Introduction
Colon cancer is the most common malignant tumor of
the digestive tract. It often occurs among adults aged above 40
years with a male-to-female ratio of 2–3:1. Colon cancer is a
leading cause of cancer deaths worldwide (1,2). With the
improvement of the living standards and diet level, the incidence
of colon cancer has been increasing year by year (3,4). Like
other cancers, the exact cause and pathogenesis of colon cancer
remain unclear (5). The biological
behavior of colon cancer is complex. Early screening, timely
detection and treatment can effectively improve the survival rate
of patients (6). Therefore, it is of
great significance to explore the pathogene-sis of colon cancer and
look for new biological markers and therapeutic targets.
MicroRNAs (miRNAs) are widely expressed in
eukaryotic organisms, regulating cell proliferation,
differentiation and apoptosis. Aberrations in the process of miRNA
biosynthesis were associated with a variety of pathophysiological
processes (7,8). In recent years, many studies have
suggested that miR-214 was abnormally expressed in many malignant
tumors such as ovarian cancer (9) and
prostate cancer (10). However, the
association of miR-214 with colon cancer has rarely been
reported.
In this study, the expression levels of miR-214 in
cancer and paracancerous tissues collected from patients with colon
cancer were analyzed. The role of miR-214 in modulating
proliferation and invasion of human colon cancer SW620 cells was
explored as well. The goal of this study was to provide a new
theoretical basis for the screening, diagnosis and treatment of
colon cancer.
Materials and methods
General data
A total of 55 patients with colon cancer were
enrolled in this study and they were treated in China-Japan Union
Hospital of Jilin University (Changchun, China) from March 2014 to
March 2015. Their cancer and corresponding paracancerous tissues
were collected. The patients, including 31 males and 24 females,
were aged 35–70 years with an average age of 55.43±12.75 years.
Patients who were pathologically diagnosed with colon cancer and
met the following criteria were included in this study: i) without
liver, kidney and other organ dysfunction before surgery; and ii)
without bleeding and clotting disorders before surgery. Patients
who met the following criteria were excluded from this study: i)
being treated prior to this study; ii) with too large tumor; and
iii) with other diseases such as lung or chest wall diseases.
This study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University. The patients or
their families signed an informed consent.
Methods
Human colon cancer SW620 cells were purchased from
Shanghai Beinuo Biotechnology Co., Ltd. (Shanghai, China) and
cultured in Leibovitz's L-15 medium purchased from Changzhou
Beiyuanxin Biotechnology Co., Ltd. (Jiangsu, China). The cell
culture was maintained at constant 37°C and pH 6.8–7.4 in an
incubator supplied with 5% CO2. A miR-214 expression
vector was constructed by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The constructed miR-214 expression vector (miR-214 positive
group) and blank vector (miR-214 negative group) were cultured,
respectively, with trypsin-digested human colon cancer cells SW620
in Leibovitz's L-15 medium at 37°C in an incubator supplied with 5%
CO2 for 24 h. After transfection, the cells in the two
groups were subjected to analytical experiments.
Total RNA extraction from tissues
Total RNA in cancer and paracancerous tissues was
extracted by using TRIzol reagent purchased from Shanghai Mingjing
Biotech Co., Ltd. (Shanghai, China), following the instructions
contained in the TRIzol kit. The concentration and purity of the
extracted RNA were measured with an MD1000 Microscale UV
Spectrophotometer manufactured by Thmorgan Biotechnology Co., Ltd.
(Beijing, China). Integrity of the RNA was assessed by 3% agarose
gel electrophoresis. The gel electrophoresis set was purchased from
Shanghai Jingke Science and Technology Co., Ltd. (Shanghai,
China).
miR-214 RT-qPCR assay
Synthesis of cDNA from the extracted total miRNA was
performed, via reverse transcription, at 37°C for 45 min and 95°C
for 5 min by using the fluorescence quantitative PCR kit purchased
from Thermo Fisher Scientific Co., Ltd. (Shanghai, China) following
the instructions contained in the kit. Total volume of the PCR
amplification reaction system was 20 µl. The PCR reaction was
performed as follows: pre-denaturation at 95°C for 10 min, 40
cycles of 95°C for 10 sec (denaturation), 60°C for 20 sec
(annealing), and 72°C for 10 sec (extension), followed by extension
at 72°C for 5 min at the end of the cycles. U6 was used as the
reference gene. All samples were run in triplicate. The results
were analyzed by using the 2−ΔΔCq method (11). The RT-qPCR primers were synthesized by
Suzhou Synbio Technologies Co., Ltd. (Suzhou, China). The primer
sequences were as follows: for miR-214 the forward sequence was
5′-AGCCACATCGCTCAGACA-3′, and the reverse sequence was
5′-CAGACGAGGCTCCGTGGT-3′; for U6 the forward sequence was
5′-CGCTTCGGCAGCACATATAC-3′, and the reverse sequence was
5′-TTCACGAATTTGCGTGTCAT-3′.
In vitro proliferation of SW620 cells
assessed by MTT assay
The transfected human colon cancer SW620 cells in
the miR-214 positive and negative groups were prepared into a
single cell suspension, followed by routine seeding in a 96-well
cell culture plate. After culturing for 6 h, 20 µl of MTT solution
(5 mg/ml) was added to a portion of the cultured cells, and
incubation was continued at 37°C for 4 h. After sucking away the
supernatant containing the medium and reagents, DMSO was added,
followed by shaking on a rocking platform shaker for 15 min. The
absorbance at a wavelength of 570 nm was measured by using an ELISA
reader. After culturing for 12, 24, 48 and 72 h, the above MTT
assay was repeated to obtain data at different time-points. The MTT
assay kits were purchased from Shanghai LMAI Bio Co., Ltd
(Shanghai, China).
Transwell in vitro migration
assay
Single cell suspensions of transfected SW620 cells
in the miR-214 positive and negative groups were prepared and
seeded in triplicate (~1×105−1×106 cells per
well) into Transwell upper chambers, respectively. The number of
cells passing through the membrane in each well was counted two
weeks later. The Transwell plates were purchased from Shanghai
Yuanzi Biotechnology Co., Ltd. (Shanghai, China).
TUNEL apoptosis assay
After culturing for 48 h, the cells (approximately
5×107 cells/ml) were fixed in 4% neutral formalin for 10
min at room temperature. Following removal of the supernatant, the
fixed cells were washed twice with PBS for 5 min each. The cells
were then incubated in PBS containing 2% hydrogen peroxide for 5
min at room temperature. Following removal of the supernatant, the
fixed cells were washed with PBS twice for 5 min each, and were
then stained by using TUNEL kits (Shanghai Runwell Technology Co.,
Ltd., Shanghai, China) according to the protocol contained in the
kit. The number of TUNEL-positive cells in five 400-fold fields of
view was counted by using the image analysis software Image-pro
Plus 5.0. The cumulative optical density value represented the
total number of TUNEL positive cells. The measurement was repeated
three times.
Statistical analysis
The statistics software SPSS 19.0 (AsiaAnalytics
Formerly SPSS China, Shanghai, China) was used for statistical
analysis. The χ2 test was applied to rate comparison.
Measurement data were expressed as mean ± standard deviation. The
t-test was used for comparison of data that were normally
distributed. P<0.05 was considered to indicate a statistically
significant difference.
Results
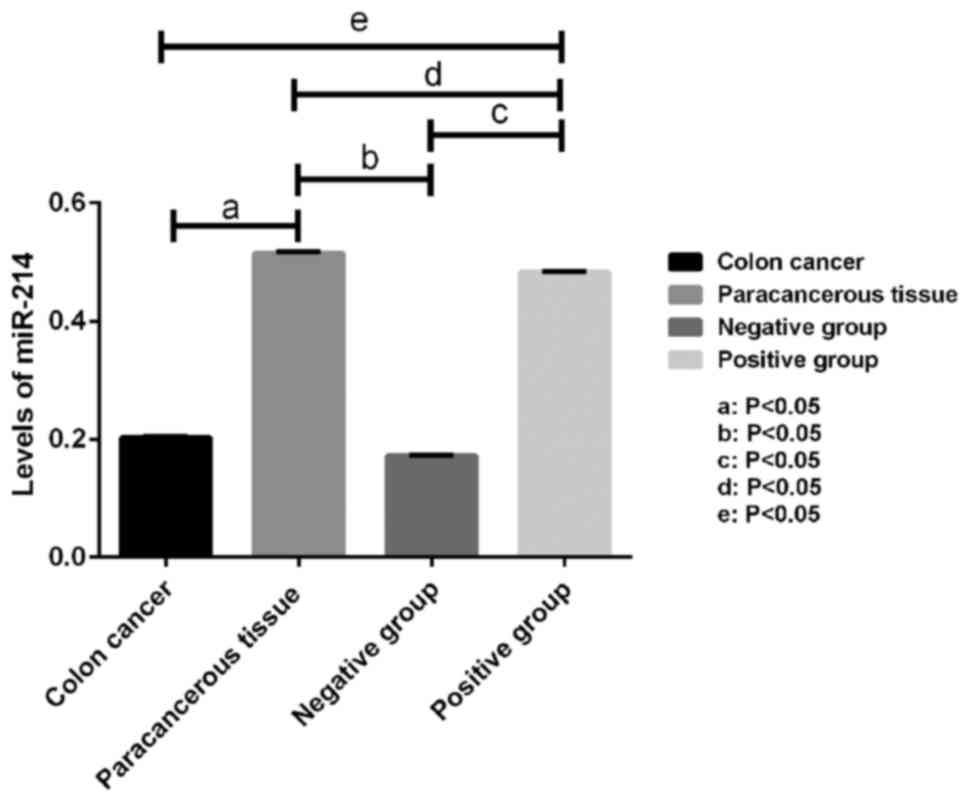
Levels of miR-214 in colon cancer and
paracancerous tissues as well as in SW620 cells
The levels of miR-214 in colon cancer and
paracancerous tissues, as well as in SW620 cells, were measured by
RT-qPCR. As shown in Fig. 1, the
expression levels of miR-214 in the colon cancer tissue
(0.203±0.001) and in SW620 cells in the miR-214 negative group
(0.172±0.001) were comparable (P>0.05), and both were
significantly lower than that in the paracancerous tissue
(0.515±0.002). The differences were statistically significant
(P<0.05). The miR-214 expression level in SW620 cells in the
miR-214 positive group (0.483±0.001) was significantly higher than
those in SW620 cells in the miR-214 negative group and in cancer
tissue, but was lower than that in paracancerous tissue
(P<0.05).
Association of miR-214 expression
level with clinical pathological characteristics
The expression level of miR-214 was not associated
with the patient's sex, age, clinical stage, depth of tumor
invasion, degree of differentiation, or lymph node metastasis
(P>0.05). However, the miR-214 level was associated with
histological classification of colon cancer. As shown in Table I, the expression level of miR-214 in
mucinous carcinoma was significantly lower than that in the
non-mucinous carcinoma (P<0.05).
 | Table I.Association of miR-214 expression
level with clinicopathological characteristics by χ2
test. |
Table I.
Association of miR-214 expression
level with clinicopathological characteristics by χ2
test.
| Clinicopathological
characteristics | n (%) | miR-214 level | χ2 | P-value |
|---|
| Patient number | 55 | 0.203±0.001 |
|
|
| Sex |
|
| 1.087 | 0.282 |
| Male | 31 (56.36) | 0.212±0.003 |
|
|
|
Female | 24 (43.74) | 0.206±0.007 |
|
|
| Age (years) |
|
| 1.174 | 0.246 |
|
<45 | 19 (34.55) | 0.203±0.002 |
|
|
| ≥45 | 36 (65.45) | 0.211±0.005 |
|
|
| Clinical stage |
|
| 0.924 | 0.359 |
|
T1/T2 | 20 (33.36) | 0.203±0.009 |
|
|
|
T3/T4 | 35 (62.64) | 0.207±0.001 |
|
|
| Invasion depth |
|
| 1.962 | 0.055 |
|
Muscularis | 14 (25.45) | 0.214±0.005 |
|
|
|
Serosa | 41 (74.55) | 0.232±0.001 |
|
|
| Differentiation |
|
| 1.394 | 0.169 |
| Well | 43 (78.18) | 0.229±0.006 |
|
|
|
Moderately and poorly | 12 (21.82) | 0.214±0.007 |
|
|
| Lymph node
status |
|
| 1.998 | 0.051 |
|
Metastasis | 32 (58.18) | 0.221±0.006 |
|
|
| No
metastasis | 23 (41.82) | 0.202±0.003 |
|
|
| Histological
classification |
|
| 34.42 | <0.001 |
| Mucinous
carcinoma | 15 (27.27) | 0.116±0.007 |
|
|
|
Non-mucinous carcinoma | 40 (72.73) | 0.303±0.004 |
|
|
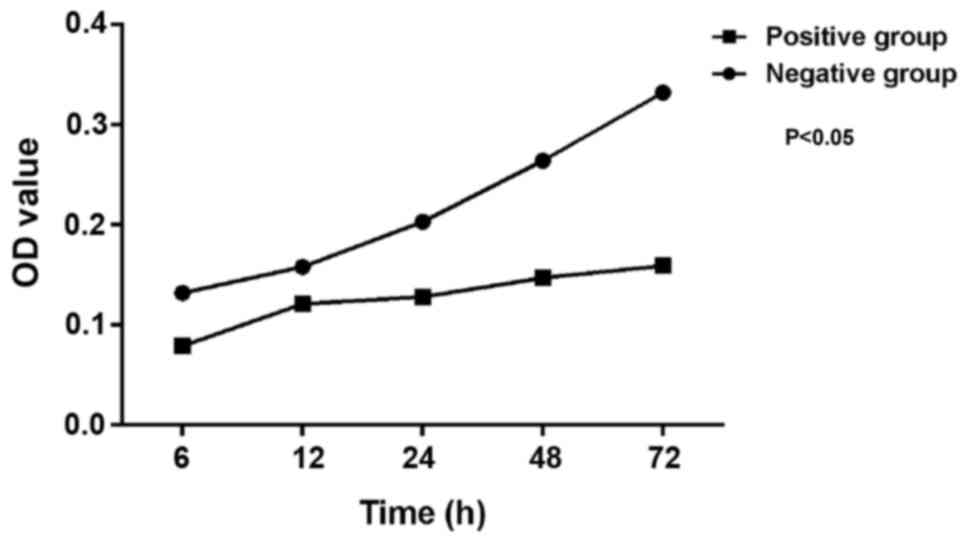
Proliferation of SW620 cells assessed
by MTT assay
The proliferation of SW620 cells in the miR-214
positive and negative groups was determined by MTT assay. As shown
in Fig. 2, the optical density (OD)
values of cells in the miR-214 positive group were lower than those
in the miR-214 negative group at five time-points of 6, 12, 24, 48
and 72 h, indicating that the proliferation rate of cells in the
miR-214 positive group was lower due to the higher miR-214 level.
The difference was statistically significant (P<0.05).
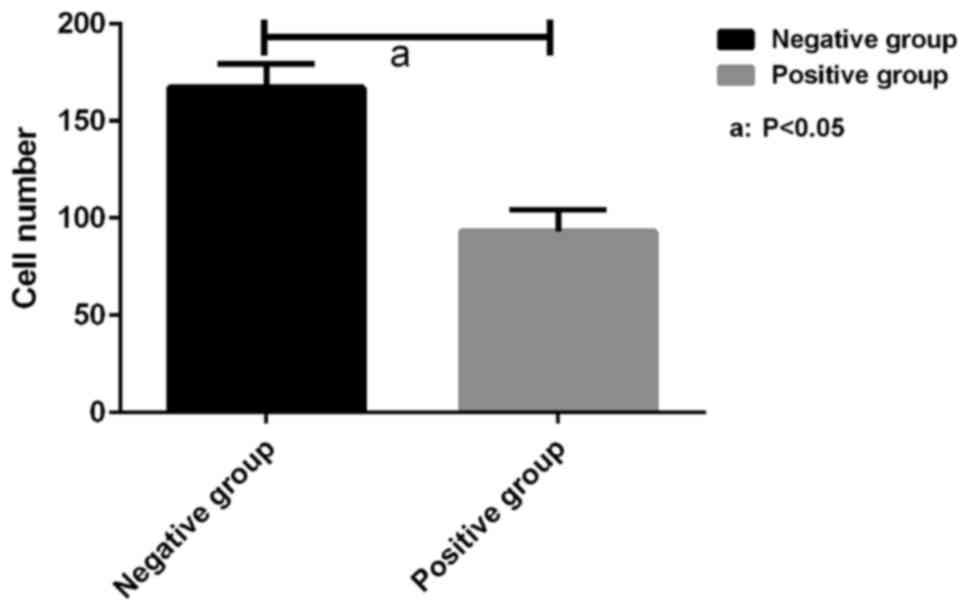
Invasion of SW620 cells measured by
Transwell in vitro migration assay
The invasiveness of SW620 cells in the miR-214
positive and negative groups was measured by Transwell in
vitro migration assay. As shown in Fig. 3, the number of SW620 cells passing
through the membrane in the miR-214 positive group (93.17±11.02)
was substantially less than that (167.32±12.15) in the miR-214
negative group (P<0.05).
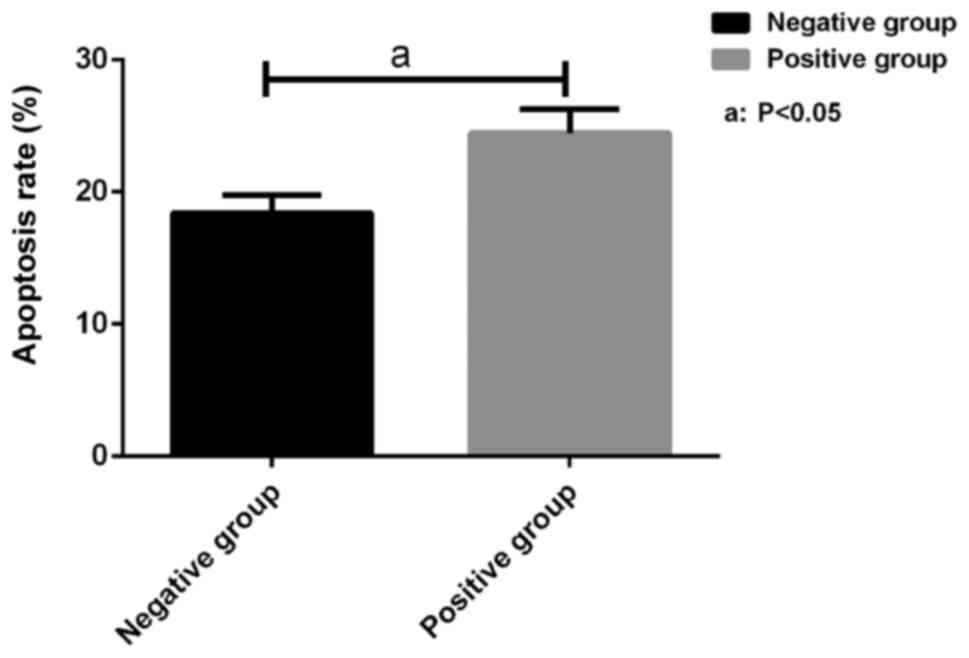
Apoptosis of SW620 cells measured by
TUNEL apoptosis assay
Apoptosis of SW620 cells in the miR-214 positive and
negative groups was measured by TUNEL apoptosis assay. As shown in
Fig. 4, the apoptosis rate of cells
in the miR-214 negative group (18.36±1.35%), was significantly
lower than (24.41±1.81%) in the miR-214 positive group. The
difference was statistically significant (P<0.05).
Discussion
Research focusing on miRNAs represents one of the
most popular topics in cancer research. The aberrant expression of
miRNAs can modify the expression of tumor-related genes, impacting
tumor onset and progression (12,13). Qiang
et al reported that plexin B1 can promote proliferation and
invasion of human cervical cancer HeLa cells, and the plexin B1
level was found to be negatively correlated with the miR-214 level
in both cervical cancer tissue and HeLa cells (14). Therefore, it was postulated that
upregulation of the miR-214 expression can inhibit HeLa cell
proliferation, migration and invasion. In this study, the
expression levels of miR-214 in colon cancer and paracancerous
tissue were evaluated aiming at exploring the relationship between
miR-214 and colon cancer. In addition, the biological function of
miR-214 on human colon cancer cells was investigated by using SW620
cells. It was found in this study that the expression level of
miR-214 in colon cancer tissue was significantly lower than that in
paracancerous tissue, suggesting that miR-214 may play a
tumor-suppressing role in colon cancer as a tumor suppressor
candidate gene. By analyzing any possible correlation between the
miR-214 expression level and the patient's clinical
characteristics, it was found that the difference in the miR-214
expression level was statistically significant only between the two
histological types. Specifically, the miR-214 level in non-mucinous
colon cancer was significantly higher than that in mucinous colon
cancer. There were no statistically significant differences in sex,
age and colon cancer clinical stage among the subjects. The
association of miR-214 with colon cancer has rarely been reported.
However, miR-214 was reported to be either upregulated or
downregulated in other types of cancer, playing a tumor-suppressing
or tumor-promoting role. For example, miR-214 was downregulated in
cutaneous squamous cell carcinoma (15), whereas it was upregulated in
nasopharyngeal carcinoma (16).
Therefore, it is possible that miR-214 may be involved in the onset
of colon cancer.
In order to further study the expression of miR-214
in colon cancer and its biological effect on colon cancer, human
colon cancer SW620 cells were transfected with a miR-214 expression
vector. The transfected cells were cultured and used in the study
of miR-214 biological effects by MTT proliferation, Transwell in
vitro migration and TUNEL apoptosis assays. It was found in
this study that the expression level of miR-214 was also lower in
SW620 cells transfected with a blank vector than that in the
paracancerous tissue. After upregulating miR-214 expression in
SW620 cells by transfection with a miR-214 expression vector, the
proliferation and invasiveness of SW620 cells were inhibited while
the apoptosis rate was increased compared with SW620 cells
transfected with a blank vector. In literature, Huang et al
reported that miR-214 was downregulated in esophageal squamous cell
carcinoma and patients with lymph node metastases experienced even
lower miR-214 level (17). Yang et
al reported that miR-214 can modulate proliferation and
invasion of gastric cancer cells (18). Li et al reported that
downregulation of the miR-214 expression can promote metastases of
intrahepatic cholangiocarcinoma (19). Wang et al reported that
inhibition of miR-214 can attenuate proliferation of hepatocellular
carcinoma (20). The findings in the
above literature were similar to our results using SW620 cells.
Therefore, it was postulated that miR-214 can inhibit proliferation
and invasion of colon cancer cells. Huang et al also
reported that miR-214 was associated with the pathological type and
clinical stage of esophageal squamous cell carcinoma, as well as
the patient's lymph node status (17). This was inconsistent with our findings
in correlation analysis of the miR-214 expression level and
clinical characteristics of patients. The reason may be related to
different types of tumors being studied or limited number of
subjects being enrolled in this study. The results in this study
demonstrated that miR-214 was downregulated in colon cancer and was
involved in modulation of proliferation and invasion of colon
cancer cells.
Wang et al reported that miR-214 can enhance
patients' sensitivity to the chemotherapeutic drug cisplatin by
inhibiting the expression of Bcl2l2 in cervical cancer HeLa and
C-33A cells (21). Xia et al
reported that downregulation of miR-214 was associated with
recurrence of hepatocellular carcinoma (22). The β-catenin signaling pathway in
hepatocellular carcinoma was modulated by miR-214 directly or
indirectly through targeting CTNNB1. Schwarzenbach et al
reported that miR-214 can be used as a marker in diagnosis of
breast cancer (23). In this study,
the role of miR-214 in diagnosis, treatment and prognosis of colon
cancer was not explored due to certain limitations. In our future
study, these questions will be addressed.
In conclusion, miR-214 was downregulated in colon
cancer. It exhibited inhibitory effect in the onset of colon cancer
as a tumor suppressor gene. The proliferation and invasion of colon
cancer SW620 cells were attenuated by miR-214, while the apoptosis
rate was increased. It is possible that miR-214 can be a new target
in the treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN wrote the manuscript and extracted total RNA from
tissues. DN performed the RT-qPCR. LM contributed to performing the
MTT assay. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University (Changchun, China).
Patients who participated in this research, signed an informed
consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kyu HH, Bachman VF, Alexander LT, Mumford
JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer
ML, et al: Physical activity and risk of breast cancer, colon
cancer, diabetes, ischemic heart disease, and ischemic stroke
events: Systematic review and dose-response meta-analysis for the
Global Burden of Disease Study 2013. BMJ. 354:i38572016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Sousa e Melo F, Kurtova AV, Harnoss JM,
Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z,
Koeppen H, et al: A distinct role for Lgr5+ stem cells
in primary and metastatic colon cancer. Nature. 543:676–680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdel-Rahman WM, Faris ME and Peltomaki P:
Molecular determinants of colon cancer susceptibility in the East
and West. Curr Mol Med. 17:34–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cayrefourcq L, Mazard T, Joosse S,
Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde
T, Pantel K, et al: Establishment and characterization of a cell
line from human circulating colon cancer cells. Cancer Res.
75:892–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tie J, Wang Y, Tomasetti C, Li L, Springer
S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al:
Circulating tumor DNA analysis detects minimal residual disease and
predicts recurrence in patients with stage II colon cancer. Sci
Transl Med. 8:346ra922016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang S, Gao L, Kathleen K, Fink A, Kalvala
A, Aguila B, Otterson G, Villalonacalero M and Duan W: micoRNA in
FA defective tumor. Cancer Res. 77 Suppl 13:Abst 44422017.
View Article : Google Scholar
|
|
8
|
Nieuwenhuizen L, de Groot PG, Grutters JC
and Biesma DH: A review of pulmonary coagulopathy in acute lung
injury, acute respiratory distress syndrome and pneumonia. Eur J
Haematol. 82:413–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Yin H, Zhang Y, Feng Y, Yan Z,
Jiang X, Bukhari I, Iqbal F, Cooke HJ and Shi Q: miR-214-mediated
downregulation of RNF8 induces chromosomal instability in ovarian
cancer cells. Cell Cycle. 13:3519–3528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer - the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2 (Delta Delta C(T)) Method. Methods. 25:402 4082001.
View Article : Google Scholar
|
|
12
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 12:e050052015. View Article : Google Scholar
|
|
14
|
Qiang R, Wang F, Shi LY, Liu M, Chen S,
Wan HY, Li YX, Li X, Gao SY and Sun BC: Plexin-B1 is a target of
miR-214 in cervical cancer and promotes the growth and invasion of
HeLa cells. Int J Biochem Cell Biol. 43:632–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamane K, Jinnin M, Etoh T, Kobayashi Y,
Shimozono N, Fukushima S, Masuguchi S, Maruo K, Inoue Y, Ishihara
T, et al: Down-regulation of miR-124/-214 in cutaneous squamous
cell carcinoma mediates abnormal cell proliferation via the
induction of ERK. J Mol Med (Berl). 91:69–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng M, Ye Q, Qin Z, Zheng Y, He W, Tang
H, Zhou Y, Xiong W, Zhou M, Li X, et al: miR-214 promotes
tumorigenesis by targeting lactotransferrin in nasopharyngeal
carcinoma. Tumour Biol. 34:1793–1800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang SD, Yuan Y, Zhuang CW, Li BL, Gong
DJ, Wang SG, Zeng ZY and Cheng HZ: MicroRNA-98 and microRNA-214
post-transcriptionally regulate enhancer of zeste homolog 2 and
inhibit migration and invasion in human esophageal squamous cell
carcinoma. Mol Cancer. 11:512012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: miR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Han Q, Zhu Y, Yu Y, Wang J and Jiang
X: Down-regulation of miR-214 contributes to intrahepatic
cholangiocarcinoma metastasis by targeting Twist. FEBS J.
279:2393–2398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z
and Jiang J: miR-214 inhibits cell growth in hepatocellular
carcinoma through suppression of β-catenin. Biochem Biophys Res
Commun. 428:525–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Liu M, Li X and Tang H: miR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia H, Ooi LL and Hui KM: miR-214 targets
β-catenin pathway to suppress invasion, stem-like traits and
recurrence of human hepatocellular carcinoma. PLoS One.
7:e442062012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwarzenbach H, Milde-Langosch K,
Steinbach B, Müller V and Pantel K: Diagnostic potential of
PTEN-targeting miR-214 in the blood of breast cancer patients.
Breast Cancer Res Treat. 134:933–941. 2012. View Article : Google Scholar : PubMed/NCBI
|