Introduction
Lung cancer is one of the most common malignant
tumors, with morbidity and mortality ranking the first among those
of malignancies, and has become Top 1 among malignant tumors in
mankind (1). Studies of World Health
Organization have shown that incidence and mortality rates of lung
cancer are increasing annually worldwide from the middle of last
century to now, and current death roll of patients due to lung
cancer has far exceeded the total number of patients who die of
breast cancer, prostate cancer and colorectal cancer (2,3).
Survival time of patients has been extended using
surgical treatment, chemotherapy, radiotherapy, targeted therapy
and other methods, but the 5-year survival rate in lung cancer
patients is only 15% (4). Studies
have found that main causes of the high incidence and mortality
rates of lung cancer are changes in related genes and signal
transduction pathways, such as increase of proto-oncogene
expression, decrease of tumor suppressor gene expression and
imbalance of intracellular signal transduction pathway, thereby
improving tumor cell proliferation, invasion and metastasis
abilities, so that tumor cells easily metastasize (5–8).
Therefore, studies of tumor-associated genes and their signal
transduction pathways in lung cancer cells are useful to identify
the mechanisms of occurrence and development of lung cancer, and
are of very important clinical significance for finding new drug
targets and methods of treatment.
Mineral dust-induced gene (mdig) is a lung
cancer-related gene that was first discovered in miners' alveolar
macrophages in 2005 (9). Mdig,
located on human chromosome 3q11.2, contains 1510 bases in full
length. It is able to encode a protein consisting of 465 amino
acids and has a molecular weight of 53 kDa (10). A slight expression of mdig was found
in normal human tissue cells, but its expression is high in various
tumor tissues and cell lines (11–14). A
further study showed that mdig can promote tumor cell proliferation
and block cell cycle progression (15).
There are few detailed reports on the effects of
mdig on proliferation and apoptosis of lung cancer cells and
mechanisms of action. Therefore, this study used ribonucleic acid
interference (RNAi) technology to silence mdig expression in lung
cancer NCI-H1650 cells and then adopted reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis to detect the impact of mdig small
interfering RNA (siRNA) on the expression of messenger RNA (mRNA)
and protein of mdig in NCI-H1650 cells. The effects of mdig
silencing on proliferation, cycle distribution, apoptosis and
apoptosis-related proteins of NCI-H1650 cells were further
studied.
Materials and methods
Materials
Materials used in the study were: NCI-H1650 human
lung cancer cells (Cell Bank of the Chinese Academy of Sciences,
Shanghai, China); Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum (Hyclone, Logan, UT, USA); TRIzol kits and
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA); bicinchoninic
acid (BCA) protein quantification kits and cell lysis buffer
(Beyotime Biotechnology, Nantong, China); reverse transcription
kits, RT-qPCR kits, primer syntheses, mdig siRNA, and negative
control siRNA (N-siRNA) (Takara, Dalian, China); mdig, cleaved
caspase-3, cleaved poly (ADP-ribose) polymerase 1 (PARP1),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary
antibodies, and horseradish peroxidase (HRP)-labeled secondary
antibodies (Proteintech Group, Inc., Wuhan, China); cycle detection
kits and Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kits (Beyotime Biotechnology, Nantong, China). The study
was approved by the Ethics Committee of Peking Union Medical
College Hospital (Beijing, China).
Cell culture and siRNA
transfection
NCI-H1650 cells were cultured in DMEM containing 10%
fetal bovine serum at 37°C in a 5% CO2 incubator, the
medium was changed every two days, and digestion and passage were
performed after cells spread to 80% of the bottom of the culture
bottle. Before transfection, NCI-H1650 cells in logarithmic phase
were inoculated into a 6-well plate at a density of
2×105/well. After 24 h, siRNA transfection was conducted
according to instructions of Lipofectamine 2000 for 48 h, with the
siRNA sequence shown in Table I. This
study included normal control group (control), negative control
group (N-siRNA) and experimental group (mdig siRNA).
 | Table I.siRNA sequences. |
Table I.
siRNA sequences.
| Name | Sequence name | siRNA sequence |
|---|
| mdig | Sense |
5′-UUGUCCGAACGUGUCACGUTT-3′ |
| siRNA | Antisense |
5′-ACGUGACACGUUCGGAGAATT-3′ |
| N-siRNA | Sense |
5′-GGGCAACGAUUCAGUUUCATT-3′ |
|
| Antisense |
5′-UGAAACUGAAUCGUUGCCCTT-3′ |
Effect of mdig siRNA on mRNA
expression of mdig in NCI-H1650 cells detected by RT-qPCR
Cells in each group were transfected for 48 h and
then collected to extract total RNA according to recommended
methods of TRIzol kits. When the absorbance ratio [the absorbance
at 260 and 280 nm (A260/280)] of samples was between 1.8 and 2.0,
the next reverse transcription reaction was carried out. Then, PCR
amplification was performed using the obtained complementary
deoxyribonucleic acid (cDNA) as a template according to primer
sequence shown in Table II. Specific
reaction conditions were as follows: pre-denaturation at 95°C for 3
min, then 95°C for 15 sec, 62°C for 30 sec, 72°C for 30 sec, with
40 cycles in total. GADPH was used as the internal reference. The
cycle threshold (Cq) value was output from the instrument, and
experimental results were analyzed using the 2−∆∆Cq
method (16).
 | Table II.RT-qPCR primer sequences. |
Table II.
RT-qPCR primer sequences.
| Gene | Primer name | Primer sequence |
|---|
| mdig | Forward |
5′-GGCAACGATTCAGTTTCACCAA-3′ |
|
| Reverse |
5′-TGTACACATTCGAGCCAACCAAG-3′ |
| GADPH | Forward |
5′-CCTGGTATGACAACGAATTTG-3′ |
|
| Reverse |
5′-CAGTGAGGGTCTCTCTCTTCC-3′ |
Impact of mdig siRNA on protein
expression of mdig in NCI-H1650 cells via western blot analysis
detection
Cells were collected from each group after 48 h of
transfection, added with cell lysis buffer, and centrifuged at
3,000 × g for 10 min at 4°C to extract proteins. The concentration
of the extracted protein was measured by BCA protein concentration
kits. Sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) was carried out, 40 µg of protein were loaded on each
well, subjected to wet membrane transfer, blocked in 5% bovine
serum albumin (BSA) solution, and incubated at 4°C with mdig and
GAPDH antibodies (diluted at 1:1,000). After washing the membrane,
proteins were added with HRP-labeled secondary antibodies dropwise
and incubated at room temperature for 2 h, followed by membrane
washing. Then, electrochemical luminescence (ECL) scotography was
performed. A gel imager was used for scanning or photographing.
Gray value was measured using Gel Pro 4.0 image analysis software
(Media Cybernetics, Inc., Rockville, MD, USA).
Role of silenced mdig in proliferation
of NCI-H1650 cells disclosed through 3-(4,5)-dimethylthiazol (-z-y1)-3,5-di-phenyl
tetrazolium bromide (MTT) assay
A single cell suspension was inoculated into a
96-well plate, with 5×103 cells in each well. Then,
siRNA experiment was performed. After 48 h, 20 µl (5 µg/µl) MTT
solution was added into each well and the plate was incubated for 4
h in the dark. After that, 100 µl dimethyl sulfoxide (DMSO)
solution was added to each well and the plate was vibrated to
dissolve purple crystals. A microplate reader (Thermo Fisher
Scientific, Waltham, MA, USA) was applied to measure absorbance
[optical density (OD) value] at 490 nm, and the cell proliferation
rate was calculated as: proliferation rate = (OD in mdig siRNA
group/OD in control group) ×100%.
Influence of silenced mdig on
NCI-H1650 cell cycle discovered via flow cytometry
Cells transfected for 48 h were collected from each
group and fixed overnight at 4°C in pre-cooled 70% ethanol. The
cells were re-suspended in 500 µl staining buffer, added with 25 µl
propidium iodide (PI) (50 µg/ml) and 10 µl ribonuclease A (RNase A)
(10 mg/ml), mixed evenly and incubated at 37°C for 30 min.
Subsequently the cells were washed with phosphate-buffered saline
once. Cell cycle analysis was performed using a flow cytometer
(Becton Dickinson and Company, Franklin Lakes, NJ, USA), and the
proportion of cells in different phases was expressed as a
percentage (%).
Effect of silenced mdig on NCI-H1650
cell apoptosis through flow cytometry and western blot
analysis
Cells were collected from each group after 48 h of
transfection, and then added with 0.3 ml binding buffer suspension
cells. Each test sample was added with 5 µl Annexin V and 5 µl PI,
incubated in the dark at room temperature for 15 min, and added
with additional 0.2 ml binding buffer. A flow cytometer (Becton
Dickinson and Company) was used to detect the apoptotic rate of
cells in each group.
Cells were transfected for 48 h and
then collected
Expression of cleaved caspase-3 and cleaved PARP1
proteins in cells in each group was detected as described
earlier.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
17.0 software (International Business Machines Corporation, Armonk,
NY, USA) was used for data analysis. Data are expressed as mean ±
standard deviation (SD). The t-test was employed for comparison
among groups. P<0.05 suggested that the difference was
statistically significant.
Results
Impact of mdig siRNA on mRNA
expression of mdig in NCI-H1650 cells
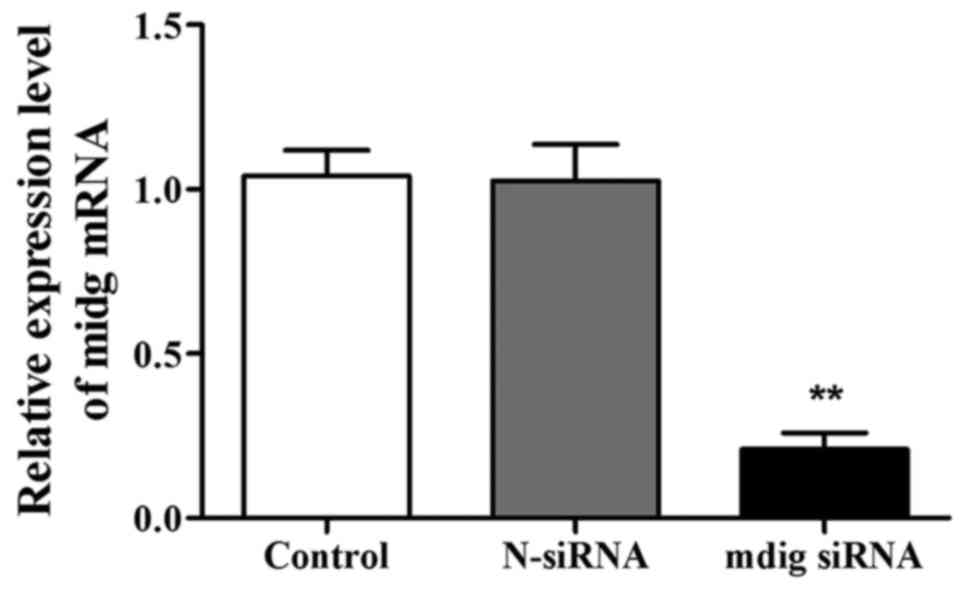
After transfection for 48 h, the expression level of
mdig mRNA in the mdig siRNA group was significantly lower than that
in control group (p<0.01) (Fig.
1). However, there was no significant difference in the
expression level of mdig mRNA between control group and N-siRNA
group, suggesting that mdig siRNA can specifically interfere with
the expression of mdig mRNA.
Effect of mdig siRNA on protein
expression of mdig in NCI-H1650 cells
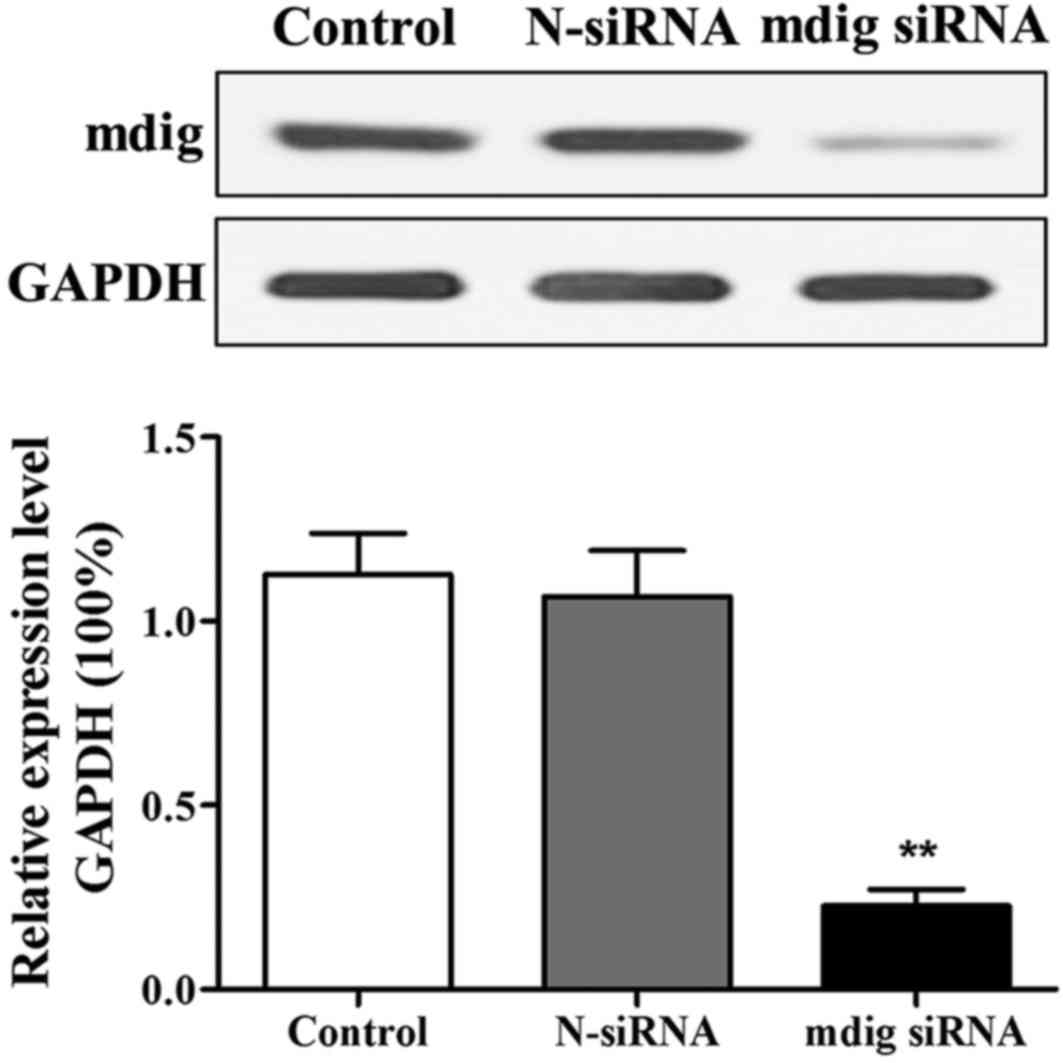
After 48 h of transfection, changes in mdig protein
level were detected through western blotting. The results revealed
that compared with that in control group, expression level of mdig
protein in cells in mdig siRNA group was distinctly decreased
(p<0.01) (Fig. 2). Changes in the
expression levels of mdig protein in both control group and N-siRNA
group were not significant, indicating that mdig siRNA is able to
specifically interfere with expression of mdig protein.
Role of mdig siRNA in of NCI-H1650
cell proliferation
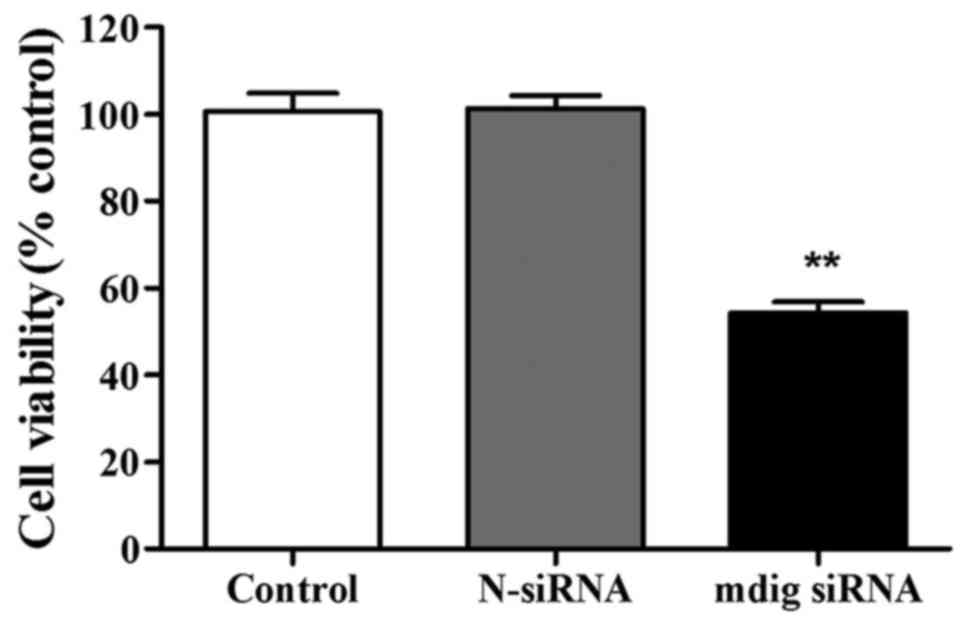
The role of mdig siRNA in the proliferation ability
of NCI-H1650 cells was detected through MTT assay. There was no
evident difference in cell viability between control group and
N-siRNA group. Cell viability was obviously inhibited in mdig siRNA
group compared to control group (p<0.01) (Fig. 3).
Effect of mdig siRNA on cell cycle of
NCI-H1650 cells
Effect of mdig siRNA on cell cycle of NCI-H1650
cells was observed via flow cytometry. Compared to control group,
the mdig siRNA group had significantly increased proportion of
cells in G1 phase (p<0.01), and clearly decreased proportion of
cells in S phase (p<0.01) (Table
III). However, in G2 phase there were no obvious changes:
proportion of cells in different phases was not changed evidently
in either the control group or N-siRNA.
 | Table III.Effect of mdig siRNA on cell cycle of
NCI-H1650 cells. |
Table III.
Effect of mdig siRNA on cell cycle of
NCI-H1650 cells.
|
| Proportion of cells
in different phases (%) |
|---|
|
|
|
|---|
| Group | G1 phase | S phase | G2/M phase |
|---|
| Control | 64.53±3.24 | 24.20±3.31 | 11.27±2.17 |
| N-siRNA | 66.38±2.95 | 22.07±3.52 | 11.55±1.98 |
| mdig siRNA |
81.45±4.33a |
9.66±1.93a | 8.89±1.13 |
Influence of mdig siRNA on of
NCI-H1650 cell apoptosis
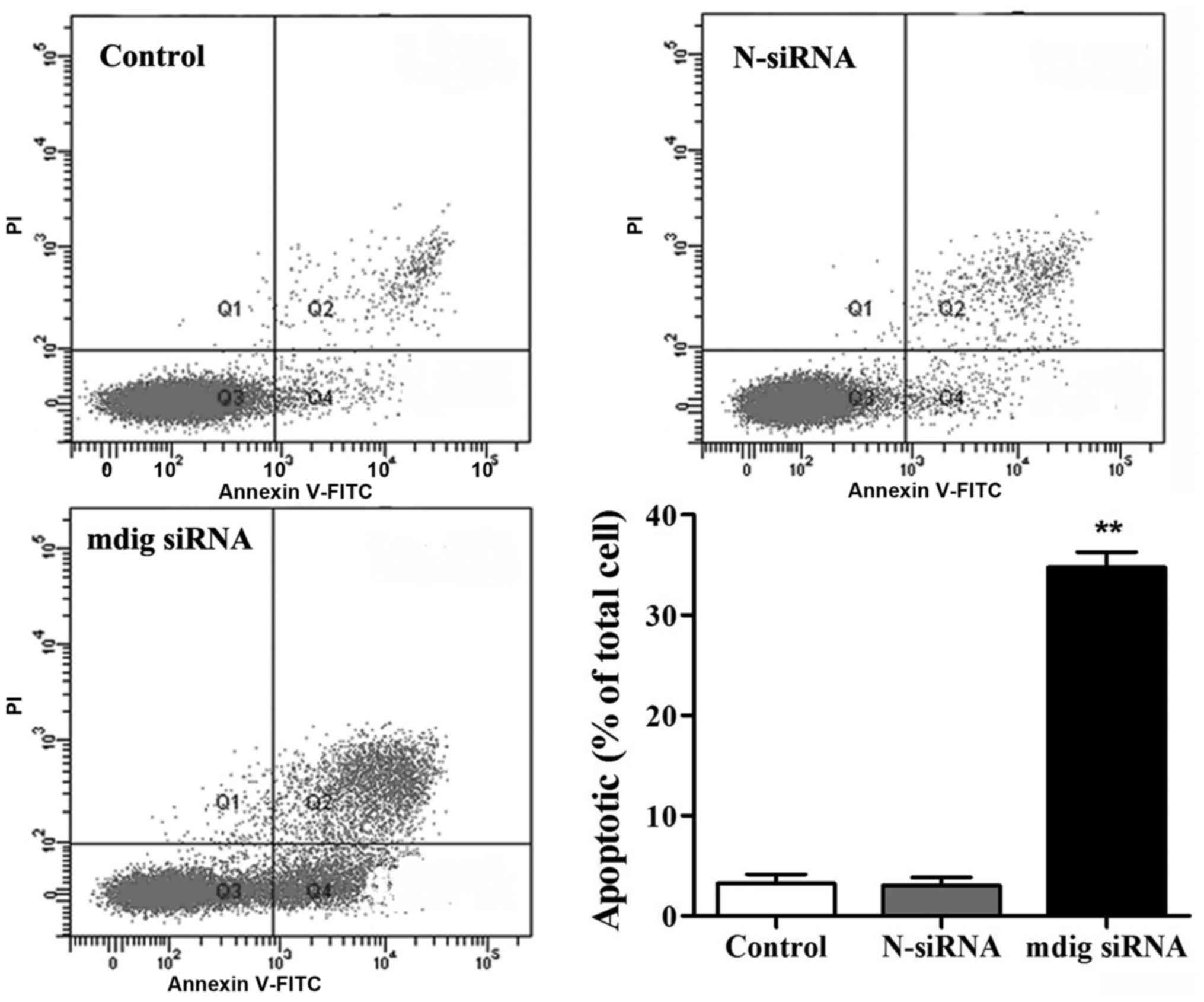
Annexin V-FITC apoptosis detection kits were used
for the detection of impact of mdig siRNA on apoptosis of NCI-H1650
cells. No obvious difference was found in apoptosis between the
control and N-siRNA groups (Fig. 4).
Compared to that in control group, the proportion of apoptotic
cells in mdig siRNA group was overtly increased (p<0.01).
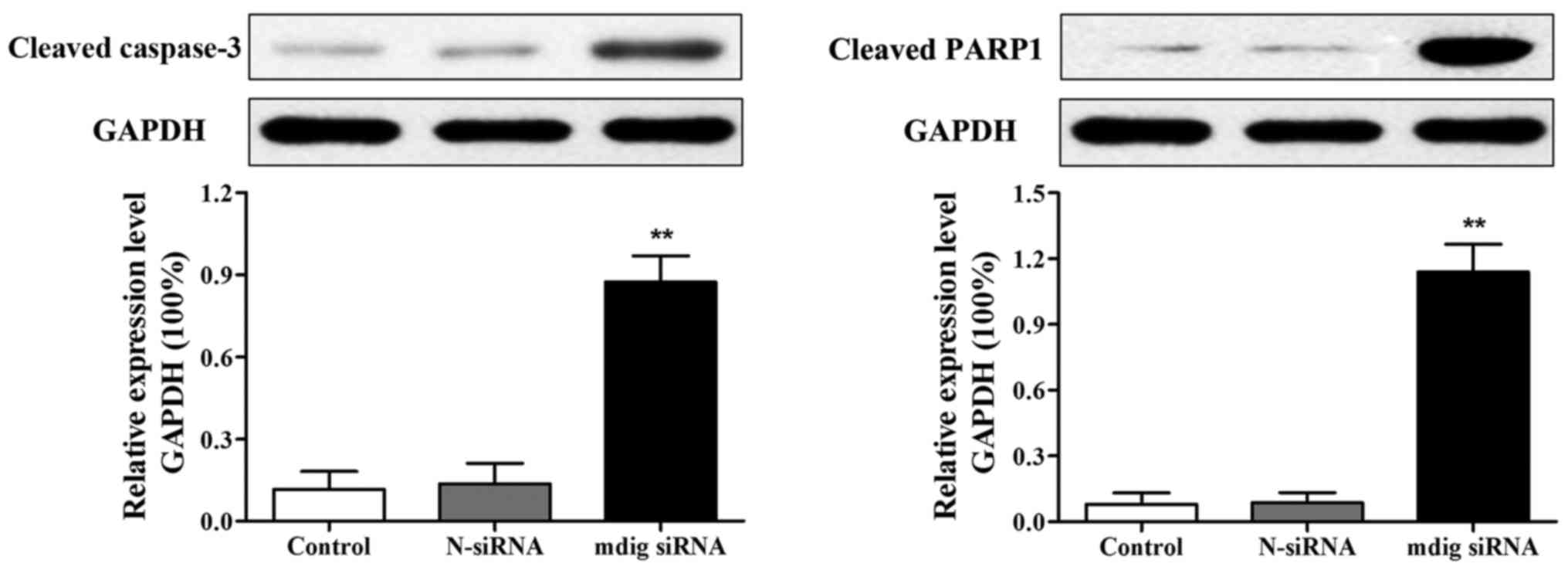
Effects of mdig siRNA on the
expression of apoptotic proteins in NCI-H1650 cells
After transfection for 48 h, the expression of
apoptotic proteins (cleaved caspase-3 and cleaved PARP1) was
measured via western blot analysis, and the results revealed that
expression levels of cleaved caspase-3 and cleaved PARP1 were
obviously increased in mdig siRNA group than those in control group
(p<0.01). No visible changes in expression levels of cleaved
caspase-3 and cleaved PARP1 were observed in control group or
N-siRNA group (Fig. 5).
Discussion
Lung cancer is a malignancy with the highest
morbidity and mortality worldwide (17,18).
Research data have shown that the incidence of lung cancer is on
the increase in China, and China may become the Top 1 in lung
cancer morbidity and mortality worldwide if no effective preventive
and control measures are taken (19).
At present, deaths caused by lung cancer are more than that due to
liver cancer and account for approximately 23% of the total deaths
caused by malignancies making lung cancer the leading cause of
death caused by malignant tumors (20). Therefore, lung cancer seriously
affects life and health, and new breakthroughs in its clinical
treatments and drugs must be made.
The mdig-encoded protein has a molecular weight of
53 kDa, and is mainly located in the nucleus. The protein contains
a conserved Jumonji C (JmjC) domain that determines the function of
mdig protein (21). A study found
that mdig is highly expressed in lung cancer, esophageal cancer,
gastric cancer and other tumors, and that mdig is a proto-oncogene
(22). A study by Zhang et al
revealed that mdig is a mineral dust-induced gene, and that the
expression level of mdig mRNA is significantly enhanced when
NCI-H1650 cells are treated with silica (23). In addition, it was found in the study
by Komiya et al that mdig is a target gene of proto-oncogene
c-myc that can induce overexpression of mdig (24). A study by Lu et al revealed
that various lung cancer cell lines have high mdig expression, and
in patients with lung cancer, expression of mdig mRNA is increased
by more than 6 times in lung cancer tissues than that in normal
tumor-adjacent tissues (25).
Caspase-3 plays a key role in the process of apoptosis; when
caspase-3 is cleaved and activated, cells enter an irreversible
process of apoptosis (26). PARP1 is
a kind of DNA repair enzyme in the nucleus, while cleaved caspase-3
can cleave and activate PARP1, leading to unrepaired cell DNA
damage, thereby resulting in apoptosis (27).
In this study, mdig siRNA was used to transfect
NCI-H1650 cells and silence mdig expression in the cell line.
RT-qPCR and western blot analysis were used to detect mRNA and
protein expression of mdig in NCI-H1650 cells. The results showed
that mdig siRNA was able to significantly decrease expression of
mRNA and protein of mdig in NCI-H1650 cells and the roles of mdig
in proliferation, cell cycle and apoptosis of NCI-H1650 cells.
Furthermore, we found that silenced mdig clearly reduced
proliferation capabilities of NCI-H1650 cells and blocked NCI-H1650
cells in G1 phase. At the same time, this study employed flow
cytometry and western blotting to observe the impact of silenced
mdig on apoptosis of NCI-H1650 cells, and the results revealed that
the apoptotic rate of NCI-H1650 cells in mdig siRNA group was
obviously increased. Western blot analysis, revealed that, silenced
mdig upregulated the expression of cleaved caspase-3 and cleaved
PARP1 proteins in NCI-H1650 cells, thereby inducing apoptosis. It
was found that in pancreatic cancer PANC-1 cells, apoptosis rate of
cells was overtly enhanced when PANC-1 cells were transfected with
mdig siRNA, suggesting that mdig is capable of inhibiting apoptosis
in PANC-1 cells (28).
In conclusion, mdig has a high expression in lung
cancer NCI-H1650 cells. In addition, mdig siRNA is able to inhibit
proliferation and promote apoptosis of NCI-H1650 cells. The
underlying mechanism may be linked to inhibited cell cycle
progression and upward expression of apoptosis proteins (cleaved
caspase-3 and cleaved PARP1).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and HL were responsible for PCR and western blot
analysis. LC and YZ helped with cell culture and siRNA
transfection. HL, ZS and YC contributed to MTT assay and flow
cytometry. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Peking Union Medical College Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marx A, Chan JK, Coindre JM, Detterbeck F,
Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, et
al: The 2015 World Health Organization Classification of Tumors of
the Thymus: Continuity and Changes. J Thorac Oncol. 10:1383–1395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Tiwari RC, Murray T, Ghafoor A,
Samuels A, Ward E, Feuer EJ and Thun MJ: American Cancer Society:
Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carrizosa DR and Gold KA: New strategies
in immunotherapy for non-small cell lung cancer. Transl Lung Cancer
Res. 4:553–559. 2015.PubMed/NCBI
|
|
8
|
Mamdani H, Induru R and Jalal SI: Novel
therapies in small cell lung cancer. Transl Lung Cancer Res.
4:533–544. 2015.PubMed/NCBI
|
|
9
|
Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi
X, Stauffer JL, Demers LM and Chen F: The Human mineral
dust-induced gene, mdig, is a cell growth regulating gene
associated with lung cancer. Oncogene. 24:4873–4882. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogasawara S, Komuta M, Nakashima O, Akiba
J, Tsuneoka M and Yano H: Accelerated expression of a Myc target
gene Mina53 in aggressive hepatocellular carcinoma. Hepatol Res.
40:330–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuneoka M, Fujita H, Arima N, Teye K,
Okamura T, Inutsuka H, Koda Y, Shirouzu K and Kimura H: Mina53 as a
potential prognostic factor for esophageal squamous cell carcinoma.
Clin Cancer Res. 10:7347–7356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Hu CM, Yuan YS, He CH, Zhao Q and
Liu NZ: Expression of Mina53 and its significance in gastric
carcinoma. Int J Biol Markers. 23:83–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishizaki H, Yano H, Tsuneoka M, Ogasawara
S, Akiba J, Nishida N, Kojiro S, Fukahori S, Moriya F, Matsuoka K,
et al: Overexpression of the myc target gene Mina53 in advanced
renal cell carcinoma. Pathol Int. 57:672–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teye K, Arima N, Nakamura Y, Sakamoto K,
Sueoka E, Kimura H and Tsuneoka M: Expression of Myc target gene
mina53 in subtypes of human lymphoma. Oncol Rep. 18:841–848.
2007.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mujica EV, Leiva ME, Rojas ME, Díaz N,
Lcaza G and Palomo GI: Discordance between nutritional status and
self perception of weight among adults from Talca, Chile. Rev Med
Chil. 137:76–82. 2009.(In Spanish). PubMed/NCBI
|
|
18
|
Tseng TS, Park JY, Zabaleta J,
Moody-Thomas S, Sothern MS, Chen T, Evans DE and Lin HY: Role of
nicotine dependence on the relationship between variants in the
nicotinic receptor genes and risk of lung adenocarcinoma. PLoS One.
9:e1072682014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wyllie AH, Kerr JFR and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma
X, Ai Y, Xu Y, Liu D, An Y, et al: China Critical Care Clinical
Trials Group: Epidemiology and outcome of severe sepsis and septic
shock in intensive care units in mainland China. PLoS One.
9:e1071812014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cakir E, Demirag E, Aydin M and Unsal E:
Clinicopathologic features and prognostic significance of lung
tumours with mixed histologic patterns. Acta Chir Belg.
109:489–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi
X, Stauffer JL, Demers LM and Chen F: The Human mineral
dust-induced gene, mdig, is a cell growth regulating gene
associated with lung cancer. Oncogene. 24:4873–4882. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Mina53, a novel c-Myc target gene, is frequently
expressed in lung cancers and exerts oncogenic property in NIH/3T3
cells. J Cancer Res Clin Oncol. 136:465–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu Y, Chang Q, Zhang Y, Beezhold K,
Rojanasakul Y, Zhao H, Castranova V, Shi X and Chen F: Lung
cancer-associated JmjC domain protein mdig suppresses formation of
tri-methyl lysine 9 of histone H3. Cell Cycle. 8:2101–2109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ,
Jiao S, Cho K and Sheng M: Caspase-3 activation via mitochondria is
required for long-term depression and AMPA receptor
internalization. Cell. 141:859–871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boulares AH, Zoltoski AJ, Yakovlev A, Xu M
and Smulson ME: Roles of DNA fragmentation factor and
poly(ADP-ribose) polymerase in an amplification phase of tumor
necrosis factor-induced apoptosis. J Biol Chem. 276:38185–38192.
2001.PubMed/NCBI
|
|
28
|
Andrade F, Casciola-Rosen LA and Rosen A:
Granzyme B-induced cell death. Acta Haematol. 111:28–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|