Introduction
In the last few years, prostate cancer (PCa) has
become a common malignant tumor among men worldwide (1). It has been demonstrated that PCa is the
second leading cause of cancer-associated mortality in western
countries and it continues to increase (2). Bone metastasis in PCa may result in
pathological fracture and bone pain, which poses a severe threat
and may have an impact on quality of life and prognosis. Currently,
radionuclide bone imaging is widely applied for the diagnosis and
monitoring of PCa (3,4). However, radionuclide bone imaging
exhibits low specificity and high cost, and there may be
radioactive effects. Thus, it is imperative to explore alternative
options for early diagnosis and treatment of bone metastasis in
PCa.
Serum markers demonstrated advantages in terms of
reproducibility, non-invasiveness and relatively low cost. Previous
studies have indicated that serum expression level of
prostate-specific antigen (PSA) is an ideal marker for the
prediction of the lesion range among patients with PCa (5). However, the level of PSA may be
disturbed in other pathological conditions, including prostatitis
and benign prostatic hyperplasia (BPH) (6), thus leading to over-diagnosis and
overtreatment (7). Additionally,
alkaline phosphatase (ALP) is also suggested to be an important
predictor for bone metastasis (8).
The expression level of serum bone sialoprotein (BSP) is an
indicator of the bone resorption process and bone cell activity.
Bone resorption marker collagen type I pyridine crosslinking
peptide (ICTP) is considered to predict bone cell function and the
bone absorption rate, which is also an important diagnostic marker
in bone metastasis (3). However,
these markers are increased in all patients with PCa, indicating
the necessity to identify specific markers for bone metastasis in
patients with PCa.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that are widely involved in post-transcriptional gene regulation
through an incomplete base pairing mechanism (9,10).
Abnormal expression of miRNAs has been identified in various
cellular processes, including cell differentiation, proliferation
and apoptosis (11,12). In the progression of bone metastasis
in patients with PCa, abnormal expression of miRNAs is common
(13,14). For instance, epidermal growth factor
receptor maintains the activation of oncogenic twist-related
protein 1 mainly by suppressing miR-1, thereby accelerating bone
metastasis (13). Furthermore,
miR-34a may regulate Wnt/transcription factor 7 signaling and
suppress bone metastasis in Ras-activated PCa cells (14).
The aim of the present study was to explore the
function of miR-21. For instance, miR-214 was identified to be
upregulated in gastric and pancreatic cancer (15,16).
Expression of miR-214 was decreased in renal carcinoma and glioma
cells (17,18). However, the expression of miR-214 has
never been explored in patients with PCa that exhibit bone
metastasis. To the best of our knowledge, the present study is the
first to compare the expression levels of serum miR-214 in patients
with PCa with bone metastasis, patients with PCa without bone
metastasis and healthy controls, thereby assessing the potential of
serum miR-214 expression as a diagnostic biomarker in patients with
PCa with bone metastasis.
Materials and methods
Patients and blood samples
Serum samples of male patients with PCa with bone
metastasis, non-bone metastasis, BPH group and healthy individuals
were obtained from the Department of Urology, Changsha Central
Hospital (Changsha, China) between June 2014 and January 2016. The
application of patient-derived materials was approved by the
Research Ethics Committee of Changsha Central Hospital (Changsha,
China) and written informed consent was obtained from all patients.
No patients received androgen-deprivation therapy or radiotherapy.
Clinical characteristics of patients are listed in Table I. The differentiation of tumor cells
was evaluated according to the Gleason score (GS) (19). Considering histological
differentiation, the patients with PCa were then divided into 3
subgroups according to the GS: Well differentiated (GS≤6),
moderately differentiated subgroup (GS=7), and poorly
differentiated (GS≥8).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Bone metastasis
group | Non-bone group | Benign prostatic
hyperplasia group | Healthy controls |
|---|
| Total subjects,
n | 75 | 65 | 45 | 56 |
| Age, years
(range) | 72 (55–86) | 70 (56–84) | 69 (55–81) | 51 (39–85) |
| BMI,
kg/m2 | 24.5±2.71 | 24.3±3.04 | 24.1±3.34 | 24.5±2.18 |
| PSA, ng/ml |
|
|
|
|
| <10,
n (%) | 23 (30.7) | 35 (53.8) | 45 (100.0) | 70 (100.0) |
| 10-20,
n (%) | 25 (33.3) | 10 (15.4) | 0 (0) | 0 (0) |
| >20,
n (%) | 27 (36.0) | 12 (18.5) | 0 (0) | 0 (0) |
| Gleason score |
|
|
|
|
| <7,
n (%) | 21 (28.0) | 17 (26.2) | – | – |
| 7, n
(%) | 26 (34.7) | 18 (27.7) | – | – |
| >7,
n (%) | 28 (41.3) | 18 (27.7) | – | – |
| Tumor stage |
|
|
|
|
| pT1/2,
n (%) | 44 (58.7) | 21 (32.3) | – | – |
| pT3/4,
n (%) | 31 (41.3) | 22 (33.8) | – | – |
| Node stage |
|
|
|
|
| N0, n
(%) | 41 (54.7) | 25 (38.5) | – | – |
| N1, n
(%) | 34 (45.3) | 22 (33.8) | – | – |
| Metastasis
stage |
|
|
|
|
| M0, n
(%) | 40 (53.3) | 46 (70.8) | – | – |
| M1, n
(%) | 35 (46.7) | 29 (44.6) | – | – |
Cell lines and samples
The human PCa cell line PC3 was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA), streptomycin (100 µg/ml) and penicillin (100 U/ml; Thermo
Fisher Scientific, Inc.) in 25-cm2 culture flasks at
37°C in a humidified atmosphere containing 5% CO2.
Detection method
Serum BSP (cat. no. LP-001185, Shanghai Lanpai Bio.
Tech. Co., Shanghai, China) and ICTP (cat. no. LP-001623, Shanghai
Lanpai Bio. Tech. Co.,) levels were detected using ELISA. ALP
activity was tested using the velocity method (20) (Nanjing Jiancheng Biological Technology
Co., Ltd., Nanjing China) and the PSA level was determined using
electrochemical immunoluminescence with a plate reader (Nanjing
Jiancheng Biological Technology Co., Ltd.). Samples were read at a
450 nm wavelength using a microplate reader (Model 3550; Thermo
Fisher Scientific, Inc.).
Cell transfection
PC3 cells were seeded in a 12- or 24-well plate for
48 h. The following day, PC3 cells were transfected with the mature
human miR-214 (5′-UGCCUGUCUACACUUGCUGUGC-3′), anti-miR-214
(antisense inhibitor, 5′-CGACAGCAAGUGUAGACAGGCA-3′), small
interfering RNA (siRNA) targeting phosphatase and tensin homolog
(PTEN) (si-PTEN-1, 5′-AAGCTGGAAAGGGACGAACT-3′; si-PTEN-2:
5′-GCTCAAGAGCAGCTACTACAT-3′; si-PTEN-3: 5′-UGCTCCGAACGTGTCACGT-3′)
or with a specific negative control siRNA (NC,
5′-CAGUACUUUGUGUAGUACAA-3′) (all purchased from GeneChem, Inc.,
Daejeon, Korea) at a concentration of 50 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. For
subsequent experiments, cells were used 48 h after
transfection.
Western blot analysis
Total proteins were isolated from cells using a
total protein extraction kit (cat no. KPG2100, Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). A BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to determine the
protein concentration. Subsequently, supernatants were extracted
from the lysates following centrifugation at 11,000 × g at 4°C for
15 min. Equal amounts of protein (30 µg/lane) were separated using
10% SDS-PAGE at 300 mA for 2 h and transferred onto a
polyvinylidene fluoride membrane. Membranes were then blocked with
5% fat-free milk in Tris-buffered saline with 0.1% Tween-20 (TBST)
buffer at room temperature for 2 h. The membranes were then
incubated with the following primary antibodies: Anti-PTEN (cat no.
9188; 1:1,000 dilution; Cell Signaling Technology, Inc., Danvers,
MA, USA) and anti-GAPDH (cat no. 5174; 1:1,000 dilution; Cell
Signaling Technology, Inc.) at 4°C overnight. Following three
washes with TBST (5 min) at room temperature, the membranes were
incubated with horseradish-peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (1:5,000; cat. no. ZB-2306,
Zhongshan Gold Bridge Biological Technology Co., Beijing, China)
for 2 h at room temperature and then washed with TBST three times
for 5 min at room temperature followed by detection with enhanced
chemiluminescent substrate (EMD Millipore, Billerica, MA, USA).
GAPDH was used as a control. The proteins were detected using
enhanced chemiluminescence (Super ECL Plus; Nanjing KeyGen Biotech
Co., Ltd.) and densitometric analysis of the bands was performed
using UVP automatic dyed gel free gel imaging system (GelDocIT TS2;
UVP, LLC, Phoenix, AZ, USA). ImageJ 1.8.0 (National Institutes of
Health, Bethesda, MD, USA) was applied to quantify the relative
protein levels. The integral optical density ratio of PTEN/GAPDH
indicated the relative expression of PTEN protein.
RNA extraction
Total RNA or miRNA from the total volume of serum
samples (5 ml) from patients with PCa with bone metastasis,
non-bone metastasis, BPH group and healthy individuals was
extracted using RNAzol® reagent (Vigorous Biotechnology
Co., Ltd., Beijing, China), according to the manufacturer's
protocol. The concentration and the purity of the RNA samples were
assayed by determining the optical density ratio at 260/280 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (10 µg) from the serum samples (5 ml) was
isolated using RNAVzol LS (Vigorous Biotechnology Co., Ltd.,
Beijing, China) according to the manufacturer's protocol. The
concentration and purity of the RNA samples were determined by the
OD260/OD280 ratio using a microplate reader
(Model 3550; Thermo Fisher Scientific, Inc.). A total of 1 µg RNA
was reverse-transcribed using a TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using specific primers for miR-214 and U6 (Sangon BioTech
Co., Ltd., Shanghai, China). U6 is a small nuclear RNA employed as
the endogenous control (21). The
sequences of the nucleotide primers used for reverse transcription
were as follows: miR-214,
5′-CGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCACAG-3′; U6,
5′-TCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAATATG-3′. The
sequences for the primers used for qPCR were as follows: miR-214,
forward, 5′-GCUGCCTGTCTACACTTG-3′; T6, forward,
5′-GCGTCGTGAAGCGTTC-3′; universal reverse primer,
5′-GTGCAGGGTCCGAGGT-3′.
The PCR amplifications were performed in a 10 µl
reaction system containing 5 µl SYBR Green Supermix, 0.4 µl forward
primer, 0.4 µl reverse primer, 2.2 µl double-distilled water and 2
µl template cDNA using SYBR Green Supermix (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) in a Bio-Rad iCycler iQ Real-Time PCR
Detection System. The thermal cycling conditions included an
initial denaturation step at 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and final extension at 60°C for 1 min. Relative
levels of miR-214 were determined using the 2−ΔΔCq
method (22).
Invasion assays
Cell invasion assays were performed using Boyden
chambers (8-µm pore filter; Corning Incorporated, Corning, NY,
USA). For the cell invasion assay, 2.0×105 cells were
cultured in a chamber (BD Biosciences, Franklin Lakes, NJ, USA)
pre-coated with 0.2% Matrigel (BD Biosciences) at 37°C. In the
lower chamber, 10% fetal bovine serum was added to the culture
medium as a chemoattractant. At 24 h, the cells in the upper
compartment were removed using cotton swabs and cells that had
invaded through the membrane were stained with a dye solution
containing 20% methanol for 30 min at 37°C and 0.1% crystal violet
for 1 h at 37°C. Images were captured under a light microscope
(Olympus Corporation, Tokyo, Japan) and 10 individual fields were
counted per insert. Three independent experiments were
performed.
Statistical analysis
Data were analyzed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). The relevant data are
expressed as the mean ± standard deviation. A two-tailed unpaired
Student's t-test was used to examine differences between two
groups. One-way analysis of variance followed by Tukey's post hoc
test was used to examine differences among multiple groups.
Receiver operating characteristic curve (ROC) curves were created
and the areas under the curve (AUCs) were determined to assess the
specificity and sensitivity of circulating miR-214 as a diagnostic
biomarker. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
As presented in Table
I, the age distribution was similar among the healthy controls,
patients with BPH and patients with PCa (P>0.05). The expression
levels of serum PSA were significantly upregulated in patients with
PCa compared with in healthy controls (P<0.001).
Expression levels of miR-214, PSA,
ALP, BSP and ICTP are significantly upregulated in the serum of
patients with PCa with bone metastasis
First, the expression levels of PSA, ALP, BSP and
ICTP were evaluated in the serum of patients with PCa with or
without bone metastasis, BPH group and healthy control group using
ELISA. The results suggested that the serum levels of PSA, BSP, ALP
and ICTP were increased in the bone metastasis group compared with
in the non-bone metastasis, BPH and control groups (P<0.05;
Fig. 1A-D). Expression levels of
miR-214 among the various groups were also evaluated using RT-qPCR.
The results indicated that the expression level of miR-214 was
significantly increased in the serum of patients with PCa with bone
metastasis compared with that of non-bone metastasis, BPH and
healthy control groups (P<0.001; Fig.
1E). The results indicated that the relative expression of
miR-214 among the various groups was as follows: 7.8±2.8 for the
bone metastasis group; 1.5±0.7 for the non-bone metastasis group;
1.2±0.4 for the BPH group and 1±0.6 for the healthy control group.
These data suggested that increased expression levels of miR-214
associated with significant bone metastasis in patients with
PCa.
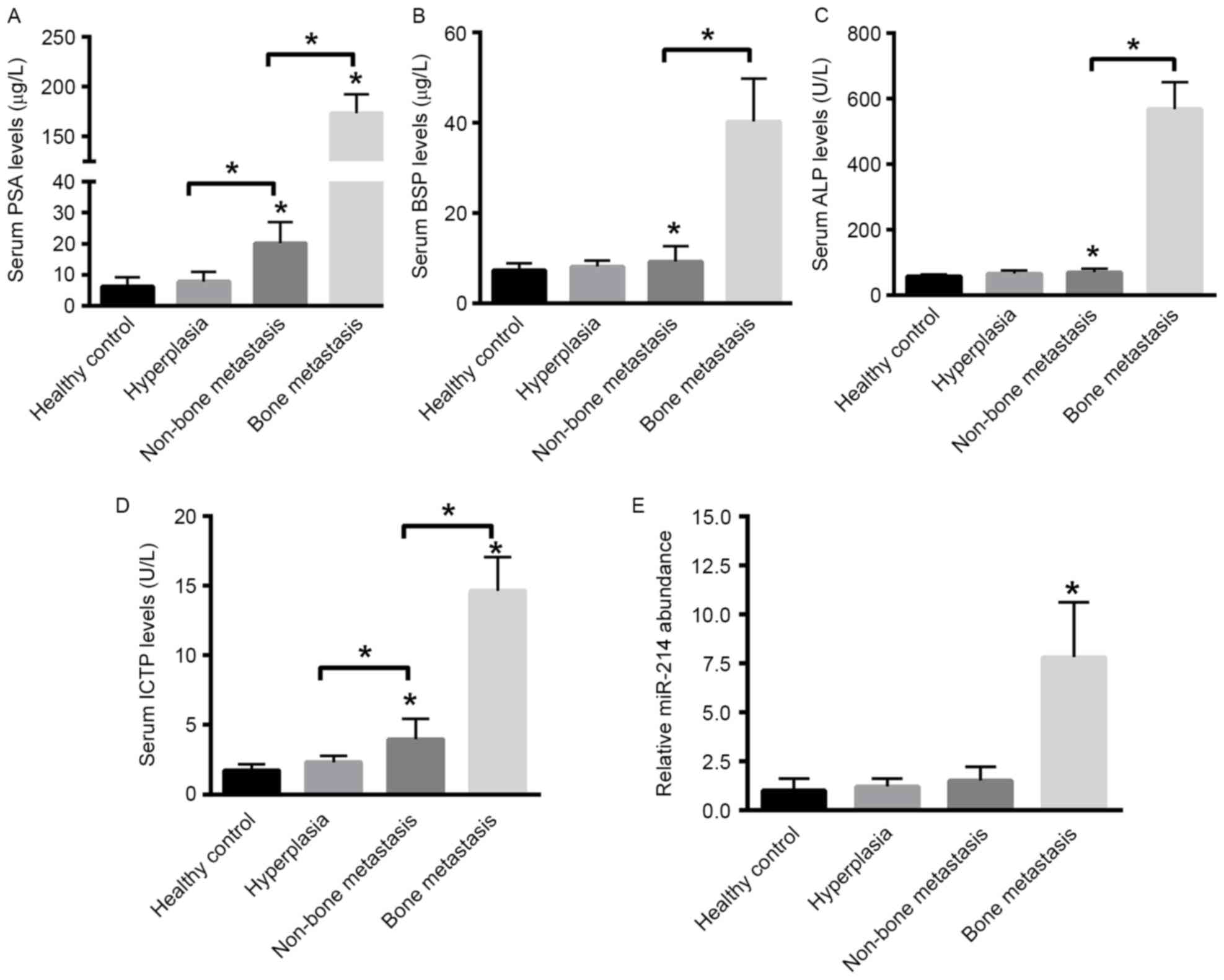 | Figure 1.Enzyme-linked immunosorbent assay of
PSA, BSP, ALP and ICTP levels and PCR analysis of serum miR-241
levels in patients with PCa with bone metastasis compared with the
patients with PCa without bone metastasis, hyperplasia and healthy
control groups. Expression levels of serum (A) PSA, (B) BSP, (C)
ALP and (D) ICTP in the bone metastasis, non-bone metastasis,
hyperplasia and healthy control groups. *P<0.05. (E) PCR
analysis of relative expression levels of serum miR-214 in the bone
metastasis, non-bone metastasis, hyperplasia and healthy control
groups. *P<0.05 vs. remaining groups. PSA, prostate-specific
antigen; BSP, bone sialoprotein; ALP, alkaline phosphatase; ICTP,
collagen type I pyridine crosslinking peptide; miR, microRNA; PCa,
prostate cancer; PCR, polymerase chain reaction. |
Increased expression levels of miR-214
are associated with tumor malignancy in patients with PCa
The expression levels of PSA, BSP, ALP and ICTP were
determined in well-differentiated (GS <7), moderately
differentiated (GS=7) and poorly differentiated tumors (GS >7)
in patients with PCa. The results suggested that the expression of
PSA, BSP, ALP and ICTP was significantly increased in the
moderately differentiated and poorly differentiated tumors compared
with well-differentiated tumors (Fig.
2A-D). As presented in Fig. 2E,
increased expression levels of miR-214 were identified in the bone
metastasis group exhibiting increased aggressive tumors in
comparison with patients with less aggressive tumors. For patients
with a GS <7, the relative expression level of miR-214 was
1.0±0.6. For patients with a GS of 7, the relative expression level
of miR-214 was increased to 1.5±0.8, whereas the relative
expression of miR-214 was increased to 2.5±1.1 in patients with a
GS >7 (Fig. 2E). ROC analysis
indicated that serum levels of miR-214 were able to distinguish the
bone metastasis group [AUC, 0.955; 95% confidence interval (CI),
0.872–1.000; P<0.001] from the non-bone metastasis group (AUC,
0.479; 95% CI, 0.291–0.667), BPH (AUC, 0.611; 95% CI, 0.415–0.807)
and healthy control group (AUC, 0.526; 95% CI, 0.332–0.719).
Additionally, miR-214 was able to distinguish patients with PCa
from healthy individuals (AUC, 0.915; 95% CI, 0.846–0.984; Fig. 2F).
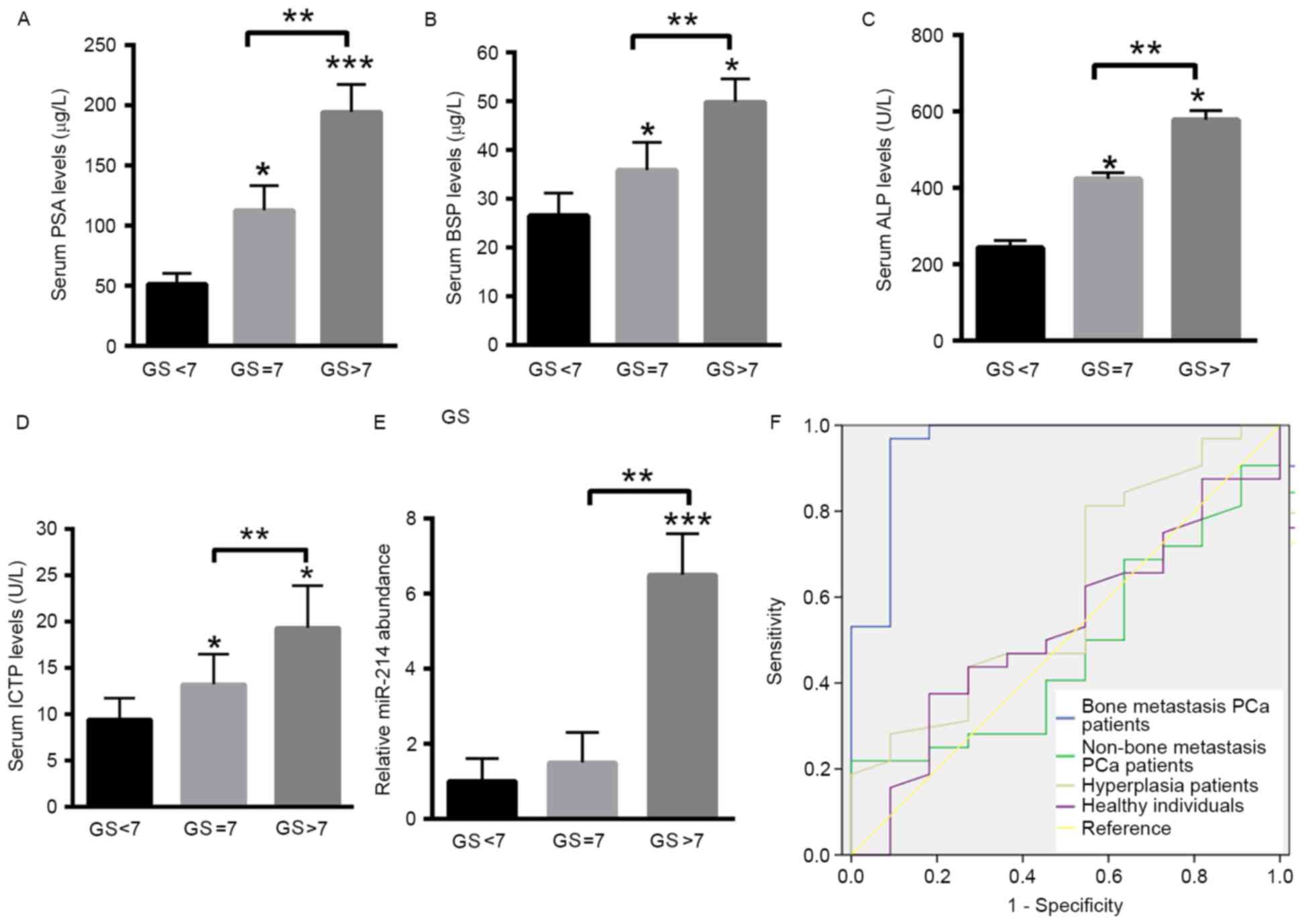 | Figure 2.Increased expression levels of miR-214
are associated with clinicopathological parameters of patients with
PCa. The expression levels of (A) PSA, (B) BSP, (C) ALP and (D)
ICTP were analyzed in well-differentiated tumors (GS <7),
moderately differentiated (GS=7) and poorly differentiated tumors
(GS >7) of patients with PCa. (E) Relative expression levels of
miR-214 in the serum of PCa patients with GS <7, GS=7 and GS
>7 of patients with PCa. ***P<0.001 vs. GS<7; **P<0.01
vs. GS=7; *P<0.05 vs. GS<7. (F) ROC analysis of miR-214 in
patients with PCa with bone metastasis compared with the patients
with PCa without bone metastasis, in hyperplasia and healthy
control groups. Data are presented as the mean ± standard
deviation. PSA, prostate-specific antigen; BSP, bone sialoprotein;
ALP, alkaline phosphatase; ICTP, collagen type I pyridine
crosslinking peptide; miR, microRNA; PCa, prostate cancer; GS,
Gleason score. |
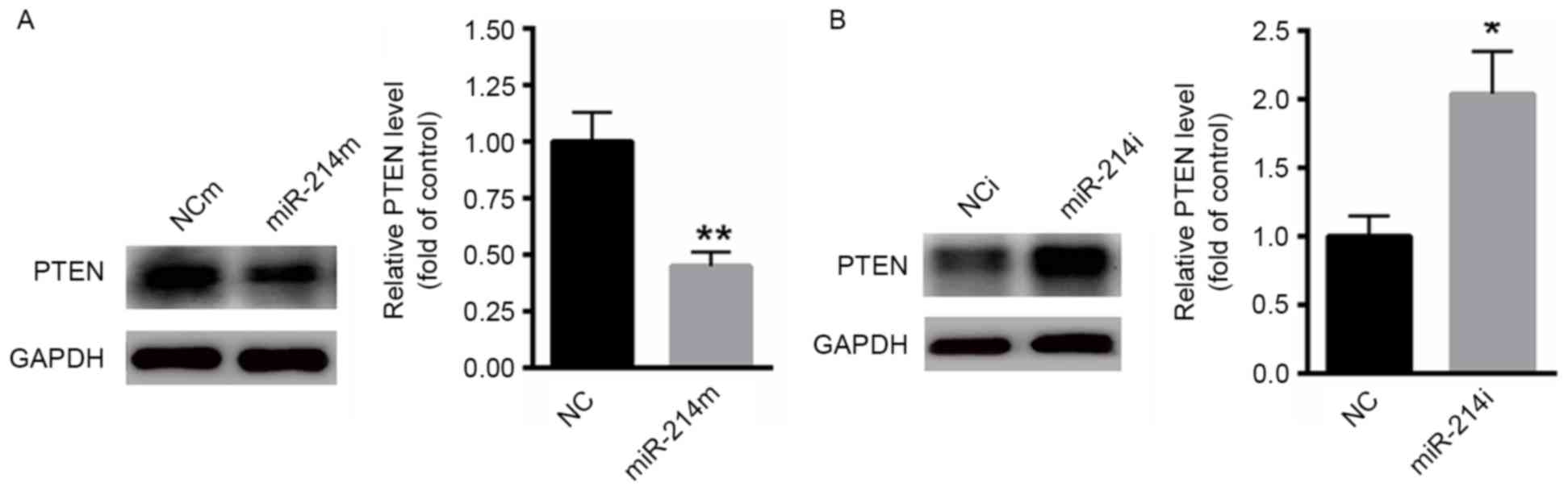
PTEN is a target gene of miR-214 in
PC3 cells
Next, the potential molecular mechanism by which
miR-214 modulates bone metastasis in PCa was investigated. It has
been demonstrated that PTEN is a target gene in various tumors,
including gastric cancer and lymphocytic leukemia (23,24). Then,
whether miR-214 was able to target PTEN in PCa cells was
investigated. As presented in Fig.
3A, overexpression of miR-214 significantly suppressed the
protein level of PTEN in PC3 cells. In contrast, inhibition of
miR-214 markedly increased the expression of PTEN in PC3 cells
(Fig. 3B).
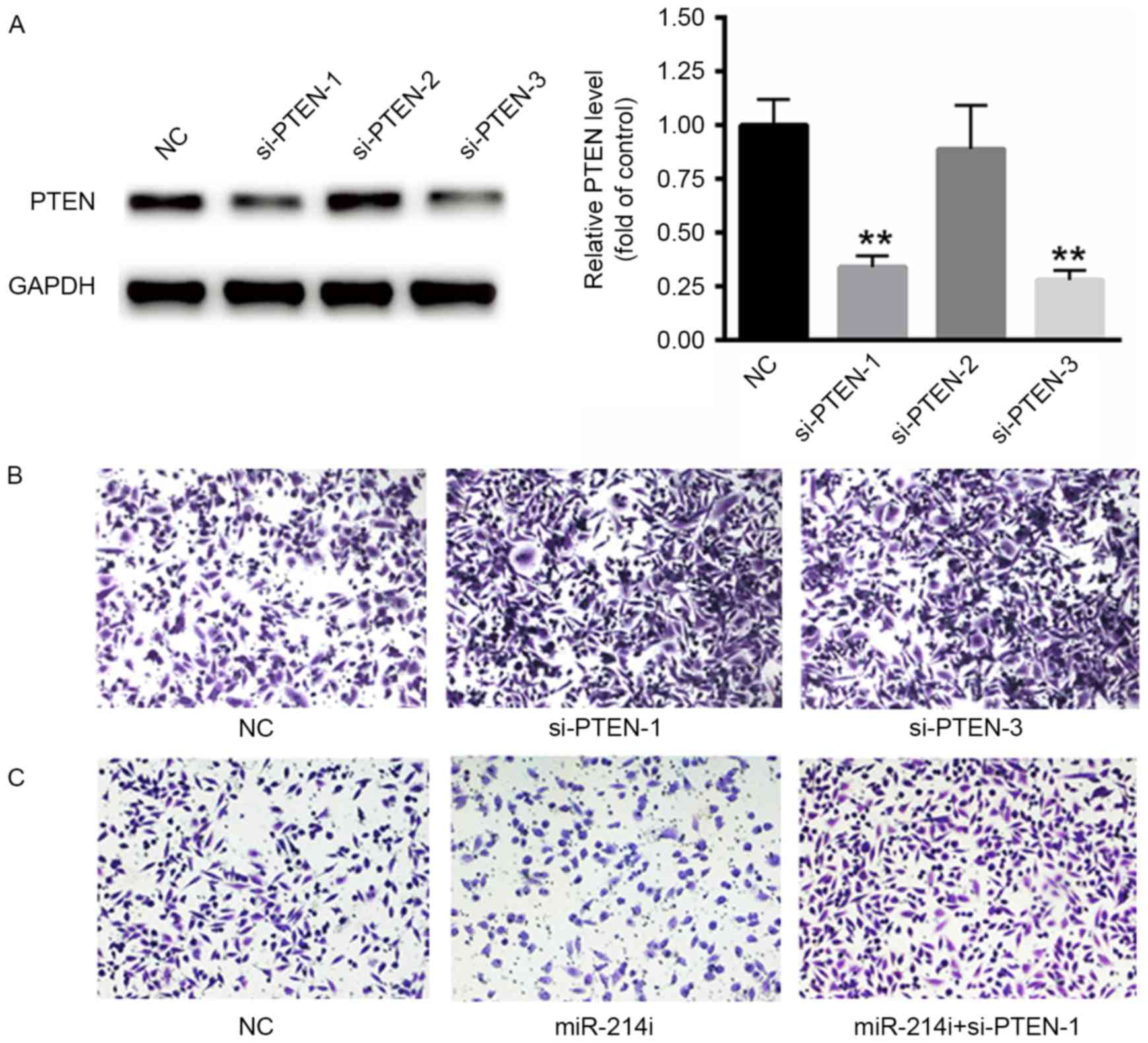
Suppression of PTEN increases the
invasive ability of PCa cells
The function of PTEN on the invasive ability of PCa
cells was evaluated using PC3 cells. First, three specific siRNAs
targeting PTEN were selected, but only two of them significantly
suppressed the mRNA levels of PTEN (Fig.
4A). Thus, si-PTEN-1 and si-PTEN-2 were selected for subsequent
experiments. Furthermore, the silencing of PTEN significantly
increased the invasive ability of PC3 cells (Fig. 4B). Downregulation of miR-214 using
miR-214i significantly suppressed the invasive ability of PCa
cells. However, such effects were eliminated in response to si-PTEN
transfection, thus suggesting that miR-214 functions as an
oncogenic miRNA partially by targeting PTEN (Fig. 4C).
Discussion
Bone metastasis is a common complication in patients
with PCa which markedly induces bone pain and pathological fracture
(25,26). However, the diagnostic tools for PCa
are relatively limited. Previous studies have suggested that miRNAs
may be potential diagnostic markers considering their key function
in different conditions, including in cardiovascular disease,
hepatocarcinoma and breast cancer (27–29).
miRNAs are identified to be dysregulated through their oncogenic
and tumor suppressive functions in the tumor (27,29). Thus,
it is necessary to investigate the function of miRNAs in the
development of bone metastasis in patients with PCa, which may lead
to novel diagnostic tools for screening bone metastasis in patients
with PCa.
Previous studies have demonstrated that abnormal
expression of miR-214 may enhance or suppress tumor progression in
various types of tumors, including breast cancer, testicular germ
cell tumor, hepatocellular carcinoma and gastric cancer (24,30–32).
Furthermore, miR-214 increased the proliferative capacity of T
cells by targeting PTEN (33). In the
present study, the expression of miR-214 in the serum of patients
with PCa with bone metastasis was investigated. The results
indicated that miR-214 was significantly upregulated in the serum
of patients with PCa with bone metastasis compared with the
non-bone metastasis, BPH and healthy control groups. However, a
previous study has indicated that the expression level of miR-214
was decreased in the urine samples of patients with PCa (34). This inconsistency may be because
miR-214 is specifically upregulated in the serum of patients with
bone metastasis.
Expression levels of additional serum bone markers
were determined. For instance, GS ≥8, PSA ≥20 mg/l and an increased
ALP level are important indicators for bone metastases in patients
with PCa (35,36). In the present study, it was
demonstrated that the serum levels of PSA, BSP, ALP and ICTP were
increased in the bone metastasis group compared with the non-bone
metastasis group, BPH and the control group. However, these serum
bone markers were increased in all patients with PCa. Additionally,
it was demonstrated that miR-214 may be increased only in patients
with PCa with bone metastasis. However, increased expression of
miR-214 was also associated with the malignant phenotype of PCa,
suggesting that its increased specificity and sensitivity may hold
promise for its potential as a biomarker in patients with PCa with
bone metastasis. Additionally, ROC analysis demonstrated that
miR-214 may significantly distinguish the bone metastasis group
from the other groups, indicating the potential for miR-214 as a
biomarker in such patients.
miRNAs exert their function mainly through binding
the 3′ untranslated region of target genes. Therefore, the
potential target genes of miR-214 were investigated. Previous
studies have demonstrated that PTEN is a target gene in various
tumors, including gastric cancer and lymphocytic leukemia (23,24). In
the present study, it was demonstrated that overexpression of
miR-214 significantly suppressed the protein levels of PTEN.
Additionally, silencing of PTEN increased the invasive ability of
PC3 cells, even when miR-214 was inhibited. These results suggested
that miR-214 increased the invasive ability of PCa cells through
targeting PTEN.
In the present study, the statistical power may be
restricted due to the limited number of experimental samples. Thus,
it is necessary to incorporate several large prospective studies to
determine the function of miR-214 in patients with PCa with bone
metastasis. Despite this limitation, the results of the present
study have identified a novel potential non-invasive biomarker for
the diagnosis of PCa with bone metastasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Hunan Province Supporting fund of Changsha Central Hospital (grant
no. HNCS-20170623).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF performed the experiments and analyzed the data.
JQ, ZJ, SX and ZZ performed part of the RT-qPCR experiments. RH
designed the experiments, analyzed the data and gave final approval
of the version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Changsha Central Hospital (Changsha, China) and
all the patients provided written informed consent for this
study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2015. CA Cancer J Clin.
65:457–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luna A, Vilanova JC and Alcalá Mata L:
Total body MRI in early detection of bone metastasis and its
indication in comparison to bone scan and other imaging techniques.
Arch Esp Urol. 68:371–390. 2015.(In Spanish). PubMed/NCBI
|
|
4
|
Ramankulov A, Lein M, Kristiansen G,
Loening SA and Jung K: Plasma osteopontin in comparison with bone
markers as indicator of bone metastasis and survival outcome in
patients with prostate cancer. Prostate. 67:330–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Draisma G, Etzioni R, Tsodikov A, Mariotto
A, Wever E, Gulati R, Feuer E and de Koning H: Lead time and
overdiagnosis in prostate-specific antigen screening: Importance of
methods and context. J Natl Cancer Inst. 101:374–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EH and Andriole GL: Prostate-specific
antigen-based screening: Controversy and guidelines. BMC Med.
13:612015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitagawa Y and Namiki M: Prostate-specific
antigen-based population screening for prostate cancer: Current
status in Japan and future perspective in Asia. Asian J Androl.
17:475–480. 2015.PubMed/NCBI
|
|
8
|
Kitajima K, Murphy RC, Nathan MA,
Froemming AT, Hagen CE, Takahashi N and Kawashima A: Detection of
recurrent prostate cancer after radical prostatectomy: Comparison
of 11C-choline PET/CT with pelvic multiparametric MR imaging with
endorectal coil. J Nucl Med. 55:223–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
ChunJiao S, Huan C, ChaoYang X and GuoMei
R: Uncovering the roles of miRNAs and their relationship with
androgen receptor in prostate cancer. IUBMB Life. 66:379–386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pekarik V, Gumulec J, Masarik M, Kizek R
and Adam V: Prostate cancer, miRNAs, metallothioneins and
resistance to cytostatic drugs. Curr Med Chem. 20:534–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stuopelytė K, Daniūnaitė K, Jankevičius F
and Jarmalaitė S: Detection of miRNAs in urine of prostate cancer
patients. Medicina (Kaunas). 52:116–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun T, McKay R, Lee GS and Kantoff P: The
role of miRNAs in prostate cancer. Eur Urol. 68:589–590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang YS, Chen WY, Yin JJ,
Sheppard-Tillman H, Huang J and Liu YN: EGF receptor promotes
prostate cancer bone metastasis by downregulating miR-1 and
activating TWIST1. Cancer Res. 75:3077–3086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen WY, Liu SY, Chang YS, Yin JJ, Yeh HL,
Mouhieddine TH, Hadadeh O, Abou-Kheir W and Liu YN: MicroRNA-34a
regulates WNT/TCF7 signaling and inhibits bone metastasis in
Ras-activated prostate cancer. Oncotarget. 6:441–457.
2015.PubMed/NCBI
|
|
15
|
Xin R, Bai F, Feng Y, Jiu M, Liu X, Bai F,
Nie Y and Fan D: MicroRNA-214 promotes peritoneal metastasis
through regulating PTEN negatively in gastric cancer. Clin Res
Hepatol Gastroenterol. 40:748–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuninty PR, Bojmar L, Tjomsland V, Larsson
M, Storm G, Östman A, Sandström P and Prakash J: MicroRNA-199a and
−214 as potential therapeutic targets in pancreatic stellate cells
in pancreatic tumor. Oncotarget. 7:16396–16408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das F, Dey N, Bera A, Kasinath BS,
Ghosh-Choudhury N and Choudhury GG: MicroRNA-214 reduces
insulin-like growth factor-1 (IGF-1) receptor expression and
downstream mTORC1 signaling in renal carcinoma cells. J Biol Chem.
291:14662–14676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang SL, Gao YL and and Chen XB:
MicroRNA-214 targets PCBP2 to suppress the proliferation and growth
of glioma cells. Int J Clin Exp Pathol. 8:12571–12576.
2015.PubMed/NCBI
|
|
19
|
Siemińska L, Borowski A, Marek B, Nowak M,
Kajdaniuk D, Warakomski J and Kos-Kudła B: Serum concentrations of
adipokines in men with prostate cancer and benign prostate
hyperplasia. Endokrynol Pol. Feb 21–2018.(Epub ahead of print).
|
|
20
|
Jansson UH, Kristiansson B, Magnusson P,
Larsson L, Albertsson-Wikland K and Bjarnason R: The decrease of
IGF-I, IGF-binding protein-3 and bone alkaline phosphatase isoforms
during gluten challenge correlates with small intestinal
inflammation in children with coeliac disease. Eur J Endocrinol.
144:417–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ofori JK, Salunkhe VA, Bagge A, Vishnu N,
Nagao M, Mulder H, Wollheim CB, Eliasson L and Esguerra JL:
Elevated miR-130a/miR130b/miR-152 expression reduces intracellular
ATP levels in the pancreatic beta cell. Sci Rep. 7:449862017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou ZJ, Fan L, Wang L, Xu J, Zhang R, Tian
T, Li JY and Xu W: miR-26a and miR-214 down-regulate expression of
the PTEN gene in chronic lymphocytic leukemia, but not PTEN
mutation or promoter methylation. Oncotarget. 6:1276–1285. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii T, Shimada K, Tatsumi Y, Fujimoto K
and Konishi N: Syndecan-1 responsive microRNA-126 and 149 regulate
cell proliferation in prostate cancer. Biochem Biophys Res Commun.
456:183–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu H, He HC, Han ZD, Wan YP, Luo HW, Huang
YQ, Cai C, Liang YX, Dai QS, Jiang FN and Zhong WD: MicroRNA-224
and its target CAMKK2 synergistically influence tumor progression
and patient prognosis in prostate cancer. Tumour Biol.
36:1983–1991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ha TY: MicroRNAs in human diseases: From
cancer to cardiovascular disease. Immune Netw. 11:135–154. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
30
|
Liu B, Tian Y, Li F, Zhao Z, Jiang X, Zhai
C, Han X and Zhang L: Tumor-suppressing roles of miR-214 and
miR-218 in breast cancer. Oncol Rep. 35:3178–3184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen BF, Suen YK, Gu S, Li L and Chan WY:
A miR-199a/miR-214 self-regulatory network via PSMD10, TP53 and
DNMT1 in testicular germ cell tumor. Sci Rep. 4:64132014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li
J, Wang Z, Chen X, Zhang W, Yokoyama S, et al: Tumor-secreted
miR-214 induces regulatory T cells: A major link between immune
evasion and tumor growth. Cell Res. 24:1164–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jindra PT, Bagley J, Godwin JG and
Iacomini J: Costimulation-dependent expression of microRNA-214
increases the ability of T cells to proliferate by targeting Pten.
J Immunol. 185:990–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamiya N, Suzuki H, Yano M, Endo T, Takano
M, Komaru A, Kawamura K, Sekita N, Imamoto T and Ichikawa T:
Implications of serum bone turnover markers in prostate cancer
patients with bone metastasis. Urology. 75:1446–1451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moslehi M, Cheki M, Salehi-Marzijarani M,
Amuchastegui T and Gholamrezanezhad A: Predictors of bone
metastasis in pre-treatment staging of asymptomatic treatment-naive
patients with prostate cancer. Rev Esp Med Nucl Imagen Mol.
32:286–289. 2013.PubMed/NCBI
|