Introduction
According to global cancer statistics in 2012, lung
cancer (LC) was the leading cause of cancer-related mortality in
developed countries (1). Furthermore,
in less-developed countries including China and India, LC rates are
predicted to continue increasing as a result of increasing endemic
use of tobacco (1). It is well-known
that the progression of LC is the result of sequential genetic and
epigenetic changes including the dysregulation of oncogenes, tumor
suppressor genes and growth factors (2). Currently, the general approach to the
treatment of LC (non-small cell LC and small cell LC) is based on
surgical resection, chemotherapy and radiation therapy according to
the stage (3). However, it is
well-known that the intrinsic and potent cytotoxicity to normal
cells of a number of anticancer agents imposes a restriction on
their prolonged use and their therapeutic effectiveness (4). As a result, exploring novel therapeutic
methods is of urgent priority.
Chinese herbs have been considered as novel ways to
treat diseases (5). With regard to
cancer, a number of previous studies have demonstrated that
extractions of specific Chinese herbs exhibit therapeutic effects
on certain types of cancer (6–8). The exact
underlying molecular mechanism of Chinese medicine-based treatments
of cancer remain elusive.
Tanshinones (Ts), including tanshinone I, tanshinone
IIA, cryptotanshinone and dihydrotanshinone I, are a class of
lipophilic diterpene compounds extracted from Salia
miltiorrhiza Bunge, a plant root used in traditional Chinese
medicine (9). Numerous studies have
demonstrated that Ts exhibit potential properties of
anti-inflammation (10), anticancer
(11–13) and cardio-cerebrovascular protection
(14). Regarding LC, previous studies
have suggested that Ts are involved in the process of inhibiting
cell proliferation and inducing apoptosis (13,15,16).
However, the underlying molecular mechanism of the effect of Ts on
LC remains unknown.
Previous studies have demonstrated that FoxO3a, a
member of the forkhead box (Fox) gene family of transcription
factors, serves a role in the apoptotic cascade (17,18), which
suggests that targeting the AMP-activated protein kinase-FoxO3a
axis is a potential therapeutic approach for cancer treatment
(19). Previous study has
demonstrated that silencing FoxO3a prevents control of the
apoptotic cascade due to the inhibition of mitochondrial membrane
depolarization. In contrast, the presence of FoxO3a is the
prerequisite for cleaved caspase-3 expression (17). On the basis of these previous studies,
we hypothesize that FoxO3a mediates the activity of Ts, regulating
LC cell proliferation and apoptosis through caspase-3
activation.
Materials and methods
Cell lines and cell culture
The human lung cancer cell line A549 was obtained
from PeproTech, Inc. (Rocky Hill, NJ, USA). The cells were
routinely cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified cell incubator containing
5% CO2.
Plasmid construction
The plasmid (pMSCV-puro) containing FoxO3a small
interfering RNA (siRNA) were constructed by Guangzhou Ribobio Co.,
Ltd. (Guangzhou, China). The sequences were as follows: siRNA1,
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′;
siRNA2, 5′-ACUCCGGGUCCAGCUCCACTT-3′ and
5′-GUGGAGCUGGACCCGGAGUTT-3′; siRNA3, 5′-GGAACGUGAUGCUUCGCAATT-3′
and 5′-UUGCGAAGCAUCACGUUCCGG-3′. Scrambled siRNA (ssiRNA) was
purchased from Invitrogen; Thermo Fisher Scientific, Inc., and with
sequences of 5′-CGUUCACGCACCAAUUCUATT-3′ and
5′-UAGAAUUGGUGCGUGAACGGA-3′.
Transfection
For transfection, 5×106 A549 cells were
seeded in 6-well culture plates until reaching 70% confluence.
Subsequently, transfection of cells with recombinant
FoxO3a-siRNA/ssiRNA plasmids was performed using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. At 8–12 h after transfection, the morphology of
transfected cells was observed using a fluorescence microscope
(Olympus Corporation, Tokyo, Japan) at ×200 magnification, and the
most successfully transfected experimental and control (CON) cells
were selected to for treatment with Ts in the subsequent
experiments.
Preparation of Ts and experimental
groups
Ts (Xian Yuensun Biological Technology, Xi'an,
Shaanxi, China) were dissolved at 25 mmol/l in absolute ethyl
alcohol and stored at 4°C until use. The transfected A549 cells
were divided into four groups and treated with Ts as follows: 5
µmol/l Ts (group I), 10 µmol/l Ts (group II), 20 µmol/l Ts (group
III), 30 µmol/l Ts (group IV) and the negative control of
ssiRNA-transfected cells without Ts administration (CON group).
Measurement of cell proliferation
Cell proliferation was determined using an MTT assay
(Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. Transfected A549 cells
were seeded onto 96-well plates (5×103 cells/well). The
cell proliferation was measured every 24 h for 3 days. The
absorbance of cells was screened at 570 nm using a microplate
reader (SM600; Shanghai Utrao Medical Instrument Co., Ltd.,
Shanghai, China).
Analysis of cell apoptosis
For analysis of apoptosis, the proportions of
apoptotic cells were determined using an Annexin V-Fluorescein
Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis Detection kit
(Beijing Solarbio Science and Technology Co., Ltd., Beijing,
China), according to the manufacturer's protocol. Briefly, the
transfected A549 cells of each group were cultured for 72 h,
collected by trypsinization (Beijing Dingguo Changseng
Biotechnology Co., Ltd., Beijing, China) and washed with PBS. The
cells were subsequently resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. Subsequently, 5 µl
Annexin V-FITC was added to the cell suspension, prior to
incubation at 4°C for 15 min in darkness. Subsequently, 10 µl PI
was added to the samples, mixed gently and incubated at 4°C for 5
min in darkness. Finally, the cells were screened using flow
cytometry (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from transfected A549 cells of each
experimental group was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The RT and PCR primers for FoxO3a
and GAPDH (internal control) were obtained from KareBay Biochem,
Inc. (Monmouth Junction, NJ, USA). The PCR primers for FoxO3a were
5′-CGTGGGTAAAAAGGTGTTCC-3′ (forward) and 5′-CAAGCCTCCAAACTCAGGAC-3′
(reverse); the primers for GAPDH were 5′-TCGTGGAAGGACTCATGACC-3′
(forward) and 5′-AGGGATGATGTTCTGGAGAG-3′ (reverse). The PrimeScript
RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) was
used to synthesize the first-strand cDNA. The cycling program was
as follows: Initiation at 37°C for 10 min, followed by 85°C for 5
sec, then 4°C for 10 min. PCR was performed using SYBR Premix Ex
Taq (Guangzhou Ribobio Co., Ltd.). Quantitative RT-PCR was
performed using the PCR 7900HT Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The cycling program
was as follows: Initiation at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and at 60°C for 34 sec. The relative
expression level of each circRNA was analyzed using the ΔΔCq method
(20).
Western blot analysis
The cells of each group were collected, centrifuged
(500 × g at 4°C for 15 min) and transferred into clean test tubes.
A 400 µl volume of lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) was added to each tube to lyse the
cells at −10°C for 30 min, then centrifuged at 500 × g at 4°C for
10 min. The formula of the lysis buffer was: Collagenase type IV (1
mg/ml), DNase type I (100 µg/ml), CaCl2 (2 mmol/l) and
MgCl2 (2 mmol/l). The protein concentration of the
lysates was examined using the Bradford method (21). Lysates (200 µl) were then boiled,
electrophoresed (4% SDS) and transferred onto nitrocellulose
membranes (CST Biological Reagents Co., Ltd., Shanghai, China).
Non-fat milk solution (10%) in Tris-buffered saline solution with
Tween-20 (0.5 ml/l) was used to block the membranes, which were
incubated at 25°C for 2 h. Subsequently, 10 µl mouse anti-human
FoxO3a monoclonal antibody (cat. no. 05-1075-25UG; 1:250; EMD
Millipore, Billerica, MA, USA) and mouse anti-human caspase-3 (cat.
no. ab1271; 1:1,000; Abcam, Cambridge, UK) were added and incubated
at room temperature for 2 h, followed by 5 µl alkaline
phosphatase-conjugated goat anti-mouse IgG secondary antibody (cat.
no. 115-055-006; 1:50; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA). Signals were screened using an automated
chemiluminescence immunoassay analyzer (Beckman Coulter, Inc.).
Statistical analysis
All statistical analysis was carried out using the
SPSS software package (version 12.0; SPSS, Inc., Chicago, IL, USA)
and GraphPad Prism 5.00 software (GraphPad Software, Inc., La
Jolla, CA). For comparisons between two groups, Student's t-test
was performed. For comparisons between multiple groups, a
Student-Newman-Keuls test was performed. P<0.05 was considered
to indicate a statistically significant difference.
Results
Recombinant FoxO3a siRNA and ssiRNA
were successfully transfected
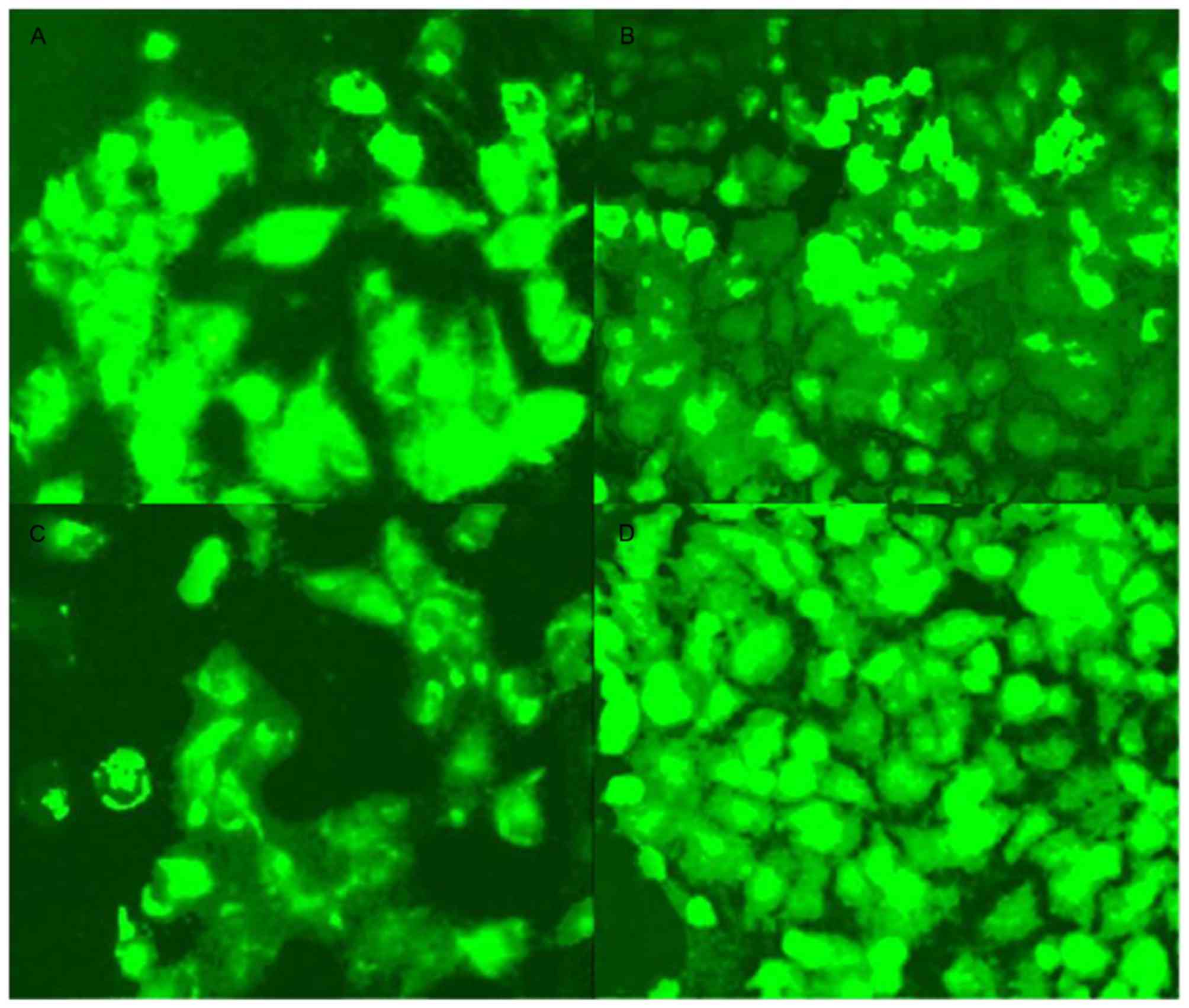
The transfected A549 cells expressed green
fluorescence as determined using fluorescence microscopy. As
presented in Fig. 1, the transfection
efficiency of siRNA2 was higher than that of siRNA1 and siRNA3
(P<0.05), with similar results in comparison with those for a
negative control. As a result, siRNA2-transfected cells were
selected as the culture containing the most successfully
transfected cells to use for further experiments.
Ts inhibit the proliferation of LC
cells
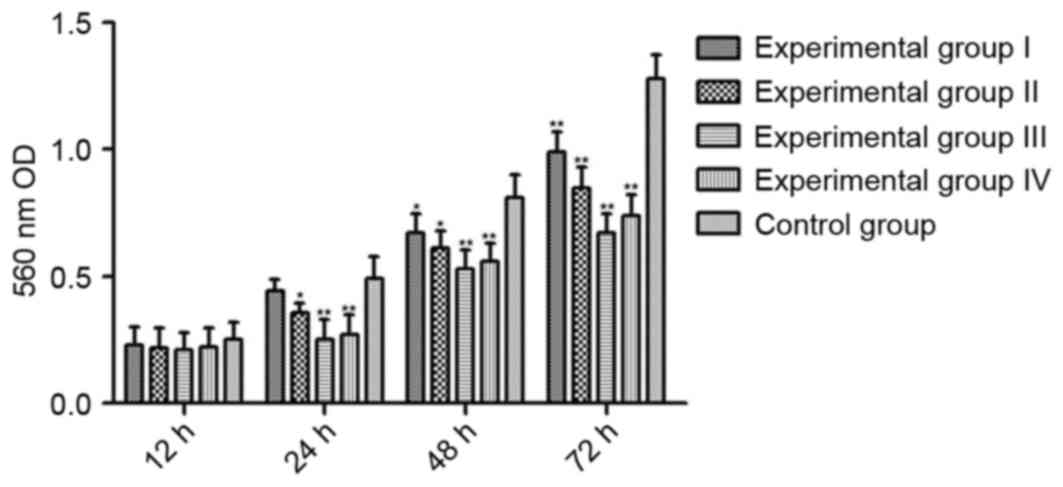
To detect cell viability, the absorbance of cells in
each group was screened at 24, 48 and 72 h using an MTT assay. As
presented in Fig. 2, Ts inhibited the
cell viability in a dose- and time-dependent manner with a maximal
dose of 20 µmol/l at 72 h treatment.
Ts induce LC cell apoptosis
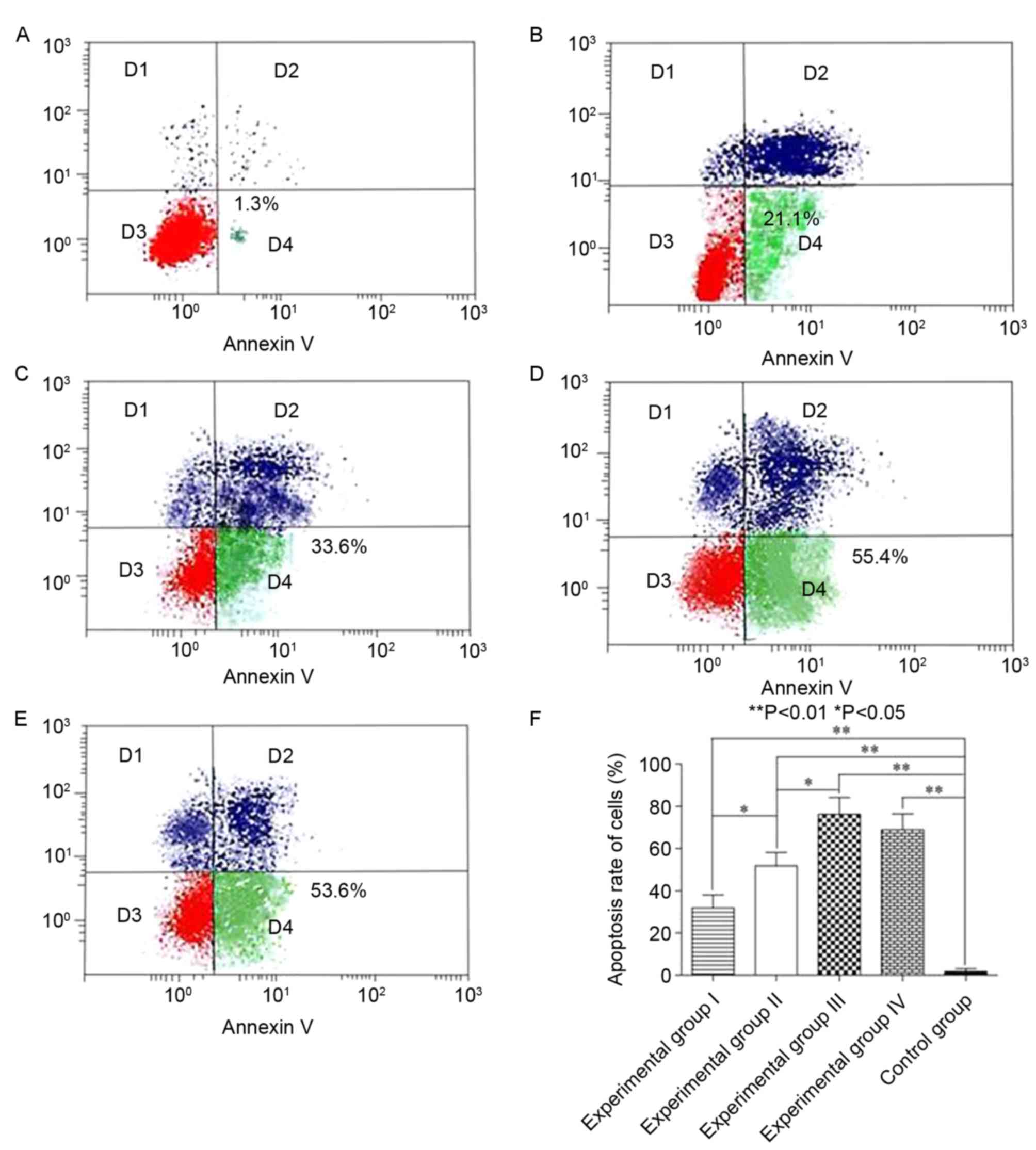
Following treatment of the cells of each group with
Ts for 72 h, the effect of Ts was examined using the Annexin
V-FITC/PI Apoptosis Detection kit. The proportion of apoptotic
cells in each experimental group was increased compared with that
of CON (P<0.05), and dose-dependence was exhibited by
experimental groups I–III (P<0.05; Fig. 3). Similarly, no statistically
significant difference was identified in experimental groups III
and IV, suggesting that 20 µmol/l may be the most appropriate
concentration to treat LC cells.
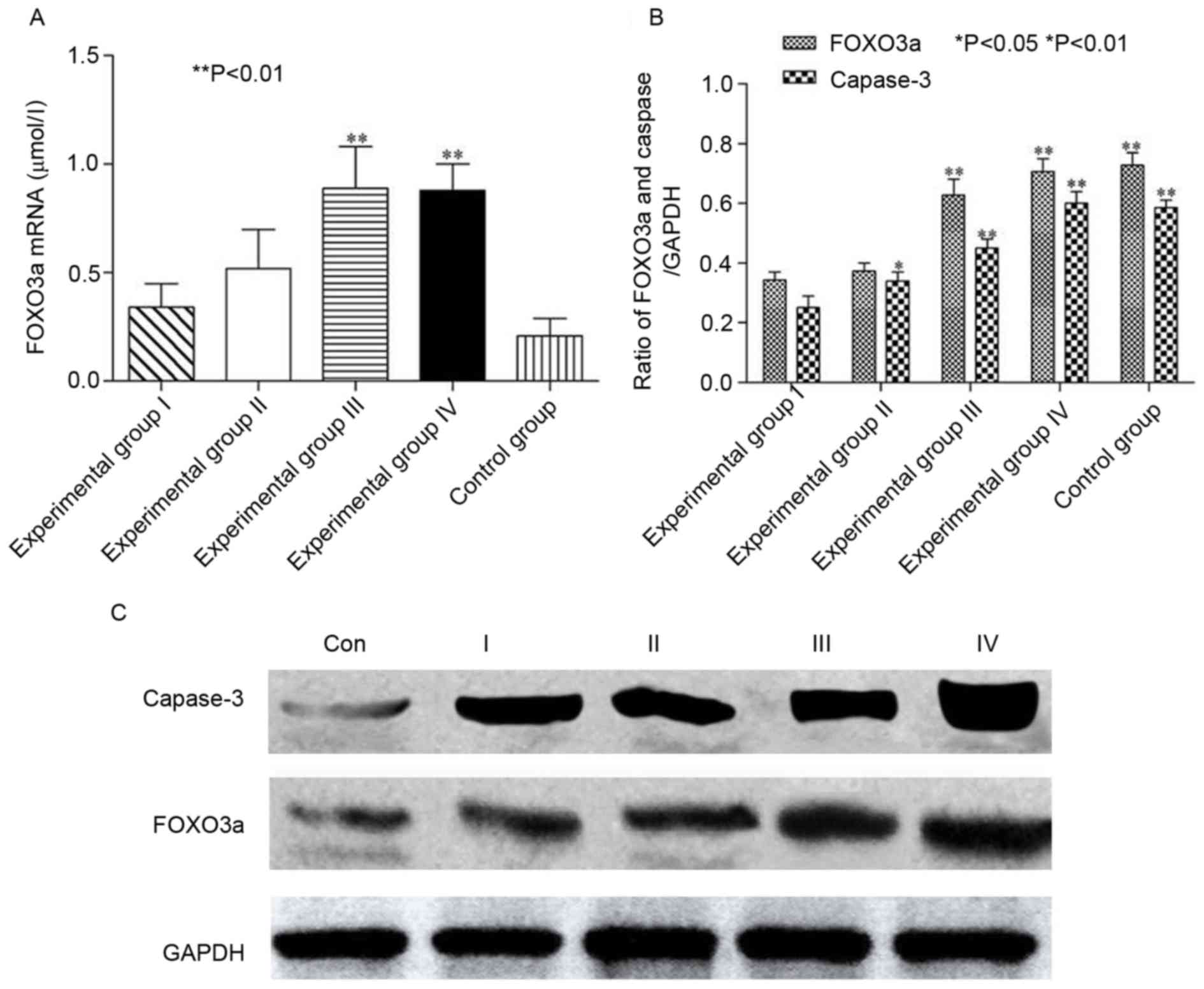
Ts upregulate the expression of FoxO3a mRNA. As
presented in Fig. 4A, the expression
of FoxO3a mRNA in experimental groups III and IV was increased
compared with that of CON (P<0.05). Similarly, a dose-dependent
association was identified in experimental groups I–III, but not in
experimental groups III and IV.
Ts modulate FoxO3a and caspase 3
As presented in Fig. 4B
and C, compared with the CON group, the expression of FoxO3a
increased as the protein concentration was increased, indicating
that FOXO3a was associated with the dosage of Ts in experimental
groups I–III (P<0.05), which was consistent with the results of
cell proliferation and apoptosis. Simultaneously, as FoxO3a protein
was restored, caspase-3 was activated, suggesting that Ts were able
to restore the function of FOXO3a, which governs apoptotic
transduction pathways by inducing caspase-3 activity.
Discussion
FoxO3a has been recognized as a potential tumor
suppressor of lung adenocarcinoma, the expression of which is
associated with the aggressiveness of the cancer (22–24).
Certain Chinese medicines, including berberine, have been
demonstrated to contribute to the inhibition of LC cell
proliferation and the induction of apoptosis by regulating the
expression of FoxO3a, which serves a role in the activation of the
p38a mitogen- activated protein kinase signaling pathway (25). Although the results of previous
studies suggest that Ts are implicated in anticancer activities
including dysregulation of cell proliferation, apoptosis, cell
cycle arrest, metastasis, differentiation and angiogenesis
(15,16,26–28), the
underlying molecular mechanism remains unknown. As a result, the
aim of the present study on LC cell treatment with Ts was to
investigate the potential signaling pathway mediated by FoxO3a.
To address the aforementioned questions, effective
transfected LC cells were established by silencing FoxO3a, whereas
the CON cells transfected with FoxO3a ssiRNA retained intact FoxO3a
in LC cells. In addition, construction of the siRNA nucleotide
sequence was optimized with processes including the presence of a
phosphate group at the 5′-terminus and the strand with a less
stable 5′-end (29). The successful
knockdown of FoxO3a provided a good foundation for the subsequent
experiments.
Dysregulation of cell proliferation is the critical
characteristic of LC, therefore four distinct concentrations of Ts
(5, 10, 20 and 30 µmol/l) were used to treat the experimental
groups. The absorbance of experimental groups with FoxO3a knockdown
decreased in a dose- and time-dependent manner when compared with
that of CON, excluding the concentration of 30 µmol/l, which
indicates that Ts inhibit LC cell proliferation. Additionally, the
results demonstrated that the optimal cell inhibition ratio was
with 20 µmol/l at 72 h, so subsequent measurements were made at the
72 h time point.
Circumventing of cell apoptosis is another feature
of LC. In accordance with a previous study of the effect of Ts on
LC (15), the results of the present
study illustrated that Ts induce cell apoptosis in a dose-dependent
manner; however, experimental group IV did not continue the trend
of dose-dependence. Cells transfected with non-specific scrambled
siRNA without Ts did not exhibit markedly early and late programs
of apoptosis. In contrast, experimental groups demonstrated
increased proportions of apoptotic cells, identifying the unique
ability of Ts to control the early and late apoptotic programs.
Furthermore, the results of the present study
demonstrated that the expression levels of FoxO3a protein in cells
treated with Ts were increased compared with those in CON cells,
consistent with the expression of FoxO3a mRNA with RT-PCR. Previous
studies have demonstrated that silencing FoxO3a depletes the
induction of caspase-3 activity (30). The results of the present study
support this hypothesis by restoring the expression of FoxO3a with
Ts treatment, inducing apoptosis by activating a downstream
molecule, caspase-3.
The results of the present study reveal that Ts may
serve a direct role in regulating LC cell proliferation and
apoptosis, mediated by FoxO3a activation; however, further studies
are required to determine the exact signaling pathways mediated by
Ts. Addressing this deficit in our knowledge may lead to novel
therapeutic approaches to treat LC clinically.
Acknowledgements
The authors would like to thank Dr. Zhuang Yongzhi,
chief physician of Daqing Oilfield General Hospital, and chief
surgeon Dr. Zhou Daming of the Oncology Department of Daqing
Oilfield General Hospital for their guidance in the present
research. The authors would also like to thank Dr. Zhang Liangyu,
deputy chief physician of the Oncology Department of Daqing
Oilfield General Hospital for their help and support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DJL, FY and DHZ made substantial contributions to
conception and design, RBY analysis and interpretation of data. DJL
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LC
|
lung cancer
|
|
Ts
|
tanshinones
|
|
siRNA
|
small interfering RNA
|
|
FoxO3a
|
forkhead box O3a
|
|
ssiRNA
|
scrambled siRNA
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Franklin WA, Gazdar AF and Bunn
PA Jr: Early detection of lung cancer: Clinical perspectives of
recent advances in biology and radiology. Clin Cancer Res. 7:5–22.
2001.PubMed/NCBI
|
|
3
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ho C, Ramsden K, Zhai Y, Murray N, Sun S,
Melosky B and Laskin J: Less toxic chemotherapy improves uptake of
all lines of chemotherapy in advanced non-small-cell lung cancer: A
10-year retrospective population-based review. J Thorac Oncol.
9:1180–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Greef J: Perspective: All systems
go. Nature. 480:S872011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Tang Q, Zhao S, Zheng F, Wu Y, Tang
G and Hahn SS: Extracellular signal-regulated kinase
signaling-mediated induction and interaction of FOXO3a and p53
contribute to the inhibition of nasopharyngeal carcinoma cell
growth by curcumin. Int J Oncol. 45:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang WG, Ye L, Ji K, Frewer N, Ji J and
Mason MD: Inhibitory effects of Yangzheng Xiaoji on angiogenesis
and the role of the focal adhesion kinase pathway. Int J Oncol.
41:1635–1642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao L, Li Q, Jiang M, Liu C, Song Z, Bao
X, Shen Y, Liu G and Hu K: Combined therapy of percutaneous
cryoablation and traditional Chinese medicine can be a promising
strategy for elderly or advanced lung cancer patients based on a
retrospective clinical study. Cryobiology. 69:174–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng S, Ren Z, Zhang Y and Qiao Y:
Anti-inflammatory mechanism research of tanshinone II A by
module-based network analysis. Biomed Mater Eng. 24:3815–3824.
2014.PubMed/NCBI
|
|
11
|
Su CC, Chen GW and Lin JG: Growth
inhibition and apoptosis induction by tanshinone I in human colon
cancer Colo 205 cells. Int J Mol Med. 22:613–618. 2008.PubMed/NCBI
|
|
12
|
Gong Y, Li Y, Abdolmaleky HM, Li L and
Zhou JR: Tanshinones inhibit the growth of breast cancer cells
through epigenetic modification of Aurora A expression and
function. PLoS One. 7:e336562012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Bioactive tanshinone I inhibits the growth of lung cancer
in part via downregulation of Aurora A function. Mol Carcinog.
52:535–543. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
16
|
Lee CY, Sher HF, Chen HW, Liu CC, Chen CH,
Lin CS, Yang PC, Tsay HS and Chen JJ: Anticancer effects of
tanshinone I in human non-small cell lung cancer. Mol Cancer Ther.
7:3527–3538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou J, Chong ZZ, Shang YC and Maiese K:
FOXO3a governs early and late apoptotic endothelial programs during
elevated glucose through mitochondrial and caspase signaling. Mol
Cell Endocrinol. 321:194–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao GX, Xu LH, Pan H, Lin QR, Huang MY,
Cai JY, Ouyang DY and He XH: The BH3-mimetic gossypol and
noncytotoxic doses of valproic acid induce apoptosis by suppressing
cyclin-A2/Akt/FOXO3a signaling. Oncotarget. 6:38952–38966. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiacchiera F and Simone C: The
AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle.
9:1091–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kruger NJ: The bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
22
|
Herzog CR, Blake DC Jr, Mikse OR,
Grigoryeva LS and Gundermann EL: FoxO3a gene is a target of
deletion in mouse lung adenocarcinoma. Oncol Rep. 22:837–843. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blake DC Jr, Mikse OR, Freeman WM and
Herzog CR: FOXO3a elicits a pro-apoptotic transcription program and
cellular response to human lung carcinogen nicotine-derived
nitrosaminoketone (NNK). Lung Cancer. 67:37–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu HB, Gao XX, Zhang Q, Liu J, Cui Y, Zhu
Y and Liu YF: Expression and prognostic implications of FOXO3a and
Ki67 in lung adenocarcinomas. Asian Pac J Cancer Prev.
16:1443–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li
L, Wu W and Hann S: p38α MAPK-mediated induction and interaction of
FOXO3a and p53 contribute to the inhibited-growth and
induced-apoptosis of human lung adenocarcinoma cells by berberine.
J Exp Clin Cancer Res. 33:362014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho TF and Chang CC: A promising ‘TRAIL’ of
tanshinones for cancer therapy. Biomedicine (Taipei). 5:232015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma ZL, Zhang BJ, Wang DT, Li X, Wei JL,
Zhao BT, Jin Y, Li YL and Jin YX: Tanshinones suppress AURKA
through up-regulation of miR-32 expression in non-small cell lung
cancer. Oncotarget. 6:20111–20120. 2015.PubMed/NCBI
|
|
28
|
Gao H, Sun W, Zhao W, Hao W, Leung CH, Lu
J and Chen X: Total tanshinones-induced apoptosis and autophagy via
reactive oxygen species in lung cancer 95D cells. Am J Chin Med.
43:1265–1279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alagia A and Eritja R: siRNA and RNAi
optimization. Wiley Interdiscip Rev RNA. 7:316–329. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang YC, Chong ZZ, Hou J and Maiese K:
The forkhead transcription factor FOXO3a controls microglial
inflammatory activation and eventual apoptotic injury through
caspase 3. Curr Neurovasc Res. 6:20–31. 2009. View Article : Google Scholar : PubMed/NCBI
|