Introduction
Ovarian cancer is the most common gynecological
malignancy, with the highest mortality among all gynecological
malignancies (1). A previous study
demonstrated that the incidence of ovarian cancer is increasing
globally (2). Epithelial ovarian
cancer is a distinct histotype of ovarian cancer that is
characterized by poor response rates to chemotherapies in the
advanced stages of disease (3). Using
a meta-analysis of prevalence rates, it was identified that females
with ovarian cancer who had undergone chemotherapy treatment
demonstrated continued symptoms of depression and anxiety (4). Ineffective or prolonged management of
treatments may contribute to the worsening of symptoms, treatment
noncompliance and even a decrease in health-associated quality of
life (5). Although previous studies
have proposed certain improvements in early diagnosis and
anticancer therapies for ovarian carcinoma (6–9),
metastasis remains the major challenge in the treatment of ovarian
carcinoma. Therefore, it is imperative to understand the mechanisms
of the metastasis in this disease.
Novel strategies for ovarian carcinoma therapy have
attracted attention from clinicians and doctors (10–12).
Targeted therapy has exhibited efficient antitumor effects
worldwide, and serves a critical role in modern treatment
modalities (13–15). Gu et al (16) demonstrated that calpain small subunit
4 (Capn4) may promote progression of non-small cell lung cancer via
the upregulation of matrix metalloproteinase 2 expression. Capn4
overexpression has been observed in and is associated with the
invasiveness of hepatocellular carcinoma (17). Notably, Capn4 may promote epithelial
ovarian carcinoma metastasis through osteopontin (OPN)-mediated
activation of the Wnt/β-catenin pathway (18). Study also found that caspase-3
up-regulation strongly suppressed tumor growth and improved mouse
survival with little liver toxicity (19). However, the associations between Capn4
and the caspase-3 signaling pathway have not been completely
elucidated.
OPN is an integrin-binding matrix phosphorylated
glycoprotein, which is frequently overexpressed in a number of
advanced human cancer tissues (20).
A previous study has indicated that OPN promotes ovarian cancer
progression and cell survival through the phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT) signaling pathway (21). In addition, OPN expression is a
biomarker in ovarian cancer progression, which contributes to the
understanding of the physiopathology of ovarian cancer progression
and tumorigenesis (22). Furthermore,
OPN overexpression has been observed in endometrioid endometrial
cancer and ovarian endometrioid cancer (23).
In the present study, the expression levels of Capn4
and OPN in clinical ovarian carcinoma tissues and a cell line were
evaluated. It was identified that Capn4 was highly expressed in
ovarian carcinoma tissues and cell lines compared with normal
ovarian tissues and cells. It was also revealed that Capn4 may
promote ovarian carcinoma metastasis through the OPN-mediated
PI3K/AKT signaling pathway. Taken together, the results of the
present study indicated that Capn4 may be regarded as a novel
potential target for ovarian carcinoma therapy.
Materials and methods
Cell culture and reagents
SKOV3 and normal ovarian epithelial cells (IOSE80)
cells were purchased from the Cell Bank of Type Culture Collection
of Chinese Academy of Science (Shanghai, China). Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
37°C humidified atmosphere with 5% CO2.
Patients and tissue samples
Normal ovarian tissues and ovarian tumor tissues
were obtained from 5 patients (42 years; range, 32–68 years) using
a biopsy at Tianjin Medical University General Hospital (Tianjin,
China) between May 2014 and June 2017. None of the patients
received chemotherapy or other antitumor treatment prior to
surgery. All patients provided written informed consent. Ethical
approval for the use of human tissue samples was provided by the
Institutional Research Ethics Committee of Tianjin Medical
University General Hospital.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays
Total RNA from SKOV3 cells, normal ovarian tissues
or ovarian tumor tissues was extracted using an RNeasy Mini kit
(Qiagen Sciences, Inc., Gaithersburg, MD, USA). The mRNA expression
levels of Capn4 and β-actin in SKOV3 cells were determined by
RT-qPCR (24). All primers were
synthesized by Thermo Fisher Scientific, Inc. (Capn4, forward,
5′-ACCCACTCCGTAACCTC-3′ and reverse, 5′-GGGTAGCAACCGTGAA-3′;
β-actin forward, 5′-CATCTCTTGCTCGAAGTCCA-3′ and reverse,
5′-ATCATGTTTGAGACCTTCAACA-3′). PCR was performed as follows:
Preliminary denaturation at 94°C for 2 min, followed by 45 cycles
of 95°C for 30 sec, the annealing temperature decreased to 55°C for
30 sec, and 72°C for 10 min in a total reaction volume of 20 µl
containing 50 ng genomic DNA, 200 µM dNTP, 2.5 units Taq DNA
polymerase (Takara Biotechnology Co., Ltd., Dalian, China) and 200
µM primers. Relative mRNA expression level changes were calculated
using the 2−ΔΔCq method (25). The results are expressed as the n-fold
comparison with the β-actin control.
Lactate dehydrogenase (LDH) assay
The ovarian tumor cells SKOV3 were cultured until a
90% monolayer was formed, and then the medium was removed. The
ovarian tumor cells were washed three times and subsequently
incubated with Triton X-100 (1%) for 30 min. LDH activity in the
lysates was measured using the Promega CytoTox 96 assay kit
(Promega™ G1780), according to the manufacturer's protocol.
Gene silencing with small interfering
RNA (siRNA)
SKOV3 cells (4×105 cells/well) were
seeded in 6-well plates for 24 h at 37°C. The medium was removed
and Opti-MEM reduced serum medium (Invitrogen; Thermo Fisher
Scientific, Inc.) was added for 24 h at 37°C. siRNA-Capn4
(siR-Capn4), siRNA-OPN (siR-OPN) or control scrambled siRNA
(siR-vector) were chemically synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). The sequences were as follows:
siR-Capn4, 5′-GCACCAUGCAGAAUACAAA-3′, siRNA-OPN,
5′-CCUGUGCCAUACCAGUUAA-3′, siR-vector, 5′-CUCGUCUCAUUGATGACAGTT-3′.
SKOV3 cells were transfected with 100 pmol siRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Cell invasion and migration
assays
SKOV3 cells and Capn4-slienced SKOV3 cells were
treated with PI3K inhibitor (SW30-Calbiochem, 200 µM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 24 h at 37°C.
For the invasion assay, SKOV3 cells were suspended at a density of
1×105 in 500 µl serum-free DMEM. The cells were then
applied to the tops of BD BioCoat Matrigel Invasion Chambers (BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocols. For the migration assay, a control insert
(BD Biosciences) was used instead of a Matrigel Invasion Chamber.
The tumor cells that had invaded or migrated were fixed with 4%
formaldehyde at room temperature for 5 min and stained with 0.5%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) at room temperature for 15 min. and counted
in at least three randomly selected fields of view for each
membrane.
Flow cytometry
Apoptosis of the SKOV3 cells was evaluated using an
Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide
(PI) Apoptosis Detection kit (BD Biosciences). SKOV3 cells were
collected and suspended with annexin V-FITC and PI, according to
the manufacturer's protocol. Fluorescence was determined with a
fluorescence-activated cell sorting flow cytometer using FCS
Express™ IVD software (version 4; De Novo Software, Los Angeles,
CA, USA).
Western blotting
SKOV3 cells (1×106) were homogenized in
lysate buffer containing protease inhibitor (cat. no. P3480;
Sigma-Aldrich; Merck KGaA) and were centrifuged at 8,000 × g at 4°C
for 10 min. The supernatant was used for analyzing protein
expression. Protein concentration was measured using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Protein samples (20 µg) were separated by SDS-PAGE (12% gel)
and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA), and incubated with rabbit
anti-mouse primary antibodies against PI3K (1:1,000; cat. no.
ab151549), phospho-(p-)PI3K (1:1,000; cat. no. ab86714), AKT
(1:1,000; cat. no. ab8805), p-AKT (1:1,000; cat. no. ab133458),
Capn4 (1:1,000; cat. no. ab28237), OPN (1:1,000; cat. no. ab8448),
caspase-3 (1:1,000; cat. no. ab13847), caspase-9 (1:1,000; cat. no.
ab52298), B-cell lymphoma (Bcl)-2 (1:1,000; cat. no. ab692), Bcl-w
(1:1,000; cat. no. ab32370) and β-actin (1:1,000; cat. no. ab5262)
(all Abcam, Cambridge, UK). Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit immunoglobulin G (cat. no. PV-6001; OriGene
Technologies, Inc., Rockville, MD, USA) was added for 24 h at 4°C.
A Ventana Benchmark automated staining system was used for
analyzing protein expression (Olympus BX51; Olympus Corporation,
Tokyo, Japan). The density of the bands was analyzed using Quantity
One software (version 4.62; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Immunohistochemistry
Paraffin-embedded 4-µm tissue sections were prepared
for further analysis. The paraffin sections were treated with
H2O2 (3%) for 10–15 min at 37°C, which
subsequently were blocked by a regular blocking solution (5%
skimmed milk powder in PBST) for 10–15 min at 37°C. Finally, the
sections were incubated with anti-Capn4 (1:1,000) at 4°C for 12 h.
All sections were washed with PBST three times and incubated with
HRP-conjugated IgG (cat. no. PV-6001; OriGene Technologies, Inc.)
for 24 h at 4°C. The relative expression of Capn4 was determined in
six random views under a light microscope. Images were captured and
analyzed using a MicroChemi chemiluminescence system (version 4.2;
Eastwin, Shenzen, China).
Statistical analysis
All data are expressed as the mean ± standard
deviation of triplicate dependent experiments and analyzed using
Student's t-test or a one-way analysis of variance (followed by
Tukey's honest significant difference test). Patients' survival was
analyzed using the Kaplan-Meier method. All data were analyzed
using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Capn4 is overexpressed in ovarian
carcinoma and is associated with poor clinical outcomes
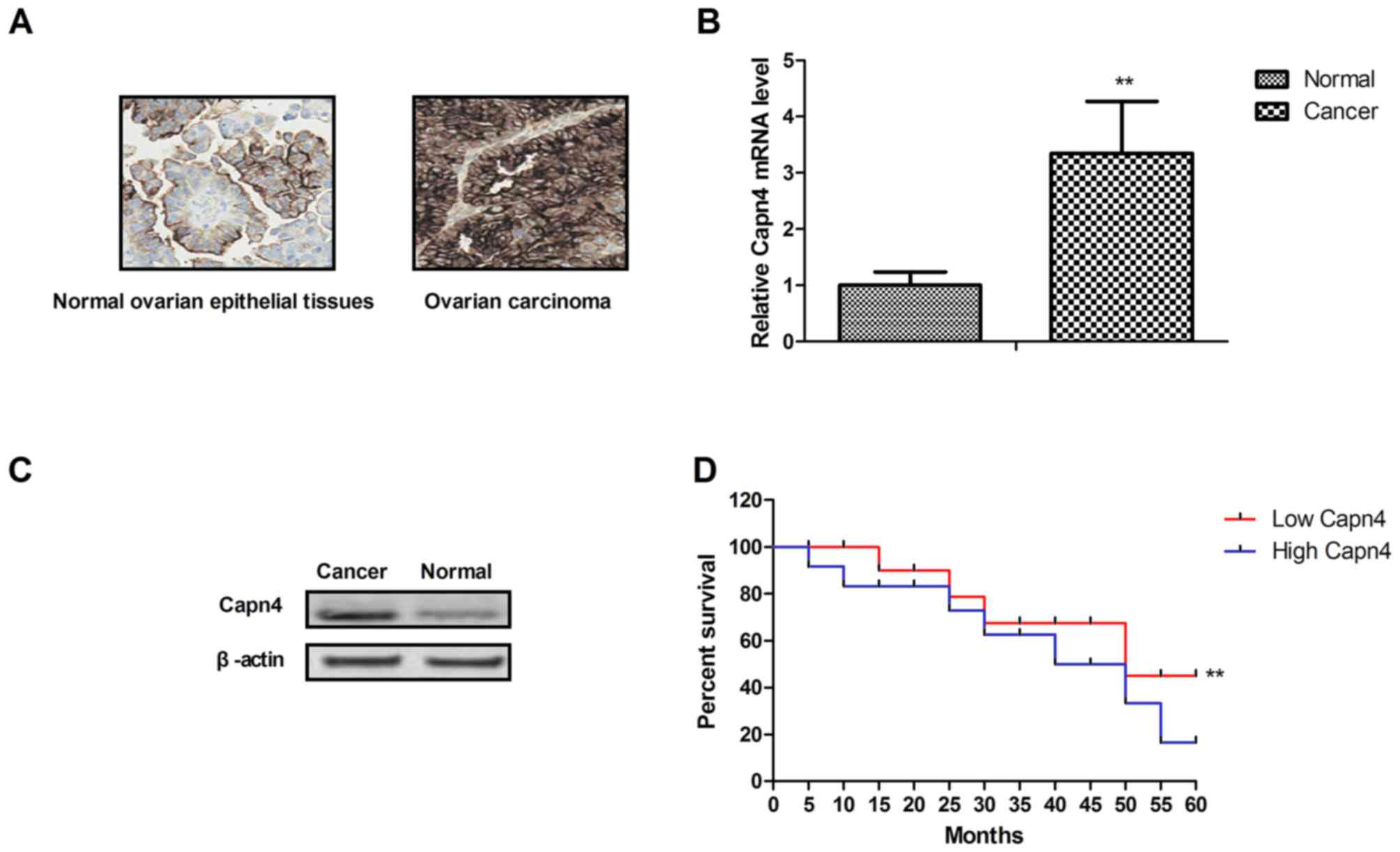
To determine the significance of Capn4, its
expression was analyzed in ovarian carcinoma (n=5) and normal
ovarian epithelial tissues from the same patients. The results
revealed that Capn4 was more highly expressed in ovarian carcinoma
tissues compared with in normal ovarian epithelial tissues
determined by immunohistochemistry (Fig.
1A). The gene and protein expression levels of Capn4 were also
increased in ovarian carcinoma tissues compared with normal ovarian
epithelial tissues (Fig. 1B and C).
It was demonstrated that Capn4 expression was markedly associated
with poor survival of patients (Fig.
1D). Taken together, these results indicated that the
overexpression of Capn4 may be associated with the progression of
ovarian carcinoma.
Capn4 silencing inhibits the viability
and aggressiveness of ovarian carcinoma cells
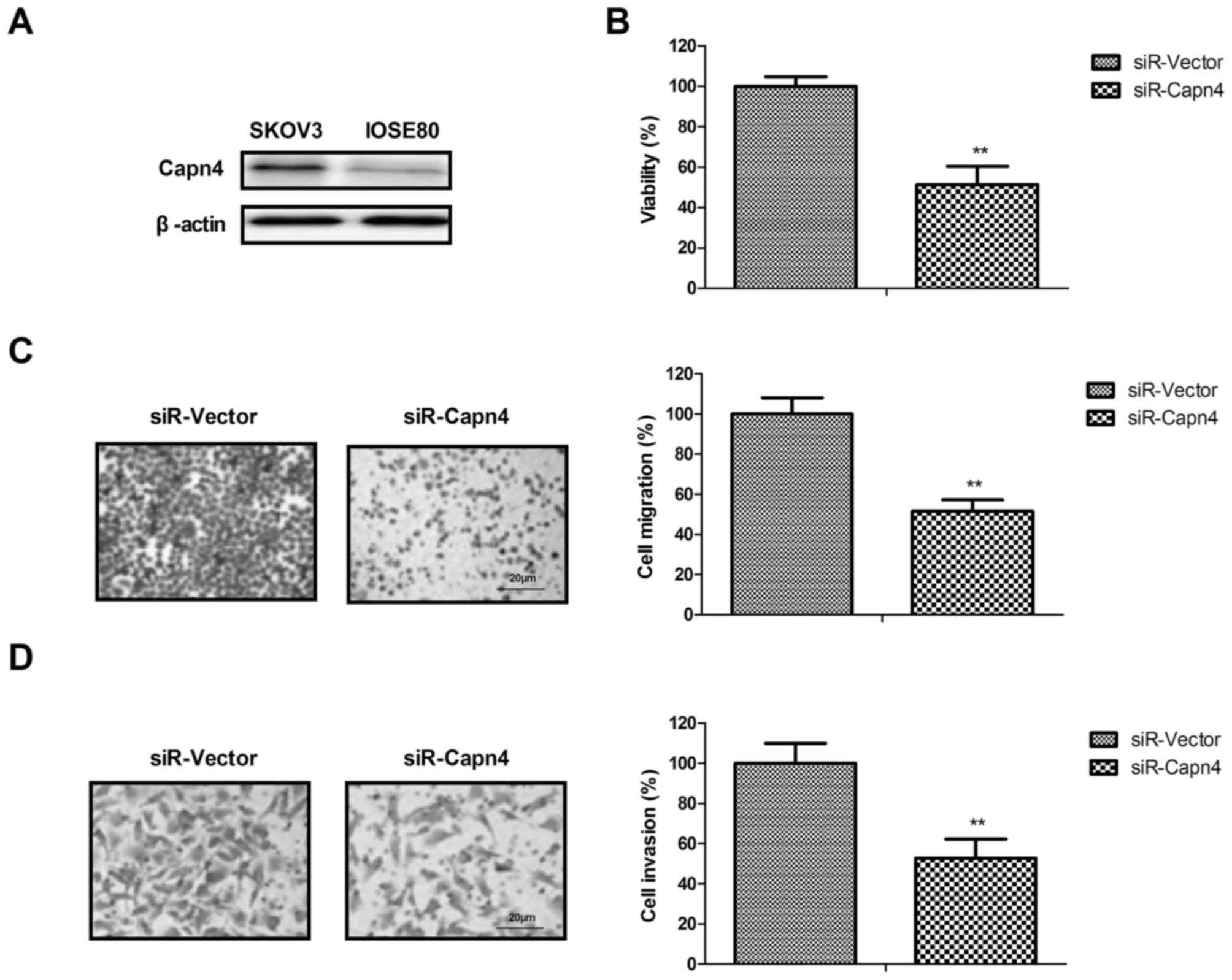
The function of Capn4 silence was analyzed in
ovarian carcinoma cells and scrambled siRNA (siR-vector) was used
to exclude the non-specific target effect. The results revealed
that Capn4 is more highly expressed in ovarian carcinoma cells
SKOV3 compared with in normal ovarian epithelial cells (IOSE80)
(Fig. 2A). It was revealed that Capn4
silencing inhibited ovarian cancer cell (SKOV3) viability in
vitro (Fig. 2B). It was also
identified that Capn4 silencing markedly inhibited the migration
and invasion of SKOV3 cells (Fig. 2C and
D). Taken together, these results indicated that Capn4
silencing may inhibit the viability and aggressiveness of ovarian
carcinoma cells.
Capn4 silencing promotes apoptosis of
ovarian carcinoma cells via the caspase-3 apoptotic signaling
pathway
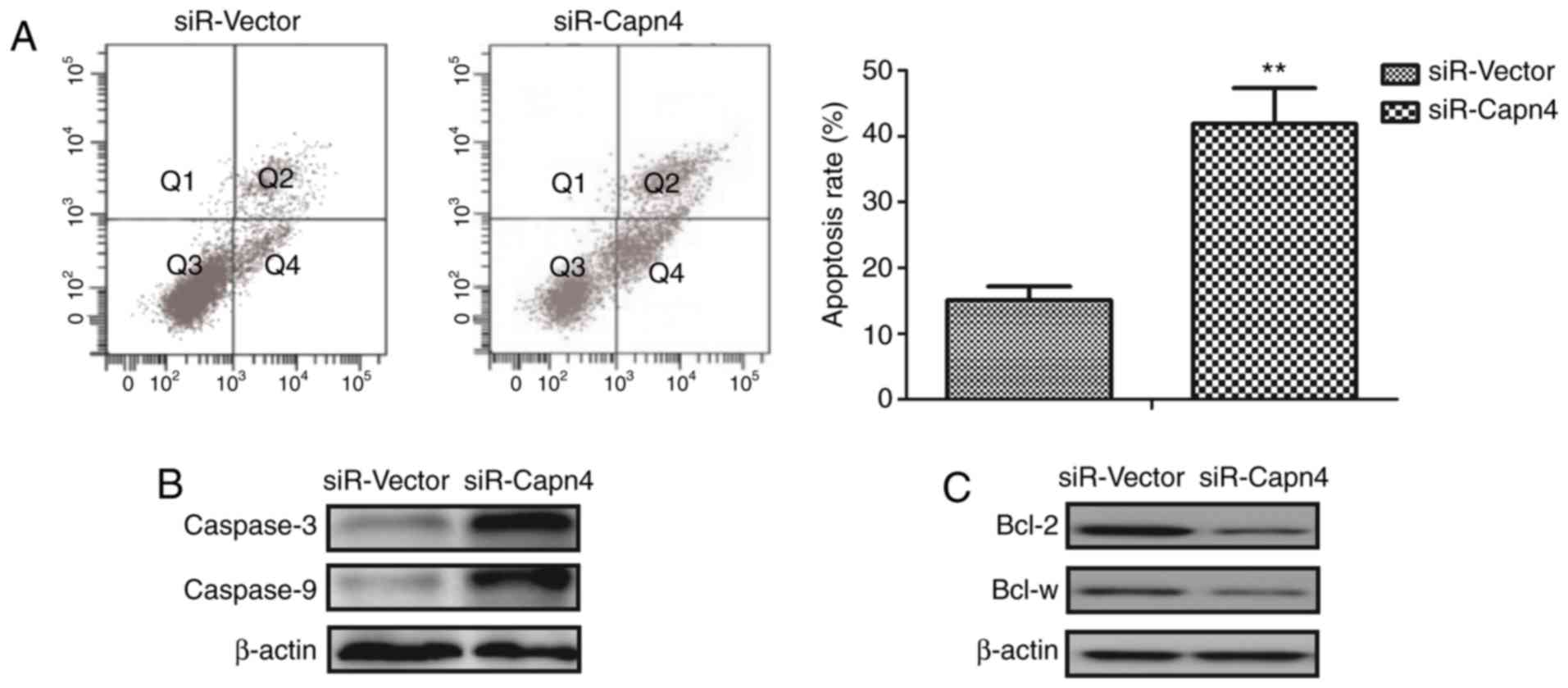
To investigate the underlying biological functions
of Capn4 in ovarian carcinoma cells, apoptosis was determined in
Capn4-silenced ovarian carcinoma cells. Results indicated that
Capn4 silencing promoted the apoptosis of SKOV3 cells induced by
cisplatin (Fig. 3A). Capn4 silencing
increased the expression levels of the pro-apoptotic caspase-3 and
caspase-9 compared with the control group (Fig. 3B). It was revealed that Capn4
silencing decreased the expression levels of the anti-apoptotic
Bcl-2 and Bcl-w compared with the control group (Fig. 3C). Taken together, these results
indicate that Capn4 silencing may promote apoptosis of ovarian
carcinoma cells via the capase-3 apoptotic signaling pathway.
Capn4 silencing inhibits ovarian
carcinoma cell metastasis via the OPN-mediated PI3K/AKT signaling
pathway
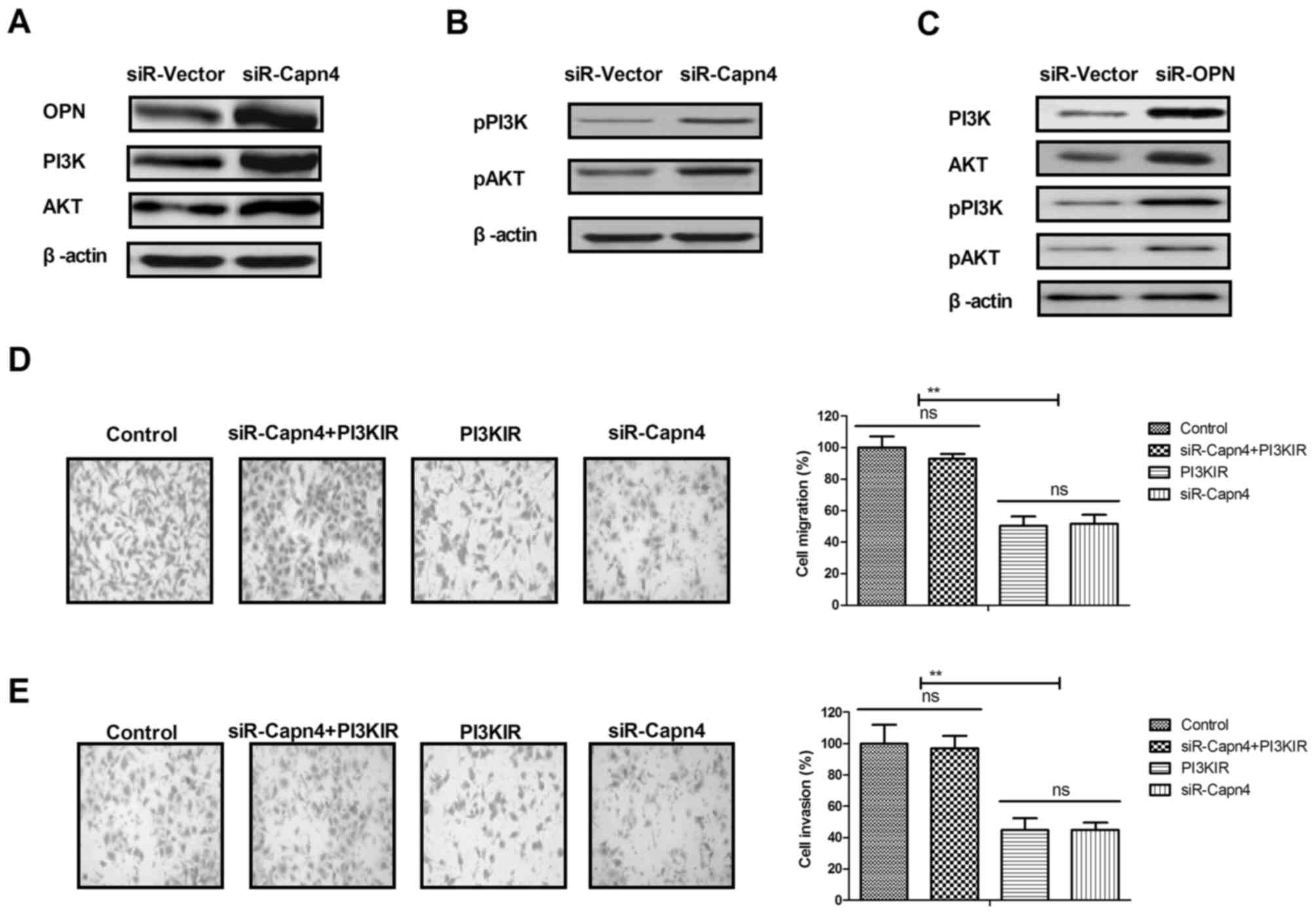
The mechanism mediated by Capn4 in ovarian carcinoma
cells was investigated. The results indicated that Capn4 silencing
increased OPN, PI3K and AKT expression in SKOV3 cells (Fig. 4A). It was identified that Capn4
silencing increased levels of p-PI3K and p-AKT in SKOV3 cells
(Fig. 4B). OPN silencing also
decreased the expression levels of p-PI3K and p-AKT in SKOV3 cells
(Fig. 4C). Results demonstrated that
PI3K inhibitor inhibited the migration and invasion of SKOV3 cells
in Capn4-silenced SKOV3 cells (Fig. 4D
and E). Taken together, these results indicate that Capn4
regulated ovarian carcinoma cell metastasis via the OPN-mediated
PI3K/AKT signaling pathway.
Discussion
Inhibiting systemic metastasis of ovarian cancer may
be a novel therapeutic option for patients with ovarian cancer
(26). Evidence has indicated that
Capn4 may represent a potential therapeutic target in the treatment
of human cancer (16,27). In the present study, Capn4 expression
levels were investigated in ovarian cancer tissues and ovarian
cancer cell lines in vitro. Previous studies indicated that
activation of the PI3K/AKT signaling pathway is involved in the
progression of ovarian serous cystadenocarcinoma (28,29).
Although it has been demonstrated that Capn4 enhances OPN
expression through activation of the Wnt/β-catenin signaling
pathway (18), to the best of our
knowledge, the association between Capn4 and the PI3K/AKT signaling
pathway has not been investigated in ovarian cancer cells. The
results of the present study indicated that Capn4 regulated ovarian
cancer cell migration and invasion via the PI3K/AKT signaling
pathway.
Targeted therapy has much potential as a treatment
approach, as it exhibits lower toxicity to normal cells, but not to
tumor cells (30–32). Capn4 is a secreted protein with
relevant interactions with numerous migration- and
invasion-associated proteins (33).
The results of the present study identified that Capn4 is
overexpressed in ovarian carcinoma tissues and ovarian carcinoma
cells compared with normal ovarian tissues and cells. Capn4
overexpression increased tumor invasion and metastasis following
tumorectomy for hepatocellular carcinoma, which may be a candidate
biomarker for future diagnosis and a target for therapy (17). It was revealed that Capn4 exhibited a
significant association with poor survival of patients with ovarian
carcinoma, and thus may be a potential target for ovarian carcinoma
therapy. Notably, Capn4 knockdown significantly inhibited viability
and aggressiveness of ovarian carcinoma cells via the OPN-mediated
PI3K/AKT signaling pathway.
OPN serves a significant function in a number of
physiological and pathological processes, including wound healing,
inflammation, immune response and tumor progression (34). OPN overexpression is associated with
ovarian cancer progression and tumorigenesis via regulation of the
PI3K/AKT signaling pathway (21). In
the present study, it was observed that Capn4 knockdown
downregulated OPN expression in ovarian cancer cells via
downregulation of the PI3K/AKT signaling pathway. The results
indicated that the PI3K inhibitor blocked Capn4-OPN-regulated
viability and migration of ovarian cancer cells. These results
suggest that Capn4 may be a potential target for the treatment of
ovarian cancer. A previous study identified that mitogen-activated
protein kinase/extracellular-signal-regulated kinase (ERK) kinase
kinase 2/ERK1/2/Capn4 signaling is involved in the migration of
breast cancer cells (27). The
results of the present study also indicated that the
Capn4/OPN/PI3K/AKT signaling pathway participates in the migration
and invasion of ovarian carcinoma cells. Additionally, a previous
study indicated that Capn4 may represent a potential therapeutic
target and a novel prognostic marker of non-small cell lung cancer
(16). In the present study, it was
identified that Capn4 is a potential target for the inhibition of
ovarian carcinoma cells.
Apoptosis serves an important function in the
treatment of cancer (35–37). In the present study, the potential
role of Capn4 in the apoptotic signaling pathway was analyzed. A
previous study has demonstrated that the overexpression of the
apoptosis-associated gene caspase-3 contributed to the apoptosis of
human ovarian cancer cell lines induced by cisplatin (38). The results of the present study
revealed that Capn4 silencing promoted the apoptosis of ovarian
cancer cells induced by cisplatin by downregulation of Bcl-2 and
Bcl-w expression. It was indicated that Capn4 silencing may promote
apoptosis of ovarian carcinoma cells via the capase-3 apoptosis
signaling pathway. However, a limitation of the present study is
that only a single cell line was used to identify the effects of
Capn4, as is the small number of ovarian carcinoma samples from
patients.
In conclusion, the results of the present study
indicated that Capn4 may be a potential target for ovarian
carcinoma therapy. The results highlight the regulatory role of
Capn4 in the growth and metastasis of ovarian cancer, and reveal
that Capn4 knockdown may upregulate the PI3K/AKT signaling pathway
via upregulation of OPN expression in ovarian carcinoma cells.
Capn4 may be regarded as a candidate therapeutic target for the
future treatment of ovarian carcinoma.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
FX designed the experiments. YC performed the
experiments. GW, YW, XG, KW, and JL prepared the investigations and
analyzed data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China). Patient provided written informed consent.
Patient consent for publication
All patients provided written informed consent and
approved publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Korkmaz T, Seber S and Basaran G: Review
of the current role of targeted therapies as maintenance therapies
in first and second line treatment of epithelial ovarian cancer; In
the light of completed trials. Crit Rev Oncol Hematol. 98:180–188.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woopen H, Pietzner K, Darb-Esfahani S,
Oskay-Oezcelik G and Sehouli J: Extraperitoneal response to
intraperitoneal immunotherapy with catumaxomab in a patient with
cutaneous lymphangiosis carcinomatosa from ovarian cancer: A case
report and review of the literature. Med Oncol. 29:3416–3420. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dybro A and Blaakær J: Treatment of
epithelian ovarian cancer should be followed by lymphadenectomy-a
systematic review. Ugeskr Laeger. 174:865–870. 2012.(In Danish).
PubMed/NCBI
|
|
4
|
Watts S, Prescott P, Mason J, McLeod N and
Lewith G: Depression and anxiety in ovarian cancer: A systematic
review and meta-analysis of prevalence rates. BMJ Open.
5:e0076182015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis LL and Carpenter JS: A systematic
review of nonpharmacologic interventions for treatment-related
symptoms in women with ovarian cancer. Clin J Oncol Nurs.
19:535–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGee J, Panabaker K, Leonard S, Ainsworth
P, Elit L and Shariff SZ: Genetics consultation rates following a
diagnosis of high-grade serous ovarian carcinoma in the Canadian
province of Ontario. Int J Gynecol Cancer. 27:437–443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maru Y, Tanaka N, Ohira M, Itami M, Hippo
Y and Nagase H: Identification of novel mutations in Japanese
ovarian clear cell carcinoma patients using optimized targeted NGS
for clinical diagnosis. Gynecol Oncol. 144:377–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon DY, Han GH, Ulak R, Ki KD, Lee JM and
Lee SK: Syndrome of inappropriate antidiuretic hormone secretion
following irinotecan-cisplatin administration as a treatment for
recurrent ovarian clear cell carcinoma. Obstet Gynecol Sci.
60:115–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sirota R, Gibson D and Kohen R: The timing
of caffeic acid treatment with cisplatin determines sensitization
or resistance of ovarian carcinoma cell lines. Redox Biol.
11:170–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gullbo J, Fryknäs M, Rickardson L, Darcy
P, Hägg M, Wickström M, Hassan S, Westman G, Brnjic S, Nygren P, et
al: Phenotype-based drug screening in primary ovarian carcinoma
cultures identifies intracellular iron depletion as a promising
strategy for cancer treatment. Biochem Pharmacol. 82:139–147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itamochi H, Kigawa J, Kanamori Y, Oishi T,
Bartholomeusz C, Nahta R, Esteva FJ, Sneige N, Terakawa N and Ueno
NT: Adenovirus type 5 E1A gene therapy for ovarian clear cell
carcinoma: A potential treatment strategy. Mol Cancer Ther.
6:227–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKay TR, Bell S, Tenev T, Stoll V, Lopes
R, Lemoine NR and McNeish IA: Procaspase 3 expression in ovarian
carcinoma cells increases survivin transcription which can be
countered with a dominant-negative mutant, survivin T34A; a
combination gene therapy strategy. Oncogene. 22:3539–3547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhan Q, Wang C and Ngai S: Ovarian cancer
stem cells: A new target for cancer therapy. Biomed Res Int.
2013:9168192013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ziebarth AJ, Nowsheen S, Steg AD, Shah MM,
Katre AA, Dobbin ZC, Han HD, Lopez-Berestein G, Sood AK, Conner M,
et al: Endoglin (CD105) contributes to platinum resistance and is a
target for tumor-specific therapy in epithelial ovarian cancer.
Clin Cancer Res. 19:170–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama K, Nakayama N and Miyazaki K:
Development of a novel ovarian cancer molecular target therapy
against cancer-related transcriptional factor, NAC1. J Obstet
Gynaecol Res. 39:18–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu J, Xu FK, Zhao GY, Lu CL, Lin ZW, Ding
JY and Ge D: Capn4 promotes non-small cell lung cancer progression
via upregulation of matrix metalloproteinase 2. Med Oncol.
32:512015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai DS, Dai Z, Zhou J, Liu YK, Qiu SJ, Tan
CJ, Shi YH, Huang C, Wang Z, He YF and Fan J: Capn4 overexpression
underlies tumor invasion and metastasis after liver transplantation
for hepatocellular carcinoma. Hepatology. 49:460–470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Sun J, Xia D, Can X, Liu L, Zhang
J, Xu H, Du N, Liu W, Shen F, et al: Capn4 enhances osteopontin
expression through activation of the Wnt/β-catenin pathway to
promote epithelial ovarian carcinoma metastasis. Cell Physiol
Biochem. 42:185–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Y, Xin X, Xia Z, Zhai X and Shen K:
Selective suppression of autocatalytic caspase-3 driven by two-step
transcriptional amplified human telomerase reverse transcriptase
promoter on ovarian carcinoma growth in vitro and in mice. Oncol
Rep. 32:225–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brakora KA, Lee H, Yusuf R, Sullivan L,
Harris A, Colella T and Seiden MV: Utility of osteopontin as a
biomarker in recurrent epithelial ovarian cancer. Gynecol Oncol.
93:361–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song G, Cai QF, Mao YB, Ming YL, Bao SD
and Ouyang GL: Osteopontin promotes ovarian cancer progression and
cell survival and increases HIF-1alpha expression through the
PI3-K/Akt pathway. Cancer Sci. 99:1901–1907. 2008.PubMed/NCBI
|
|
22
|
Tilli TM, Franco VF, Robbs BK, Wanderley
JL, da Silva FR, de Mello KD, Viola JP, Weber GF and Gimba ER:
Osteopontin-c splicing isoform contributes to ovarian cancer
progression. Mol Cancer Res. 9:280–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashiguchi Y, Tsuda H, Bandera CA,
Nishimura S, Inoue T, Kawamura N, Berkowitz RS and Mok SC:
Comparison of osteopontin expression in endometrioid endometrial
cancer and ovarian endometrioid cancer. Med Oncol. 23:205–212.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckert MA, Pan S, Hernandez KM, Loth RM,
Andrade J, Volchenboum SL, Faber P, Montag A, Lastra R, Peter ME,
et al: Genomics of ovarian cancer progression reveals diverse
metastatic trajectories including intraepithelial metastasis to the
fallopian tube. Cancer Discov. 6:1342–1351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhang Z, Zhou X, Li L, Liu Q, Wang
Z, Bai X, Zhao Y, Shi H, Zhang X and Ye L: The oncoprotein HBXIP
enhances migration of breast cancer cells through increasing
filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer
Lett. 355:288–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Hua K, Zhou X, Jin H, Chen X, Lu
X, Yu Y, Zha X and Feng Y: Activation of the PI3K/AKT pathway
mediates FSH-stimulated VEGF expression in ovarian serous
cystadenocarcinoma. Cell Res. 18:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou C, Qiu L, Sun Y, Healey S, Wanebo H,
Kouttab N, Di W, Yan B and Wan Y: Inhibition of EGFR/PI3K/AKT cell
survival pathway promotes TSA's effect on cell death and migration
in human ovarian cancer cells. Int J Oncol. 29:269–278.
2006.PubMed/NCBI
|
|
30
|
Rodriguez-Brenes IA, Wodarz D and Komarova
NL: Quantifying replicative senescence as a tumor suppressor
pathway and a target for cancer therapy. Sci Rep. 5:176602015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Collet G, Szade K, Nowak W, Klimkiewicz K,
El Hafny-Rahbi B, Szczepanek K, Sugiyama D, Weglarczyk K,
Foucault-Collet A, Guichard A, et al: Endothelial precursor
cell-based therapy to target the pathologic angiogenesis and
compensate tumor hypoxia. Cancer Lett. 370:345–357. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marech I, Leporini C, Ammendola M,
Porcelli M, Gadaleta CD, Russo E, De Sarro G and Ranieri G:
Classical and non-classical proangiogenic factors as a target of
antiangiogenic therapy in tumor microenvironment. Cancer Lett.
380:216–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai Z, Zhou SL, Zhou ZJ, Bai DS, Xu XY, Fu
XT, Chen Q, Zhao YM, Zhu K, Yu L, et al: Capn4 contributes to
tumour growth and metastasis of hepatocellular carcinoma by
activation of the FAK-Src signalling pathways. J Pathol.
234:316–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin
BD, Fu HT and Zhong RQ: Diagnostic value of osteopontin in ovarian
cancer: A meta-analysis and systematic review. PLoS One.
10:e01264442015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mishra J, Drummond J, Quazi SH, Karanki
SS, Shaw JJ, Chen B and Kumar N: Prospective of colon cancer
treatments and scope for combinatorial approach to enhanced cancer
cell apoptosis. Crit Rev Oncol Hematol. 86:232–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braunhut SJ, McIntosh D, Vorotnikova E,
Zhou T and Marx KA: Detection of apoptosis and drug resistance of
human breast cancer cells to taxane treatments using quartz crystal
microbalance biosensor technology. Assay Drug Dev Technol. 3:77–88.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tilly JL and Kolesnick RN: Sphingolipids,
apoptosis, cancer treatments and the ovary: Investigating a crime
against female fertility. Biochim Biophys Acta. 1585:135–138. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang XK, Xing H, Zheng F, Gao QL, Wang W,
Lu YP and Ma D: Role of apoptosis-associated genes and caspase-3 in
cisplatin-resistant human ovarian cancer cell lines. Zhonghua Fu
Chan Ke Za Zhi. 38:158–161. 2003.(In Chinese). PubMed/NCBI
|