear membrane infolding, trilamellar
membrane structure, coated vesicle, microtubule, centriole,
annulate lamellae and basal granule, are similar to those of RB
(13).
Studies have demonstrated that miR-204 is expressed
at low level in RB tissues (14), but
its specific biological effect and mechanism in RB have not been
clarified and need further studies. In this study, the miR-204
expression in RB and para-carcinoma tissues was detected, its
associations with clinicopathological features of patients were
analyzed, and the expression, clinical significance and biological
effect on RB cells were explored via the in vitro experiment
of transient transfection of Y79 cells with miR-204 mimics, so as
to provide clinical reference bases for early diagnosis, treatment
and prognosis evaluation of RB.
Materials and methods
General information
A total of 110 cases of RB tissues were collected
after ophthalmectomy in the First Affiliated Hospital of Hunan
Normal University (People's Hospital of Hunan Province) (Changsha,
China) from April 2013 to June 2017. Another 100 cases of
para-carcinoma normal tissues (>1 cm away from the tumor lesion)
were collected. Among the 110 patients with RB, there were 51 males
and 59 females, aged from 4 months to 11 years with an average age
of 3.2±1.6 years, 46 cases with monocular RB and 64 cases with
binocular RB. The degree of tissue differentiation was judged based
on the international intraocular RB classification criteria
(15), and there were 21 cases of
undifferentiated type and 89 cases of differentiated type. In terms
of clinical stage, there were 41 cases of intraocular stage, 36
cases of glaucoma stage, and 33 cases of extended stage. Moreover,
there were 69 cases with neural infiltration and 41 cases without
neural infiltration. Inclusion criteria: patients who did not
receive any treatment before surgery, patients whose tissue
sections were diagnosed by the chief physician of the Pathology
Department in the First Affiliated Hospital of Hunan Normal
University (People's Hospital of Hunan Province), patients with
complete clinical data. Exclusion criteria: patients with a history
of mental disease and a family history of mental disease, or
patients complicated with severe heart, lung, liver and renal
dysfunction. Only one eye was taken from patients with binocular RB
as specimen, and immediately placed in liquid nitrogen for 5 h and
then stored at −80°C for cryopreservation. This study was approved
by the Ethics Committee of the First Affiliated Hospital of Hunan
Normal University (People's Hospital of Hunan Province), and the
patients and their families were informed and signed the informed
consent.
Main instruments and reagents
RNA extraction kit (TRIzol method) and liposome
(Lipofectamine 2000) for cell transfection were purchased from
Invitrogen (Invitrogen: Thermo Fisher Scientific, Inc., Waltham,
MA, USA). miR-204, B-cell lymphoma 2 (Bcl-2) and Sirt1 reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) kits
were purchased from Takara Bio, Inc. (Otsu, Japan). M-MLV reverse
transcription kit was purchased from Applied Biosystems (Applied
Biosystems: Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
UV-1900 double-beam UV Spectrophotometer was purchased from Yoke
Instrument Co., Ltd. (Shanghai, China). The SYBR-Green qPCR Master
Mix kit was purchased from Thermo Fisher Scientific, Inc.
(Shanghai, China). RB Y79 and HXO-Rb44 cell lines and human retinal
microvascular endothelial cell line ACBRI-181 were purchased from
Shanghai Guandao Bio-engineering, Ltd. (Shanghai, China). miR-204
and its corresponding negative control plasmid were purchased from
Shanghai BioLeaf Biotech Co., Ltd. (Shanghai, China). Blue methyl
thiazolyl tetrazolium (MTT), dimethyl sulfoxide (DMSO) and Annexin
V-FITC/PI apoptosis detection kits were from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). Real-time
fluorescence quantitative PCR and CytoFLEX S series flow cytometry
were from Beckman Coulter, Inc. (Brea, CA, USA). The primers used
in real-time fluorescence quantitative PCR for miR-204, Bcl-2
messenger RNA (mRNA), Sirt1 mRNA and β-actin were synthesized by
Invitrogen: Thermo Fisher Scientific, Inc. The required primer
sequences are shown in Table I.
 | Table I.Primer sequences for the reference
genes miR-204, Bcl-2 mRNA, Sirt1 mRNA and β-actin. |
Table I.
Primer sequences for the reference
genes miR-204, Bcl-2 mRNA, Sirt1 mRNA and β-actin.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| miR-204 |
5′-AACCUGAUCCCGUCUGAGAUUG-3′ |
5′-CCGGAUCAAGAUUAGUUCGGUU-3′ |
| Bcl-2 |
5′-CCTTTGTGTAACTGTACGGCC-3′ |
5′-CTTTGGCAGTAAATAGCTGATTCGAC-3′ |
| Sirt1 |
5′-CAAAGGAGCAGATTAGTAGGCG-3′ |
5′-CTCTGGCATGTCCCACTATCAC-3′ |
| β-actin |
5′-ATCATGTTTGAGACCTTCAACA-3′ |
5′-CATCTCTTGCTCGAAGTCCA-3′ |
Cell culture and transfection
Cell culture: the human RB Y79, HXO-Rb44 cell lines
and human retinal microvascular endothelial cell line ACBRI-181
were cultured in high-glucose Dulbecco's modified Eagle's medium
(DMEM) supplemented with 15% fetal bovine serum and 1%
penicillin/streptomycin in an incubator under 5% CO2 at
37°C, followed by culture at constant temperature and saturated
humidity. Cells were stabilized for 2–3 generations, and the medium
was replaced in time. Cell transfection: the cell transfection was
performed with reference to the instructions of Lipofectamine 2000
for cell transfection. The cells in the logarithmic growth phase
were inoculated into a 6-well plate at a concentration of
1×l05 cells/well, and the transfection was performed
when 25–50% cells were fused on the next day. In the experimental
group, 80 pmol/l miR-204 mimics and 10 µl Lipofectamine 2000 were
added into the well plate. In the control group, 80 pmol/l negative
control and 10 µl Lipofectamine 2000 were added into each well. The
plate in both two groups was placed in a constant-temperature
incubator under 5% CO2 at 37°C for incubation.
RT-qPCR detection
The total RNA was extracted from tissues and cells
using the RNA extraction kit. The absorption value of RNA extracted
was determined by UV-1900 double-beam UV Spectrophotometer and the
integrity of total RNA was determined by 1% agarose gel
electrophoresis. Total RNA (1 µl) was taken and reversely
transcribed into cDNA according to the instructions of the M-MLV
reverse transcription kit. The reaction system was as follows: 10
µl RNA and 10 µl oligo(dT) were evenly mixed in a thin-walled tube,
followed by heating at 65°C for 30 min, 10 µl 2X SYBR-Green qPCR
Master Mix solution were added and mixed evenly, and then
RNase-free water was added until the total volume was 20 µl,
followed by reaction at 37°C for 2.5 h and heating at 65°C for 30
min. After cooling for 1 min, 20 µl cDNA were diluted to 100 µl
with deionized water and stored at −80°C for use. cDNA was used for
the RT-qPCR detection of miR-204, Bcl-2 and Sirt1, with β-actin as
an internal reference. PCR conditions were as follows:
pre-denaturation at 94°C for 3 min, 35 cycles, denaturation at 95°C
for 10 sec, annealing at 65°C for 8 sec, extension at 72°C for 1
min and then extension again at 72°C for 10 min. After the
reaction, the Cq value of each reaction tube was obtained. The
relative expression levels of miR-204, Bcl-2 mRNA and Sirt1 mRNA
were analyzed via 2−ΔCq for relative gene quantification
(16).
Cell proliferation and apoptosis
detection
Cell proliferation was detected by the MTT method.
The cells in the logarithmic growth phase were prepared into the
cell suspension (1×105) and inoculated into a 96-well
plate at a density of 3×103 cells/well. The cell
viability was detected by MTT once every 24 h, 4 times until 96 h.
For the detection, 20 µl MTT solution (5 mg/ml) were added into
each well. After cells being incubated in the incubator for 4 h,
the culture medium was discarded and 150 µl DMSO were added into
each well. The crystals were dissolved via vibration for 10 min at
room temperature. The optical density (OD) at the wavelength of 490
nm in each well was repeatedly detected 3 times using a microplate
reader. Apoptosis was detected by flow cytometry. The cells
transfected for 48 h were collected and digested by trypsin, and
then washed with 0.01 mol/l cold phosphate-buffered saline (PBS).
The supernatant was discarded after centrifugation at 111.8 × g
under the temperature of 25°C. The cells were re-suspended with 100
µl 1X binding buffer, and then transferred to the flow detection
tube. A total of 5 µl 7-AAD and 5 µl PE Annexin V were added into
each tube, followed by reaction for 15 min at room temperature
without light. Then 400 µl 1X binding buffer were added. Flow
cytometry was completed within 1 h, and each specimen was
repeatedly detected 3 times.
Statistical methods
The SPSS 19.0 software (IBM Corp., Beijing, China)
was used for statistical analysis. The measurement data were
expressed as mean ± standard deviation (SD) and compared among
groups by t-test. The mean values among multiple groups were
compared via the one-way analysis of variance, and the Dunnett's
test was the post hoc test used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-204 in RB and
para-carcinoma normal tissues
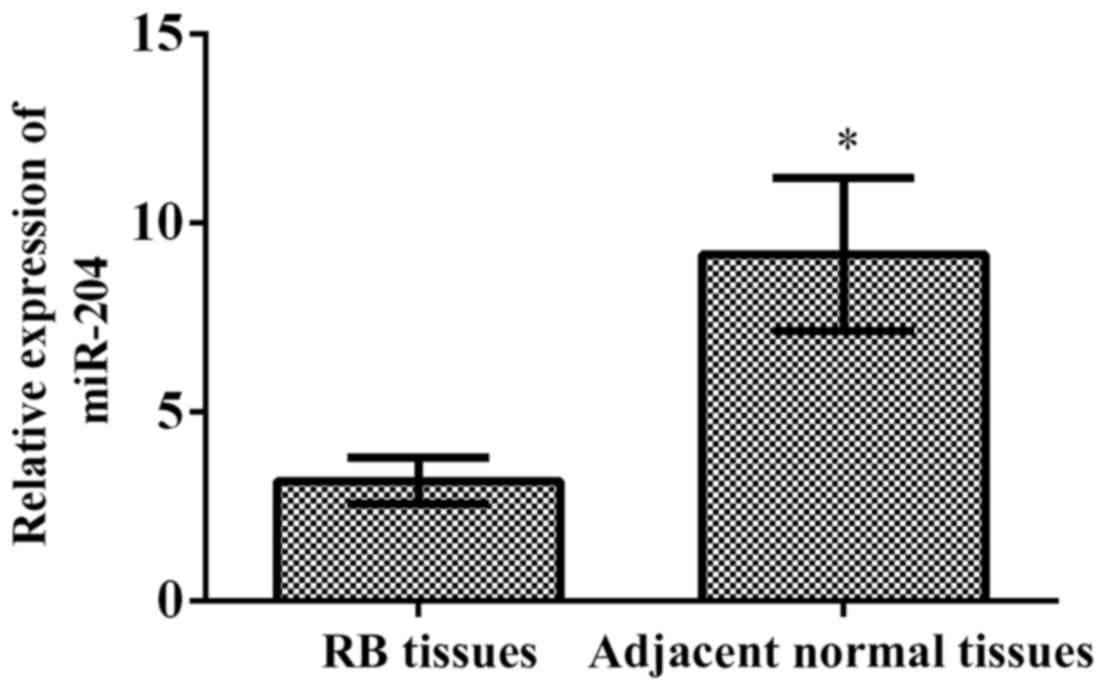
The results of RT-qPCR showed that the relative
expression of miR-204 in RB tissues was 3.164±0.611, and in
para-carcinoma normal tissues was 9.164±2.016. The relative
expression of miR-204 in RB tissues was significantly lower than
that in para-carcinoma normal tissues (p<0.001) (Fig. 1).
Association of the relative expression of miR-204 in
RB tissues with clinicopathological parameters of patients. The
relative expression of miR-204 in RB tissues had no significant
association with age, sex, affected eyes and clinical stage
(p>0.05), but had significant association with the degree of
tissue differentiation, neural infiltration and lymph node
metastasis (p<0.001) (Table
II).
 | Table II.Association of the relative expression
levels of miR-204 with clinicopathological parameters of RB
patients (x±s). |
Table II.
Association of the relative expression
levels of miR-204 with clinicopathological parameters of RB
patients (x±s).
| Item | n | miR-204 | t | P-value |
|---|
| Age (years) |
|
| 1.717 | 0.088 |
|
<4 | 67 | 3.211±0.542 |
|
|
| ≥4 | 43 | 3.016±0.638 |
|
|
| Sex |
|
| 0.338 | 0.735 |
| Male | 51 | 3.134±0.673 |
|
|
|
Female | 59 | 3.173±0.535 |
|
|
| Affected eye |
|
| 1.929 | 0.056 |
| Single
eye | 46 | 2.984±0.531 |
|
|
| Both
eyes | 64 | 3.204±0.629 |
|
|
| Degree of tissue
differentiation |
|
| 5.277 | <0.001 |
|
Undifferentiated type | 21 | 3.495±0.871 |
|
|
|
Differentiated type | 89 | 2.772±0.468 |
|
|
| Clinical stage |
|
| 1.358 | 0.261 |
|
Intraocular stage | 41 | 3.241±0.731 |
|
|
|
Glaucoma | 36 | 3.161±0.467 |
|
|
| Extended
stage | 33 | 3.011±0.547 |
|
|
| Neural
infiltration |
|
| 9.845 | <0.001 |
| Yes | 69 | 2.614±0.337 |
|
|
| No | 41 | 3.841±0.941 |
|
|
| Lymph node
metastasis |
|
| 9.462 | <0.001 |
|
Yes | 43 | 2.547±0.271 |
|
|
| No | 67 | 3.816±0.851 |
|
|
Expression of miR-204 in human RB
cells
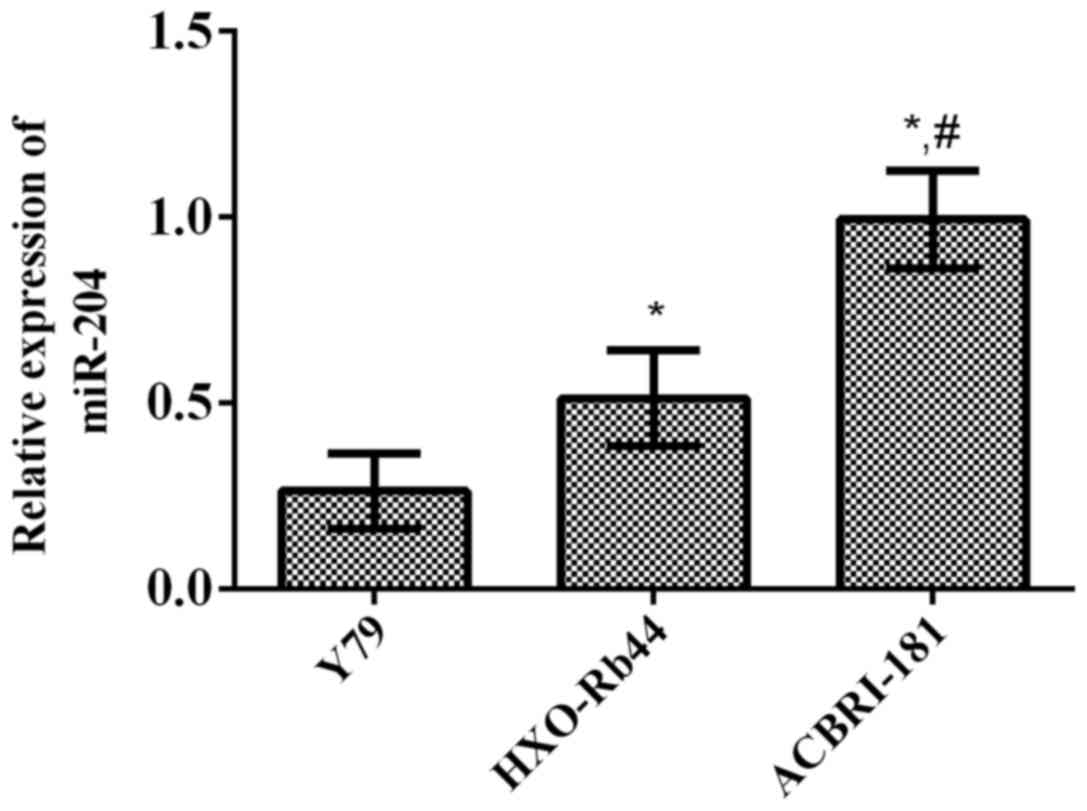
The results of RT-qPCR revealed that the relative
expression levels of miR-204 in human RB cell lines (Y79 and
HXO-Rb44) and human retinal microvascular endothelial cell line
(ACBRI-181) were 0.264±0.101, 0.513±0.129 and 0.994±0.131,
respectively. It can be seen that the relative expression levels of
miR-204 in human RB cell lines (Y79 and HXO-Rb44) were
significantly lower than that in human retinal microvascular
endothelial cell line (ACBRI-181) (p<0.01 and p<0.001,
respectively) (Fig. 2).
Transfection of human RB Y79 cells
with miR-204
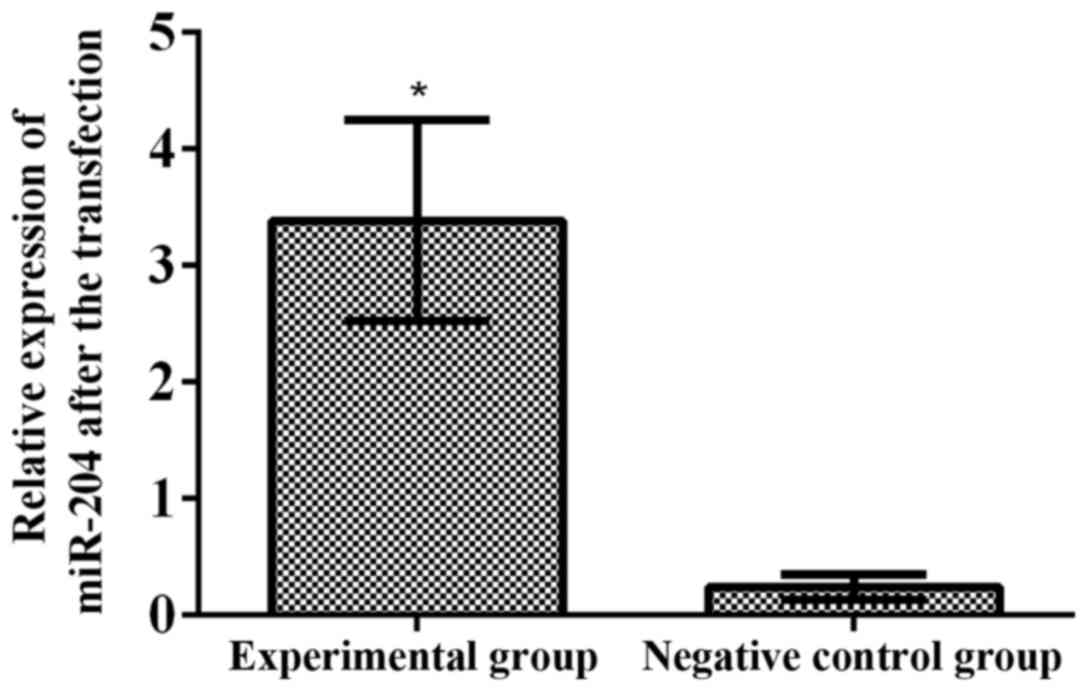
To further study the effect of miR-204 on the
biological behavior of RB, Y79 cells were transfected with miR-204.
The results of RT-qPCR showed that the relative expression level of
miR-204 in the experimental group (3.386±0.863) was significantly
higher than that in the negative control group (0.244±0.107) after
transfection (p<0.001) (Fig.
3).
Effect of miR-204 overexpression on
proliferation capacity of human RB Y79 cells
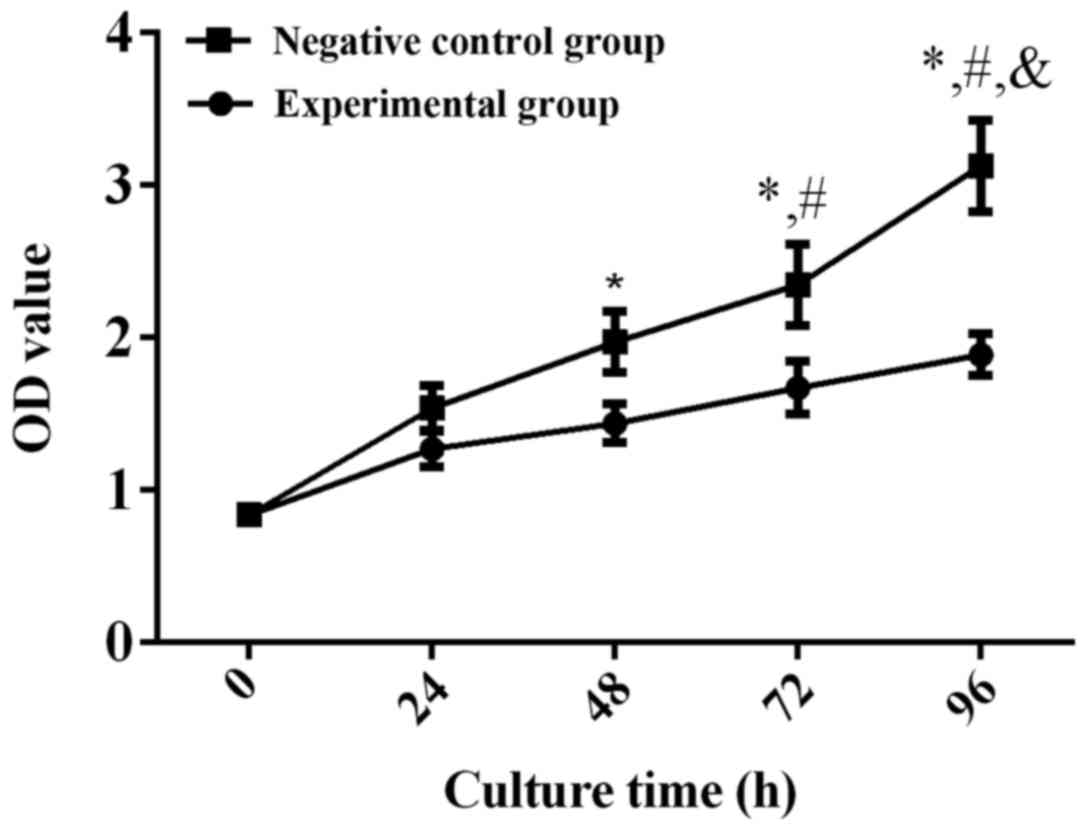
To further study the effect of miR-204 on the
proliferation capacity of human RB Y79 cells after transfection,
the proliferation of Y79 cells was detected via MTT assay at 24,
48, 72 and 96 h after transfection with miR-204. The results showed
that the measured value of OD in the experimental group was
significantly lower than that in the negative control group at 48 h
(p<0.001), indicating that miR-204 can significantly inhibit the
proliferation capacity of human RB Y79 cells (Fig. 4).
Effect of miR-204 overexpression on
apoptosis of human RB Y79 cells
To further study the effect of miR-204 on the
apoptosis of human RB Y79 cells after transfection, the proportion
of apoptotic human RB Y79 cells was detected via flow cyto- metry.
The results revealed that the proportion of apoptotic cells in the
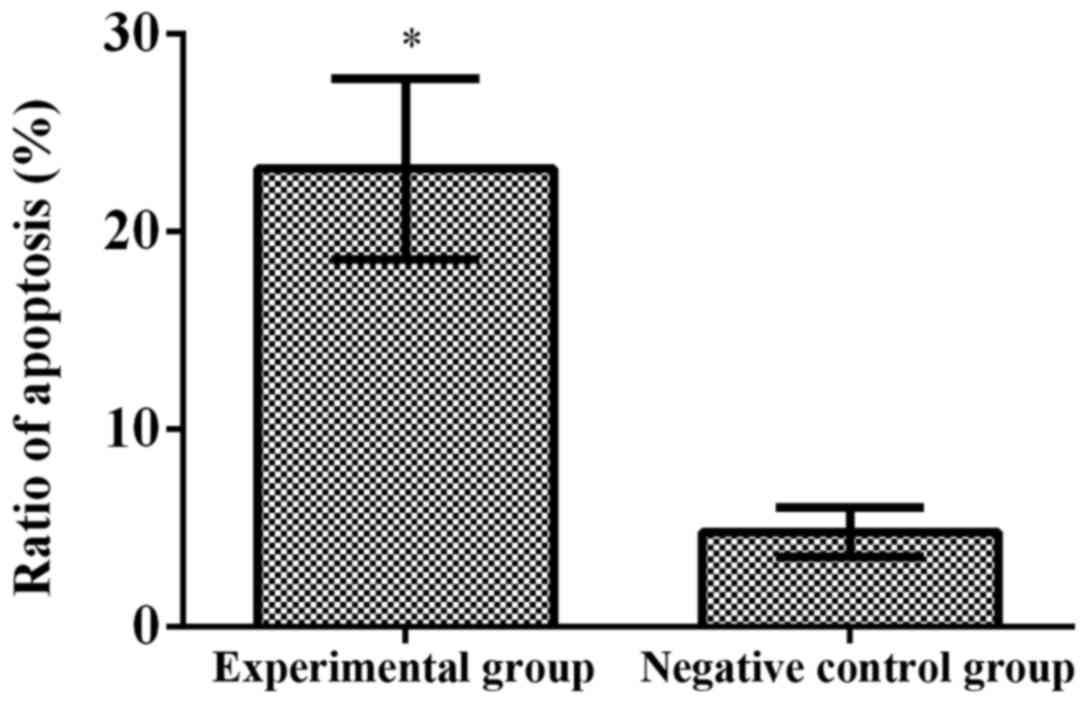
experimental group (23.16±4.58%) was obviously higher than that in
the negative control group (4.77±4.58%) after transfection with
miR-204 (p<0.001) (Fig. 5).
Effect of miR-204 overexpression on
Bcl-2 and Sirt1 expression levels in human RB Y79 cells
To explore the biological mechanism of miR-204 in
regulating the proliferation and apoptosis of RB cells, changes in
the expression of Bcl-2 mRNA and Sirt1 mRNA in human RB Y79 cells
after overexpression of miR-204 were detected via RT-qPCR. The
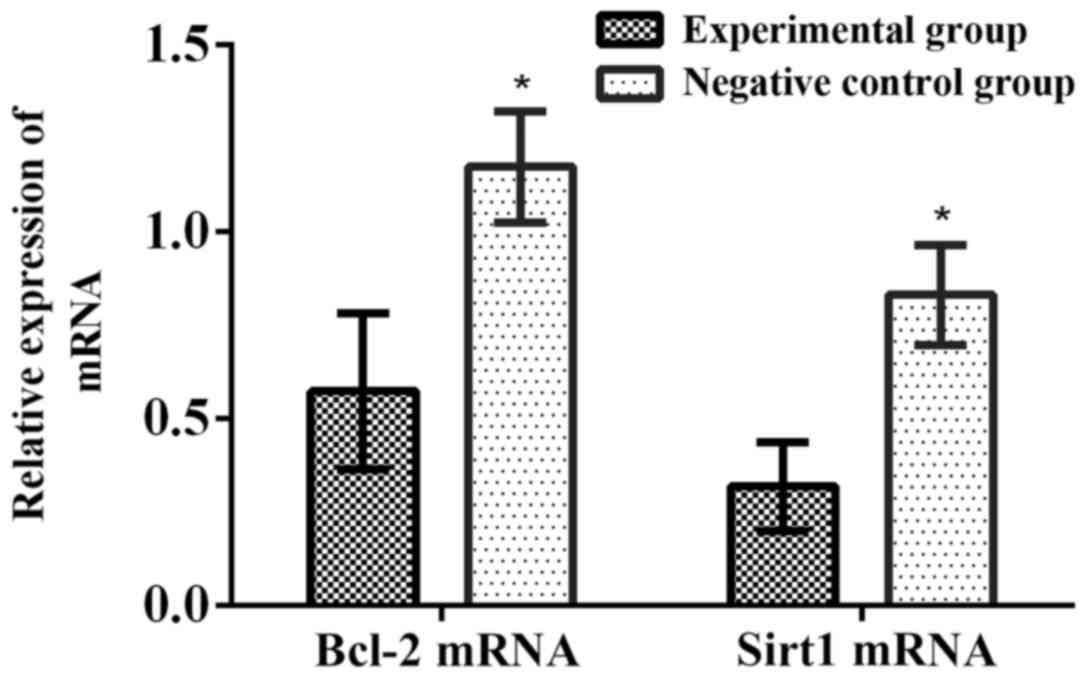
results demonstrated that compared with those in control group, the
relative expression levels of Bcl-2 mRNA and Sirt1 mRNA in the
experimental group obviously declined (p<0.001) (Fig. 6).
Discussion
RB is a kind of common primary malignant tumor
occurring frequently in the retina of children. The clinical
treatment methods of RB are mainly ophthalmectomy, chemotherapy,
radiotherapy and cryotherapy. Despite the continuous improvement of
the treatment methods, the therapeutic effect on most child
patients is limited, and both visual function and quality of life
of the child patients are often seriously affected after treatment
(17). The improvement of medical
level and the gradual development of molecular biology help provide
new thoughts for the gene-targeted therapy of RB. The specific
mechanism of the occurrence and development of RB has not been
clarified, but the proliferation and apoptosis of tumor cells is
considered as one of the mechanisms of RB occurrence (18). Therefore, studying the specific
pathogenic mechanism of RB and investigating the molecular
biological markers and the therapeutic targets closely related to
the occurrence and development of RB is important for the early
diagnosis and molecular therapy of RB.
miRNA is a non-coding RNA molecule, ~19–25
nucleotides in length, which can be involved in molecular
biological processes, such as cell proliferation, apoptosis,
differentiation, metabolism and death, through regulating gene
expression at the transcriptional or post-transcriptional level
(19). Studies have demonstrated that
miRNA plays a key role in the development process of a variety of
cells, which has close association with cell differentiation,
morphogenesis and tumorigenesis (20). miRNA plays a role as oncogene or
cancer suppressor gene in the occurrence of various human tumors,
and can serve as an effective molecular biological index for early
diagnosis, treatment and prognosis evaluation of tumors (21). The results in this study manifested
that the relative expression level of miR-204 in RB tissues was
significantly lower than that in para-carcinoma normal tissues, and
had significant associations with clinicopathological parameters
(degree of tissue differentiation, neural infiltration and lymph
node metastasis) of RB patients (p<0.001), indicating that
miR-204 may play an important role in the occurrence and
development of RB. Montagnana et al (22) have shown that miR-204 inhibits
proliferation and promotes apoptosis of tumor cells in endometrial
and pancreatic cancer. In this study, miR-204 was transiently
transfected in vitro to be overexpressed in human RB Y79
cell lines. The results of MTT assay revealed that the measured
value of OD in the experimental group at 48 h was obviously lower
than that in the negative control group, and the results of flow
cytometry showed that the proportion of apoptotic cells in the
experimental group was remarkably higher than that in the negative
control group after transfection, suggesting that miR-204 can
inhibit proliferation capacity and promote apoptosis of human RB
Y79 cells.
Bcl-2 protein is an important regulatory factor in
the process of apoptosis, which, like most oncogenes, can inhibit
apoptosis and be involved in the occurrence and development of
tumors (23). Canu et al
(24) have shown that in human
gastric cancer cells, miR-204 can reduce the Bcl-2 gene expression,
enhance the therapeutic effect of 5-fluorouracil and promote
apoptosis. Sirt1 is a highly-conserved deacetylase dependent on
NAD+, which can regulate cell proliferation, stress
responses and DNA damage (25).
According to the study of Yuan et al (26), Sirt1 is one of the downstream target
genes of miR-204 in human gastric cancer cells, and miR-204
inhibits the invasion and metastasis of gastric cancer cells
through downregulating the Sirt1 expression, thus exerting an
antitumor effect. It was found in this study that compared with
those in the negative control group, the relative expression levels
of Bcl-2 and Sirt1 mRNAs in the experimental group were
significantly decreased, indicating that miR-204 may inhibit
proliferation and promote apoptosis of RB cells through
downregulating the expression of Bcl-2 and Sirt1 in RB.
In this study, the effects of miR-204 on
proliferation and apoptosis of RB cells were observed, and the
roles of miR-204 during these processes were also analyzed, so as
to provide a theoretical basis for the gene-targeted therapy of RB.
In this research, the mechanism of miR-204 in RB cells was explored
preliminarily, but its pathway mechanism could not be verified in
more detail, so there were certain limitations. Cells in
vitro rather than in vivo were used in the present study
as research objects for ethical reasons. The environment in the
body is complex, so whether miR-204 has such effects on RB cells in
the human body and whether it is affected by the surrounding
relevant genes need further clinical research and animal
experiments.
In conclusion, miR-204 may be involved in the
occurrence and development of RB, which is significantly associated
with clinical tissue differentiation, neural infiltration and lymph
node metastasis in patients. miR-204 may inhibit proliferation and
promote apoptosis of RB cells through downregulating the Bcl-2 and
Sirt1 expression levels in RB. Therefore, miR-204 may become a new
biological index for early diagnosis, prognosis evaluation and
biotherapy of RB.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD drafted the manuscript. JD and XL were
responsible for the acquisition and interpretation of the data. JD
revised it critically for important intellectual content. JD and XL
were responsible for the conception and design of the study. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Hunan Normal University (People's
Hospital of Hunan Province) (Changsha, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenberg A and Mahalingam D:
Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to
response. J Gastrointest Oncol. 9:143–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eldehna WM, Al-Wabli RI, Almutairi MS,
Keeton AB, Piazza GA, Abdel-Aziz HA and Attia MI: Synthesis and
biological evaluation of certain hydrazonoindolin-2-one derivatives
as new potent anti-proliferative agents. J Enzyme Inhib Med Chem.
33:867–878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garsed DW, Alsop K, Fereday S, Emmanuel C,
Kennedy CJ, Etemadmoghadam D, Gao B, Gebski V, Garès V, Christie
EL, et al: Nadia Traficante, for the Australian Ovarian Cancer
Study Group: Homologous recombination DNA repair pathway disruption
and retinoblastoma protein loss are associated with exceptional
survival in high-grade serous ovarian cancer. Clin Cancer Res.
24:569–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi DL and Cobrinik D: MDM2 but not MDM4
promotes retinoblastoma cell proliferation through p53-independent
regulation of MYCN translation. Oncogene. 36:1760–1769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medina-Cleghorn D and Nomura DK: Chemical
approaches to study metabolic networks. Pflugers Arch. 465:427–440.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang WF, Xiong YW, Zhu TT, Xiong AZ, Bao
HH and Cheng XS: MicroRNA let-7g inhibited hypoxia-induced
proliferation of PASMCs via G0/G1 cell cycle arrest by targeting
c-myc. Life Sci. 170:9–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HF, Wang YC and Han YD: MicroRNA-34a
inhibits liver cancer cell growth by reprogramming glucose
metabolism. Mol Med Rep. 17:4483–4489. 2018.PubMed/NCBI
|
|
8
|
Suzuki HI, Young RA and Sharp PA:
Super-enhancer-mediated RNA processing revealed by integrative
microRNA network analysis. Cell. 168:1000–1014.e15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Fan F, Huo Y and Xu X:
Identifying the optimal blood pressure target for ideal health. J
Transl Int Med. 4:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castro-Magdonel BE, Orjuela M, Camacho J,
García-Chéquer AJ, Cabrera-Muñoz L, Sadowinski-Pine S,
Durán-Figueroa N, Orozco-Romero MJ, Velázquez-Wong AC,
Hernández-Ángeles A, et al: miRNome landscape analysis reveals a 30
miRNA core in retinoblastoma. BMC Cancer. 17:458–470. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Golabchi K, Soleimani-Jelodar R, Aghadoost
N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H and
Mirzaei H: MicroRNAs in retinoblastoma: Potential diagnostic and
therapeutic biomarkers. J Cell Physiol. 233:3016–3023. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao N, Wang FX, Wang G and Zhao QS:
Targeting the HMGA2 oncogene by miR-498 inhibits non-small cell
lung cancer biological behaviors. Eur Rev Med Pharmacol Sci.
22:1693–1699. 2018.PubMed/NCBI
|
|
13
|
Yang G, Fu Y, Zhang L, Lu X and Li Q:
miR106b regulates retinoblastoma Y79 cells through Runx3. Oncol
Rep. 38:3039–3043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo R, Shen W, Su C, Jiang S and Wang J:
Relationship between the pathogenesis of glaucoma and miRNA.
Ophthalmic Res. 57:194–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scelfo C, Francis JH, Khetan V, Jenkins T,
Marr B, Abramson DH, Shields CL, Pe'er J, Munier F, Berry J, et al:
An international survey of classification and treatment choices for
group D retinoblastoma. Int J Ophthalmol. 10:961–967.
2017.PubMed/NCBI
|
|
16
|
Cheng J, Chen Y, Zhao P, Li N, Lu J, Li J,
Liu Z, Lv Y and Huang C: Dysregulation of miR-638 in hepatocellular
carcinoma and its clinical significance. Oncol Lett. 13:3859–3865.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu ZP, Zhou KY, Chen LL, Xiao ZH and Chen
YZ: A preliminary study of retinoblastoma-related serum tumor
markers. Zhongguo Dang Dai Er Ke Za Zhi. 19:318–321. 2017.(In
Chinese). PubMed/NCBI
|
|
18
|
Guo L, Huang C and Ji QJ: Aberrant
promoter hypermethylation of p16, survivin, and retinoblastoma in
gastric cancer. Bratisl Lek Listy. 118:164–168. 2017.PubMed/NCBI
|
|
19
|
Colden M, Dar AA, Saini S, Dahiya PV,
Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S:
MicroRNA-466 inhibits tumor growth and bone metastasis in prostate
cancer by direct regulation of osteogenic transcription factor
RUNX2. Cell Death Dis. 8:e25722017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li JH, Sun SS, Fu CJ, Zhang AQ, Wang C, Xu
R, Xie SY and Wang PY: Diagnostic and prognostic value of
microRNA-628 for cancers. J Cancer. 9:1623–1634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams FF, Hoffmann T, Zuber J, Heckl D,
Schambach A and Schwarzer A: Pooled generation of lentiviral
tetracycline-regulated microRNA embedded short hairpin RNA
libraries. Hum Gene Ther Methods. 29:16–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montagnana M, Benati M, Danese E, Giudici
S, Perfranceschi M, Ruzzenenete O, Salvagno GL, Bassi A, Gelati M,
Paviati E, et al: Aberrant MicroRNA expression in patients with
endometrial cancer. Int J Gynecol Cancer. 27:459–466. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen C, Liu TS, Zhao SC, Yang WZ, Chen ZP
and Yan Y: XIAP impairs mitochondrial function during apoptosis by
regulating the Bcl-2 family in renal cell carcinoma. Exp Ther Med.
15:4587–4593. 2018.PubMed/NCBI
|
|
24
|
Canu V, Sacconi A, Lorenzon L, Biagioni F,
Lo Sardo F, Diodoro MG, Muti P, Garofalo A, Strano S, D'Errico A,
et al: MiR-204 down-regulation elicited perturbation of a gene
target signature common to human cholangiocarcinoma and gastric
cancer. Oncotarget. 8:29540–29557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Wang H, Hao Y, Yang S, Tian H, Sun
B and Liu Y: A novel reaction-based fluorescent probe for the
detection of cysteine in milk and water samples. Food Chem.
262:67–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan X, Wang S, Liu M, Lu Z, Zhan Y, Wang
W and Xu AM: Histological and pathological assessment of miR-204
and SOX4 levels in gastric cancer patients. Biomed Res Int.
2017:68946752017. View Article : Google Scholar : PubMed/NCBI
|