Introduction
Cancers of adolescents and young adults (AYAs) have
distinct biological features compared to both pediatric and adult
cancers (1). AYAs face many life
events, such as school admission, employment, marriage, childbirth,
and raising children. Cancers in AYAs greatly influence their lives
after cancer treatment. Therefore, AYAs with cancer need specific
support during and after cancer treatment (2). However, cancer in AYAs accounts for only
a few cancers and there are various types (3). Consequently, insufficient attention has
been paid to cancers in AYAs. Unmet service needs are present in
cancers in AYAs, particularly financial, mental health, and support
group services (4).
There is no universal definition of AYAs. The
National Cancer Institute Surveillance, Epidemiology, and End
Results (SEER) program in the United States (3) and other countries (5) initially defined AYAs as those aged
between 15 and 29 years old. Recently, however, the program defined
widened that range to 15–39 years (6).
In Japan, two reports of cancers in AYAs, defined as
from 15 to 39 years old, were published using data from the
Monitoring Cancer Incidence in Japan Project for 2009–2011
(7) and 2011–2014 (8). No detailed studies have described the
epidemiology, treatment outcomes, and social status of cancers in
AYAs in Japan.
The distribution of cancer types in adults that are
30–39 years old is similar to that in those 40 years and older
(7–9),
and life events such as schooling, employment, and marriage are
mainly experienced at 15–29 years of age. Therefore, we defined
AYAs as being from 15–29 years old and analyzed the incidence,
cancer types, treatment methods, and outcomes of cancer in this
group. We also analyzed the family and social status of AYA
patients in our institution.
Materials and methods
Definition of AYAs and cancer
classification
We defined AYAs as being 15–29 years old and
categorized AYAs according to sex by age groups in 5-year
increments (15–19, 20–24, and 25–29 years). Cancer types were
classified according to the SEER AYA Site Recode Classification/WHO
2008 Definition (10). We included
cases of carcinoma in situ and benign tumors in the central
nervous system and intracranial neoplasms.
Patients and data collection
This study was approved by the Ethics Review
Committee of the School of Medicine of Niigata University (receipt
no. 2611). In this retrospective study, we collected data from a
hospital-based cancer registry and from electronic medical charts
at Niigata University Medical and Dental Hospital from 2007 to
2015.
We analyzed the types of cancer, treatment methods
and outcomes, fertility preservation, marital status, whether the
patient had children, school admission, and employment status. The
treatment methods were classified as surgery, radiation,
chemotherapy, endocrine treatment, other treatment, and no
treatment. Fertility preservation was classified as sperm banking,
fertility-sparing surgery, chemotherapy withdrawal, egg freezing,
others, and unknown. Marital status was classified as married, not
married, divorced, widowed, and unknown. Marital status, parenting
status, and employment were surveyed at the date of diagnosis and
last date confirmed. Employment was classified as full-time work,
full-time school, part-time work, part-time school, housewife,
unemployed, and unknown.
Statistical analyses
Survival curves were drawn using the Kaplan-Meier
method. The log-rank test was used to compare survival in each
group. Fisher's exact test was used to compare the two groups. For
multiple comparisons, the Bonferroni correction was used. P<0.05
was considered to be significant in two groups comparison,
P<0.017 in three group comparisons (e.g., age group),
P<0.0033 in six group comparisons (e.g., cancer types). All
calculations were performed using SPSS Statistics, version 22.0
software (IBM Corp., Armonk, NY, USA).
Results
Types of cancer and distribution
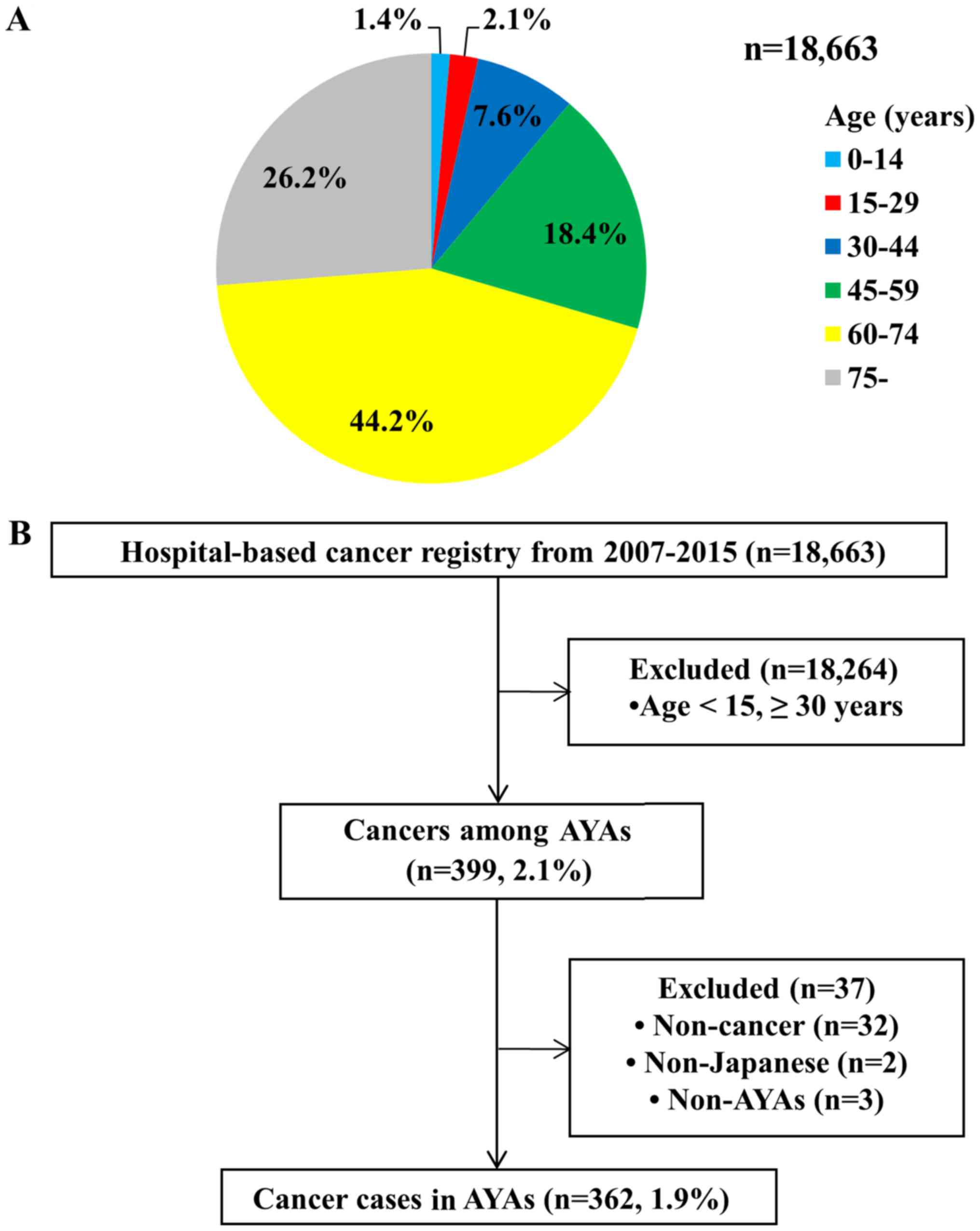
We identified 399 cancer cases in AYAs (2.1%) from
the 18,663 cases in the hospital-based cancer registry from 2007 to
2015 (Fig. 1A). Of these, patients
were excluded if non-cancer was the final diagnosis after surgery
or pathological analyses (n=32; e.g., severe dysplasia in the
uterine cervix), they were not Japanese (n=2), or they were younger
than 15 years old at the date of diagnosis (n=3). Ultimately, we
identified 362 (1.9%) cancer cases in AYAs (Fig. 1B). Table
I shows the age- and sex-specific distributions according to
the SEER AYA Site Recode Classification. There were more cases of
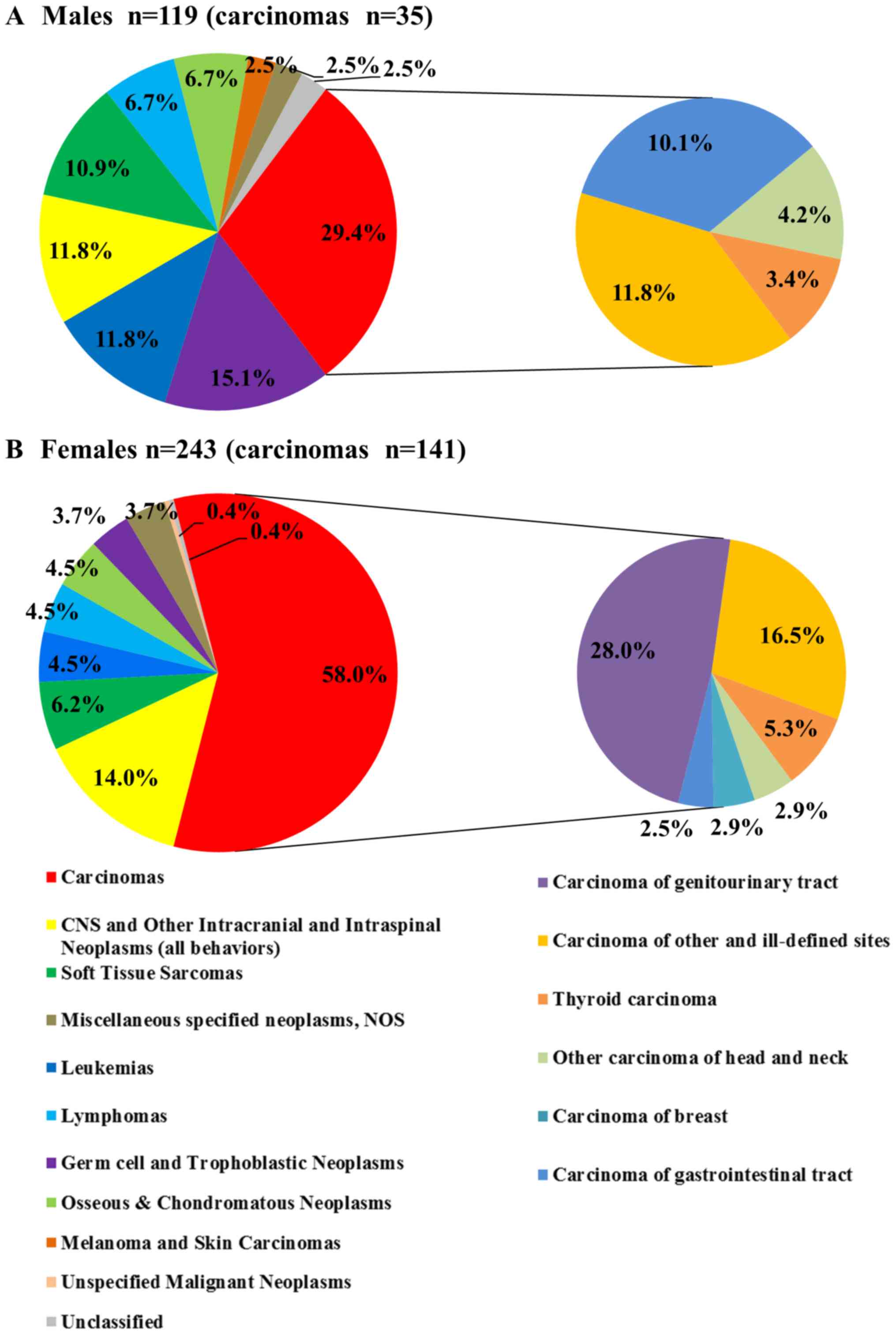
females (243 cases) than males (119 cases) (Fig. 2).
 | Table I.Cancer classification (age and sex
specific distribution). |
Table I.
Cancer classification (age and sex
specific distribution).
| Site group | Total, n (%) | Male, n (%) | Female, n (%) | Age group, 15–19, n
(%) | Age group, 20–24, n
(%) | Age group 25–29, n
(%) |
|---|
| Leukemias | 25 (6.9) | 14 (3.9) | 11 (3.0) | 8
(2.2) | 9
(2.5) | 8
(2.2) |
| Acute
lymphoid leukemia | 7
(1.9) | 4
(1.1) | 3
(0.8) | 3
(0.8) | 3
(0.8) | 1
(0.3) |
| Acute
myeloid leukemia | 15 (4.1) | 7
(1.9) | 8
(2.2) | 4
(1.1) | 5
(1.4) | 6
(1.7) |
| Chronic
myeloid leukemia | 3
(0.8) | 3
(0.8) | 0 | 1
(0.3) | 1
(0.3) | 1
(0.3) |
| Other and
unspecified leukemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphomas | 19 (5.2) | 8
(2.2) | 11 (3.0) | 6
(1.7) | 5
(1.4) | 8
(2.2) |
|
Non-Hodgkin lymphoma | 15 (4.1) | 7
(1.9) | 8
(2.2) | 4
(1.1) | 4
(1.1) | 7
(1.9) |
| Hodgkin
lymphoma | 4
(1.1) | 1
(0.3) | 3
(0.8) | 2
(0.6) | 1
(0.3) | 1
(0.3) |
| CNS and other
intracranial and intraspinal neoplasms (all behaviors) | 48 (13.3) | 14 (3.9) | 34 (9.4) | 10 (2.8) | 18 (5.0) | 20 (5.5) |
|
Astrocytoma | 11 (3.0) | 2
(0.6) | 9
(2.5) | 1
(0.3) | 3
(0.8) | 7
(1.9) |
|
Specified
low-grade astrocytic tumors | 3
(0.8) | 0 | 3
(0.8) | 0 | 1
(0.3) | 2
(0.6) |
|
Astrocytoma, NOS
Glioblastoma and anaplastic astrocytoma | 4
(1.1) | 2
(0.6) | 2
(0.6) | 0 | 1
(0.3) | 3
(0.8) |
|
Astrocytoma,
NOS | 4
(1.1) | 0 | 4
(1.1) | 1
(0.3) | 1
(0.3) | 2
(0.6) |
| Other
glioma | 10 (2.8) | 3
(0.8) | 7
(1.9) | 0 | 3
(0.8) | 7
(1.9) |
|
Ependymoma | 4
(1.1) | 1
(0.3) | 3
(0.8) | 2
(0.6) | 1
(0.3) | 1
(0.3) |
|
Medulloblastoma and other
PNET | 2
(0.6) | 1
(0.3) | 1
(0.3) | 0 | 1
(0.3) | 1
(0.3) |
|
Medulloblastoma | 2
(0.6) | 1
(0.3) | 1
(0.3) | 0 | 1
(0.3) | 1
(0.3) |
|
Supratentorial
PNET | 0 | 0 | 0 | 0 | 0 | 0 |
| Other
specified intracranial and intraspinal neoplasms | 14 (3.9) | 3
(0.8) | 11 (3.0) | 3
(0.8) | 7
(1.9) | 4
(1.1) |
|
Unspecified intracranial and
intraspinal neoplasms | 7
(1.9) | 4
(1.1) | 3
(0.8) | 4
(1.1) | 3
(0.8) | 0 |
|
Unspecified
malignant intracranial and intraspinal neoplasms | 4
(1.1) | 3
(0.8) | 1
(0.3) | 3
(0.8) | 1
(0.3) | 0 |
|
Unspecified
ben/border intracranial and intraspinal neoplasms | 3
(0.8) | 1
(0.3) | 2
(0.6) | 1
(0.3) | 2
(0.6) | 0 |
| Osseous and
chondromatous neoplasms | 19 (5.2) | 8
(2.2) | 11 (3.0) | 12 (3.3) | 4
(1.1) | 3
(0.8) |
|
Osteosarcoma | 12 (3.3) | 4
(1.1) | 8
(2.2) | 9
(2.5) | 1
(0.3) | 2
(0.6) |
|
Chondrosarcoma | 2
(0.6) | 0 | 2
(0.6) | 0 | 1
(0.3) | 1
(0.3) |
| Ewing
tumor | 3
(0.8) | 2
(0.6) | 1
(0.3) | 1
(0.3) | 2
(0.6) | 0 |
| Other
specified and unspecified bone tumors | 2
(0.6) | 2
(0.6) | 0 | 2
(0.6) | 0 | 0 |
| Soft Tissue
Sarcomas | 28 (7.7) | 13 (3.6) | 15 (4.1) | 10 (2.8) | 7
(1.9) | 11 (3.0) |
|
Fibromatous neoplasms | 5
(1.4) | 2
(0.6) | 3
(0.8) | 1
(0.3) | 0 | 4
(1.1) |
|
Rhabdomyosarcoma | 2
(0.6) | 1
(0.3) | 1
(0.3) | 2
(0.6) | 0 | 0 |
| Other
soft tissue sarcoma | 21 (5.8) | 10 (2.8) | 11 (3.0) | 7
(1.9) | 7
(1.9) | 7
(1.9) |
|
Specified soft
tissue sarcoma | 19 (5.2) | 8
(2.2) | 11 (3.0) | 7
(1.9) | 6
(1.7) | 6
(1.7) |
|
Specified
(excluding Kaposi sarcoma) | 19 (5.2) | 8
(2.2) | 11 (3.0) | 7
(1.9) | 6
(1.7) | 6
(1.7) |
|
Kaposi
sarcoma | 0 | 0 | 0 | 0 | 0 | 0 |
|
Unspecified soft
tissue sarcoma | 2
(0.6) | 2
(0.6) | 0 | 0 | 1
(0.3) | 1
(0.3) |
| Germ cell and
Trophoblastic Neoplasms | 27 (7.5) | 18 (5.0) | 9
(2.5) | 14 (3.9) | 8
(2.2) | 5
(1.4) |
| Germ
cell and trophoblastic neoplasms of gonads | 13 (3.6) | 7
(1.9) | 6
(1.7) | 6
(1.7) | 4
(1.1) | 3
(0.8) |
| Germ
cell and trophoblastic neoplasms of nongonadal sites | 14 (3.9) | 11 (3.0) | 3
(0.8) | 8
(2.2) | 4
(1.1) | 2
(0.6) |
|
Intracranial (all
behaviors) | 11 (3.0) | 8
(2.2) | 3
(0.8) | 6
(1.7) | 4
(1.1) | 1
(0.3) |
|
Other
nongonadal | 3
(0.8) | 3
(0.8) | 0 | 2
(0.6) | 0 | 1
(0.3) |
| Melanoma and Skin
Carcinomas | 3
(0.8) | 3
(0.8) | 0 | 0 | 1
(0.3) | 2
(0.6) |
|
Melanoma | 2
(0.6) | 2
(0.6) | 0 | 0 | 1
(0.3) | 1
(0.3) |
| Skin
carcinomas | 1
(0.3) | 1
(0.3) | 0 | 0 | 0 | 1
(0.3) |
| Carcinomas | 176 (48.6) | 35 (9.7) | 141 (39.0) | 15 (4.1) | 45
(12.4) | 116 (32.0) |
| Thyroid
carcinoma | 17
(4.7) | 4
(1.1) | 13 (3.6) | 3 (0.8) | 6
(1.7) | 8
(2.2) |
| Other
carcinoma of head and neck | 12
(3.3) | 5
(1.4) | 7
(1.9) | 0 | 7
(1.9) | 5
(1.4) |
|
Nasopharyngeal
carcinoma | 1
(0.3) | 1
(0.3) | 0 | 0 | 1
(0.3) | 0 |
|
Other sites in
lip, oral cavity and pharynx | 10
(2.8) | 4
(1.1) | 6
(1.7) | 0 | 5
(1.4) | 5
(1.4) |
|
Nasal cavity, mid
ear, sinuses, larynx, other ill-defined head/neck | 1
(0.3) | 0 | 1
(0.3) | 0 | 1
(0.3) | 0 |
|
Carcinoma of trachea,
bronchus, and lung | 0 | 0 | 0 | 0 | 0 | 0 |
|
Carcinoma of breast | 7
(1.9) | 0 | 7
(1.9) | 0 | 1
(0.3) | 6
(1.7) |
|
Carcinoma of genitourinary
tract | 68
(18.8) | 0 | 68
(18.8) | 1 (0.3) | 12 (3.3) | 55
(15.2) |
|
Carcinoma of
kidney | 2
(0.6) | 0 | 2
(0.6) | 0 | 1
(0.3) | 1
(0.3) |
|
Carcinoma of
bladder | 0 | 0 | 0 | 0 | 0 | 0 |
|
Carcinoma of
gonads | 4
(1.1) | 0 | 4
(1.1) | 0 | 4
(1.1) | 0 |
|
Carcinoma of
cervix and uterus | 62
(17.1) | 0 | 62
(17.1) | 1 (0.3) | 7
(1.9) | 54
(14.9) |
|
Carcinoma of other
and ill-defined sites, genitourinary tract | 0 | 0 | 0 | 0 | 0 | 0 |
|
Carcinoma of gastrointestinal
tract | 18
(5.0) | 12 (3.3) | 6
(1.7) | 2 (0.6) | 6
(1.7) | 10 (2.8) |
|
Carcinoma of colon
and rectum | 7
(1.9) | 5
(1.4) | 2
(0.6) | 1 (0.3) | 3
(0.8) | 3
(0.8) |
|
Carcinoma of
stomach | 5
(1.4) | 3
(0.8) | 2
(0.6) | 0 | 1
(0.3) | 4
(1.1) |
|
Carcinoma of liver
and intrahepatic bile ducts | 2
(0.6) | 2
(0.6) | 0 | 1 (0.3) | 1
(0.3) | 0 |
|
Carcinoma of
pancreas | 3
(0.8) | 1
(0.3) | 2
(0.6) | 0 | 1
(0.3) | 2
(0.6) |
|
Carcinoma other
and ill-defined sites, gastrointestinal tract | 1
(0.3) | 1
(0.3) | 0 | 0 | 0 | 1
(0.3) |
|
Carcinoma of other and
ill-defined sites | 5 4
(14.9) | 14 (3.9) | 40
(11.0) | 9 (2.5) | 13 (3.6) | 32
(8.8) |
|
Adrenocortical
carcinoma | 1
(0.3) | 0 | 1
(0.3) | 0 | 0 | 1
(0.3) |
|
Carcinoma of other
and ill-defined sites, NOS | 53
(14.6) | 14 (3.9) | 39
(10.8) | 9 (2.5) | 13 (3.6) | 31 (8.6) |
| Miscellaneous
specified neoplasms, NOS | 12
(3.3) | 3
(0.8) | 9
(2.5) | 2 (0.6) | 1
(0.3) | 9
(2.5) |
| Other
pediatric and embryonal tumors, NOS | 3
(0.8) | 1
(0.3) | 2
(0.6) | 1 (0.3) | 0 | 2
(0.6) |
|
Wilms tumor | 0 | 0 | 0 | 0 | 0 | 0 |
|
Neuroblastoma | 0 | 0 | 0 | 0 | 0 | 0 |
|
Other pediatric
and embryonal tumors, NOS | 3
(0.8) | 1
(0.3) | 2
(0.6) | 1 (0.3) | 0 | 2
(0.6) |
| Other
specified and embryonal tumors, NOS | 9
(2.5) | 2
(0.6) | 7
(1.9) | 1 (0.3) | 1
(0.3) | 7
(1.9) |
|
Paraganglioma and
glomus tumors | 0 | 0 | 0 | 0 | 0 | 0 |
|
Other specified
gonadal tumors | 0 | 0 | 0 | 0 | 0 | 0 |
|
Myeloma, mast
cell, miscellaneous lymphoreticular neoplasms, NOS | 2
(0.6) | 0 | 2
(0.6) | 0 | 0 | 2
(0.6) |
|
Other specified
neoplasms, NOS | 7
(1.9) | 2
(0.6) | 5
(1.4) | 1 (0.3) | 1
(0.3) | 5
(1.4) |
| Unspecified
Malignant Neoplasms | 1
(0.3) | 0 | 1
(0.3) | 0 | 0 | 1
(0.3) |
| # Unclassified | 4
(1.1) | 3
(0.8) | 1
(0.3) | 2 (0.6) | 1
(0.3) | 1
(0.3) |
| Total | 362 (100) | 119 (32.9) | 243 (67.1) | 79 (21.8) | 99
(27.3) | 184 (50.8) |
Fig. 2 shows the
distribution of cancer types in AYAs by sex. In males, carcinomas
(29.4%) were the most common type, followed by germ cell and
trophoblastic neoplasms (15.1%), leukemias (11.8%), and CNS and
other intracranial and intraspinal neoplasms (all behaviors)
(11.8%) (Fig. 2A). In females,
carcinomas (58.0%) were the most common, followed by CNS and other
intracranial and intraspinal neoplasms (all behaviors) (14.0%) and
soft tissue sarcomas (6.2%) (Fig.
2B). The carcinomas were subclassified in this analysis. In
males, carcinoma of other and ill-defined sites (11.8%) were the
most common, followed by carcinoma of gastrointestinal tract
(10.1%) and other carcinoma of head and neck (4.2%) (Fig. 2A). In females, carcinoma of
genitourinary tract (28.0%) was the most common, followed by
carcinoma of other and ill-defined sites (16.5%) and thyroid
carcinoma (5.3%) (Fig. 2B). Most of
the genitourinary tract cancer was cervical cancer (n=61, 25.1%).
The proportion of carcinomas was significantly higher in females
than in males (carcinomas vs. all other types, P<0.001), but
females had a lower proportion of germ cell and trophoblastic
neoplasms and leukemias than did males (germ cell and trophoblastic
neoplasms vs. all other types, leukemias vs. all other types,
P<0.05).
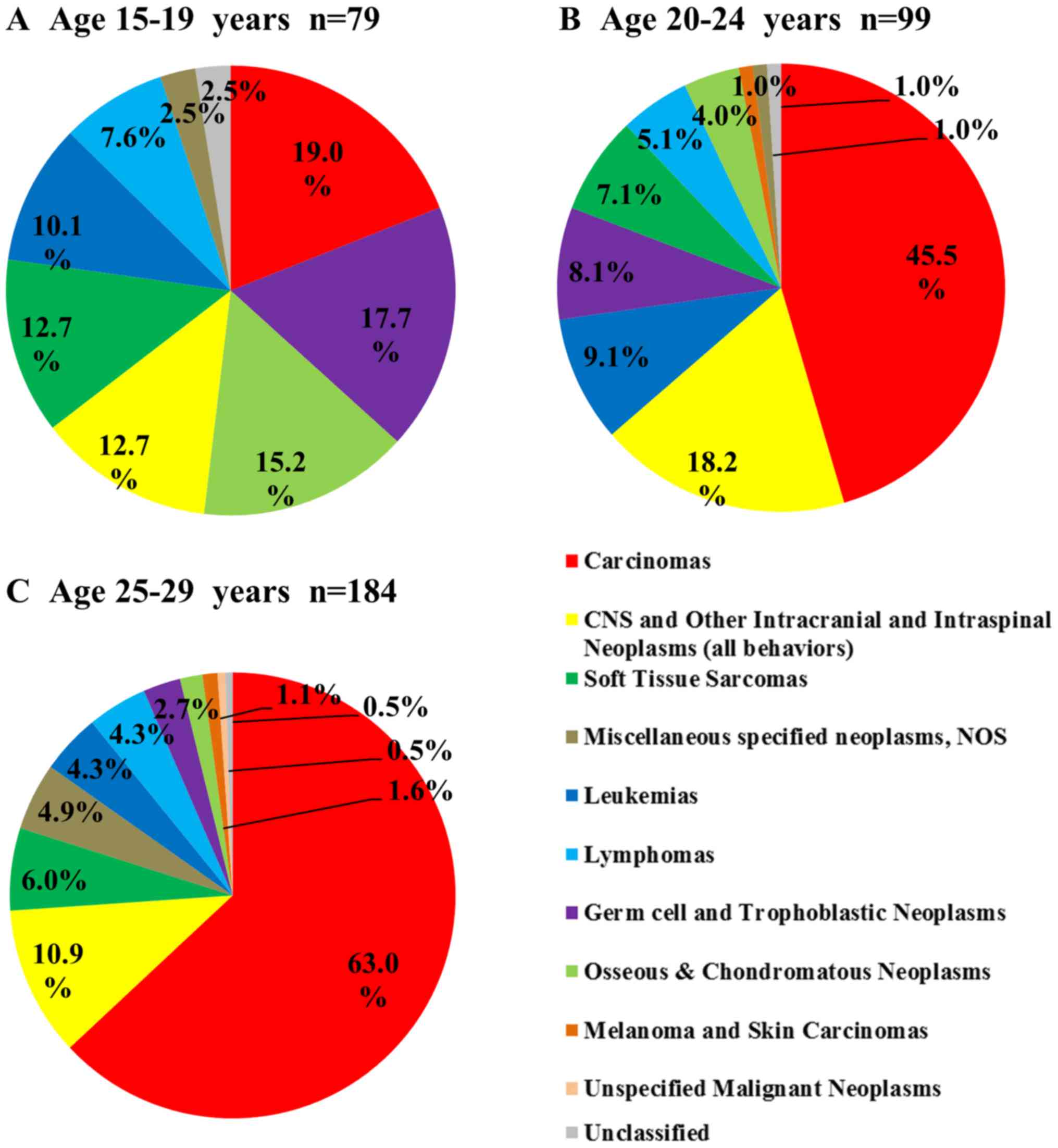
Fig. 3 shows the
distribution of cancer types in each age group. The number of cases
increased with age group with 79 (21.8%), 99 (27.3%), and 184
(50.8%) in the 15–19, 20–24, and 25–29 years groups, respectively.
Carcinomas were the most common in all age groups, and the
percentage of carcinomas significantly increased with age group
(19.0, 45.5, and 63.0%, respectively; carcinomas vs. all other
types, P<0.017). Three types of cancer decreased with age: germ
cell and trophoblastic neoplasms (17.7, 8.1 and 2.7%,
respectively), osseous and chondromatous neoplasms (15.2, 4.0 and
1.6%, respectively), and soft tissue sarcomas (12.7, 7.1 and 6.0%,
respectively).
Treatments and outcomes
Of the 362 cases, we excluded 12 cases that were
admitted to the hospital but not treated because of a second
opinion or referral to other hospitals from the analyses of
treatments, outcomes, and family/social status.
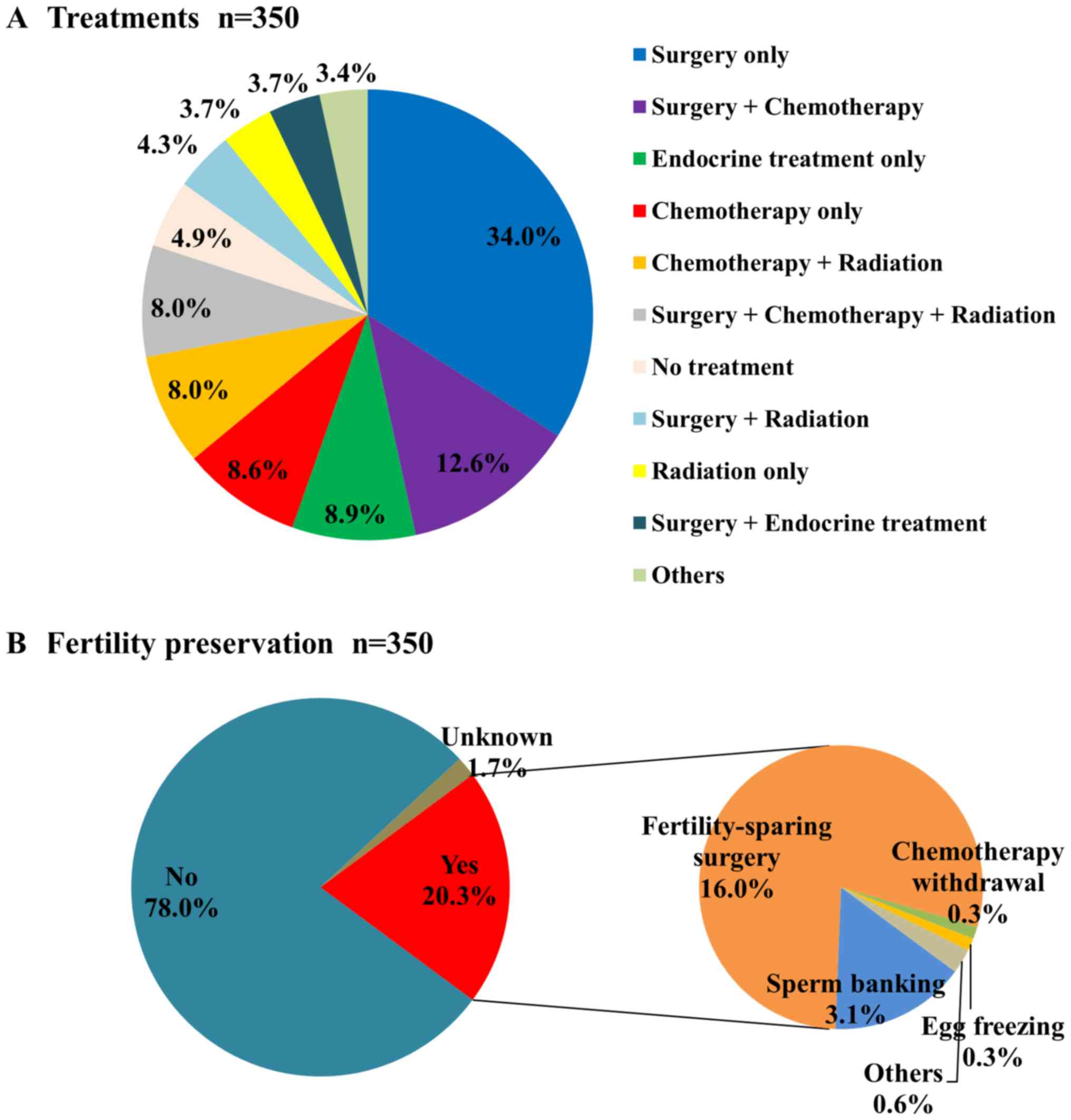
Surgery (n=228) was the most common treatment
method, followed by chemotherapy (n=136), radiation (n=90),
endocrine treatment (n=51), no treatment (n=17), and other
treatments (n=5). Radiation included total body irradiation (n=16)
for leukemia and lymphoma. Other treatments included thermal
therapy (1 case), WT1 antigen vaccination (1 case), immunotherapy
(1 case), and steroid ointment treatment (2 cases). Fig. 4A shows the treatments involving
combined modes. Surgery + chemotherapy (n=44) was the most common
combined modality, followed by chemotherapy + radiation (n=28),
surgery + chemotherapy + radiation (n=28), surgery + radiation
(n=15), surgery + endocrine treatment (n=13), and others
(n=12).
Of the patients, 20.3% underwent fertility
preservation (Fig. 4B). More females
underwent fertility preservation (females 24.7%, males 9.2%).
Fertility-sparing surgery (16.0%) was the most common method of
fertility preservation, mainly for cervical cancers, followed by
sperm banking (3.1%).
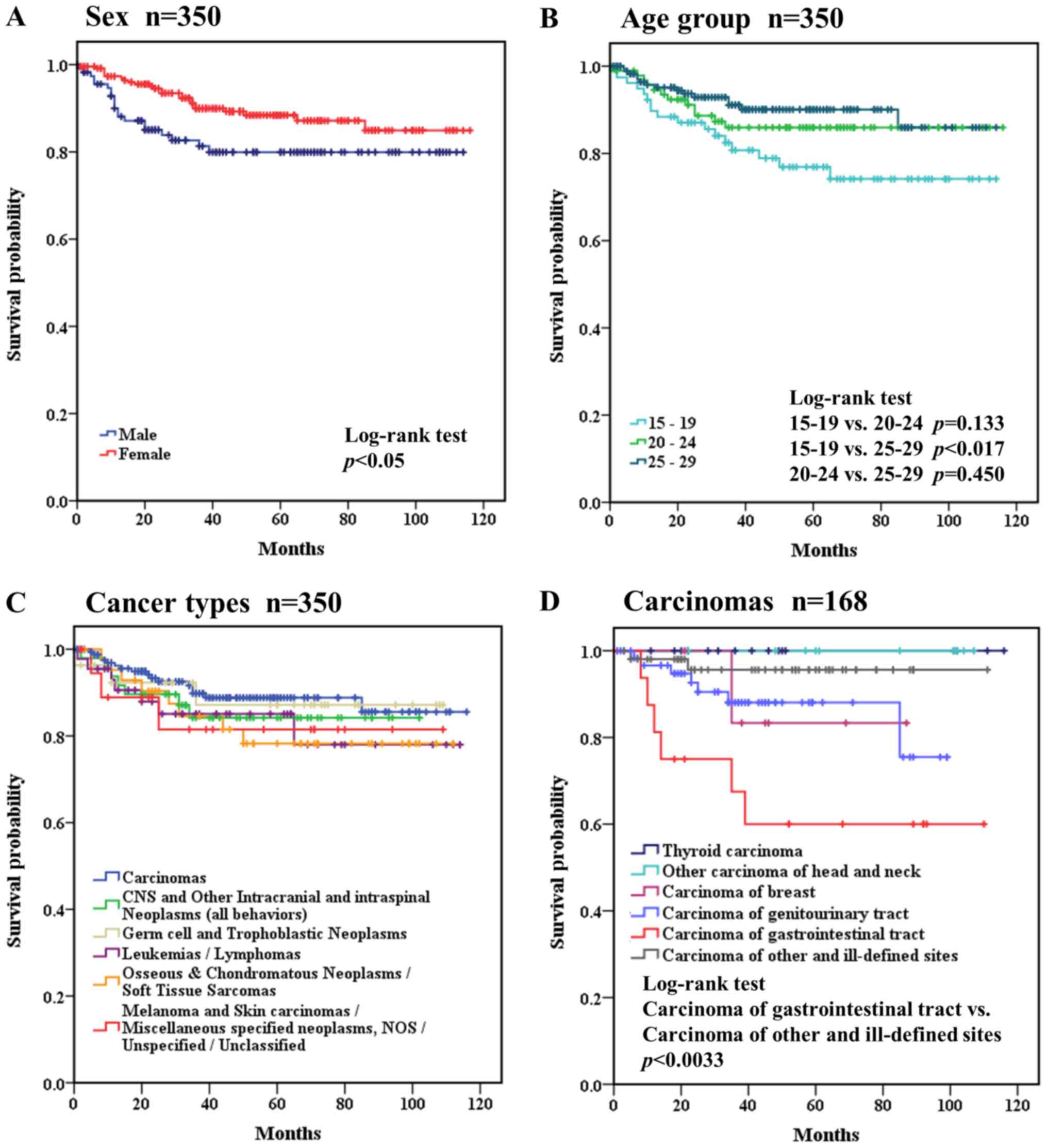
Fig. 5 shows the
treatment outcomes of the cancers in AYAs. The 5-year survival rate
was 85.6% in all AYA cases (n=350) and was better in females
(88.4%) than in males (79.9%). Overall survival curves were drawn
for several factors, including sex, age group, and cancer types
(Fig. 5). The treatment outcomes were
significantly (P<0.05) better in females (Fig. 5A). Comparing the age groups, the 15–19
years group had a significantly (P<0.017) worse outcome than the
25–29 years group (Fig. 5B). Cancer
types were categorized as i) leukemias/lymphomas; ii) CNS and other
intracranial and intraspinal neoplasms (all behaviors); iii)
osseous and chondromatous neoplasms/soft tissue sarcomas; iv) germ
cell and trophoblastic neoplasms; v) melanoma and skin
carcinomas/miscellaneous specified neoplasms, NOS/unspecified
malignant neoplasms/unclassified; and vi) carcinomas. Comparing
cancer types, the treatment outcomes were not significantly
different among the six groups (Fig.
5C). Carcinomas (n=168) were further classified and analyzed
separately, as shown in Fig. 5D. No
patients died of thyroid carcinoma or other carcinoma of head and
neck. Carcinoma of gastrointestinal tract had a significantly worse
outcome than that of carcinoma of other and ill-defined sites
(P<0.0033, Fig. 5D).
Family and social status
At the date last confirmed, the proportion of
married AYAs significantly increased with age group (married vs.
all other marital statuses, 15–19, 20–24, 25–29 years old,
P<0.017). The proportion of married cases at a diagnosis of
cancer was 0, 9.5, and 29.9% in the 15–19, 20–24, and 25–29 years
groups, respectively (Fig. 6A); at
the date last confirmed, these values were 2.6, 21.1, and 44.1%.
The percentage of patients raising children increased with age
group and was 0/1.3% (at diagnosis/date last confirmed), 7.4/15.8,
and 19.8%/33.9%, respectively (Fig.
6B). When marriage status and parenting status were compared by
sex, the proportions of patients who were married and raising
children were higher among females than males both at diagnosis and
at date last confirmed (P<0.01; Fig. 6A and B). The proportion of patients
who were married or raising children was higher at date last
confirmed than at diagnosis among females (P<0.001), but not
among males (Fig. 6A and B). Patients
with carcinoma of genitourinary tract were more likely to be
married (60.0%) or raising children (47.7%) compared with the other
types of cancer (21.4, 15.8%; P<0.001). Patients with fertility
preservation were also more likely to be married (50.7%) or raising
children (39.4%) compared with the other groups (no fertility
preservation and unknown status, 22.9 and 17.2%, respectively;
P<0.001).
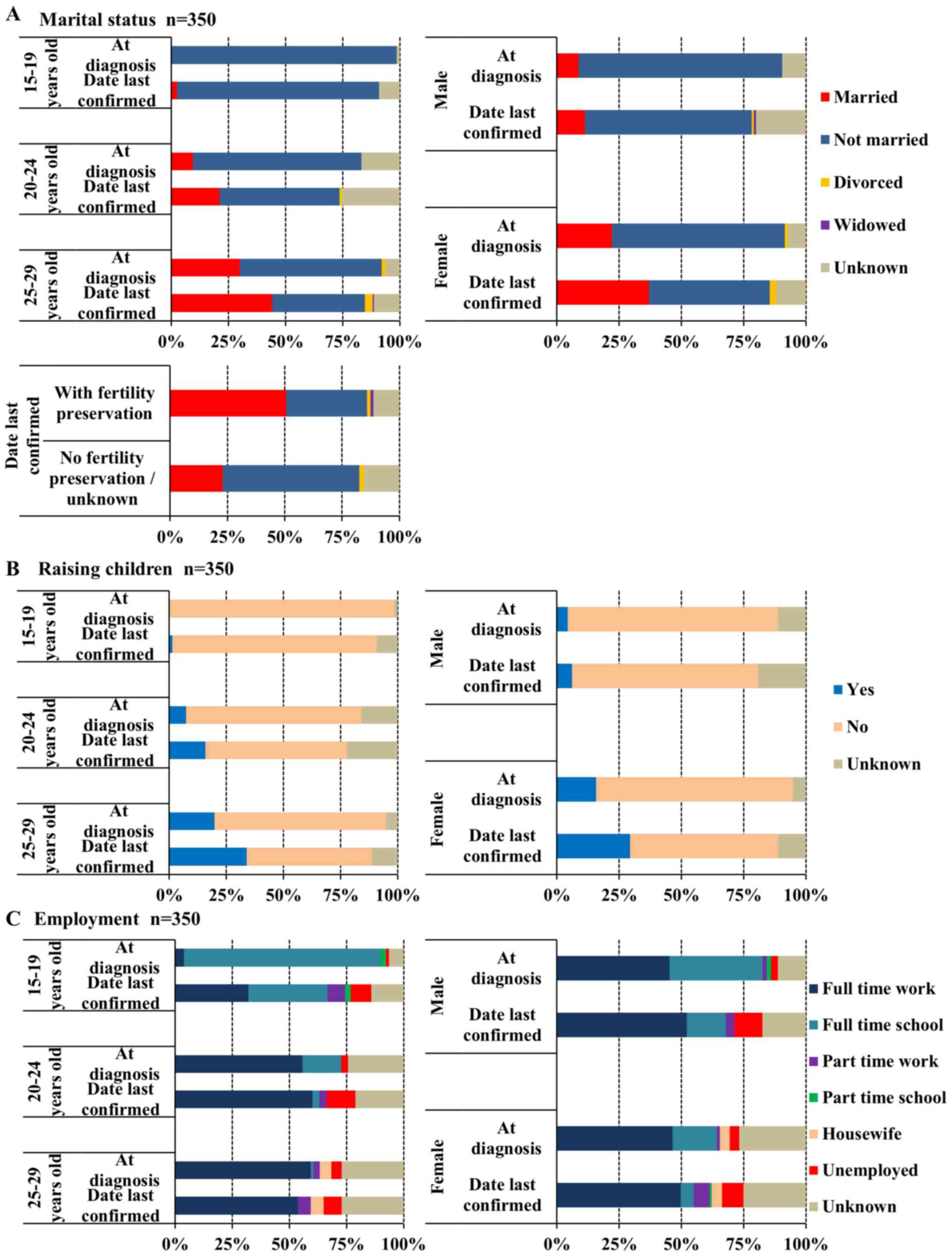 | Figure 6.Family and social status of
adolescents and young adults by age group and sex. (A) Marital
status (n=350): Married at diagnosis (male vs. female), P<0.01;
date last confirmed, P<0.001/married (at diagnosis vs. date last
confirmed): male, P=0.33; female, P<0.001. (B) Raising children
(n=350): Yes (male vs. female) at diagnosis, P<0.001, date last
confirmed, P<0.001/yes (at diagnosis vs. date last confirmed):
Male, P=0.38; female, P<0.001. (C) Employment (n=350):
Unemployed (at diagnosis vs. date last confirmed): 15–19 years,
P<0.05; 20–24 years, P<0.05; 25–29 years, P=0.135. |
The proportion of unemployed patients significantly
increased after cancer treatment in the 15–19 and 20–24 year age
groups (unemployed vs. all other employment statuses, P<0.05),
but not in the 25–29 year age group, and was greatest in the 20–24
year age group at the date last confirmed (at diagnosis/date last
confirmed: 1.3/9.0, 3.2/12.6, and 4.5%/7.9% in the 15–19, 20–24,
and 25–29 year age groups; Fig. 6C).
At the date last confirmed, the proportion of unemployed patients
was not significantly different among the three age groups
(unemployed vs. all other employment statuses).
Discussion
This study analyzed 362 AYAs (15 to 29 years old)
with cancer seen in Niigata University Medical and Dental Hospital
between 2007 and 2015. The most common cancers in both males and
females were carcinomas. The 5-year survival was 79.9% in males and
88.4% in females. More than 40% of the patients in the 25–29 years
group were married, at the date last confirmed.
We compared our data with relatively old SEER data
from 1975 to 2000 (3), due to the
recent widening of the definition of AYA by SEER. In the older SEER
data, 2.0% of AYAs (15–29 years) had cancers, compared to 2.3% in
Korea (1999 to 2010) (11). At our
institution, 1.9% of AYAs (15–29 years) had cancers. Overall, the
incidence of cancers in AYAs was similar in Japan, the USA, and
Korea.
At our institution, the most common cancers were
carcinomas, particularly in females, followed by germ cell and
trophoblastic neoplasms in males and CNS and other intracranial and
intraspinal neoplasms in females. In comparison, in a national
incidence study in Japan (7),
leukemia and germ cell and other gonadal tumors were most common in
the 15–19 and 20–29 years groups, respectively, although the
disease classification differed slightly. This difference could be
specific to our institution. In Korea, the most common cancer was
carcinoma, followed by leukemias. In the USA, lymphoma was most
common, followed by invasive skin cancer.
At our institution, carcinoma of genitourinary tract
was most common and accounted for 28.0% of the cancers in female
AYAs. Most such cancers were cervical cancer, including carcinoma
in situ. Cervical cancer is caused by human papilloma virus
(HPV) infection (12), and is
preventable by vaccination (13–15).
However, the Japanese Ministry of Health, Labor and Welfare
withdrew its recommendation for vaccination against HPV because of
controversy regarding its adverse effects (16,17).
Consequently, the high incidence of cervical cancer will continue.
Screening for cervical cancer in women over 20 years old should be
continued in Japan.
Surgery was the most common treatment modality
because of the high percentage of carcinomas in AYAs. While 20.3%
of the patients underwent fertility preservation, mainly due to
fertility-sparing surgery for cervical cancer in females and sperm
banking in males, 80% of the patients did not receive any fertility
preservation. Sperm banking was performed only in 9.2% of males,
which was markedly lower than the 17.8% in male AYAs reported in
the USA (18). Egg freezing was
performed in one case only. Sperm banking and egg freezing should
be performed widely in AYA cancer patients in Japan. The National
Comprehensive Cancer Network (19)
and American Society of Clinical Oncology (20) guidelines recommend discussing the
possibility of infertility and use of fertility preservation before
initiating treatment.
The 5-year survival was better in females (88.4%)
than in males (79.9%), and was better than those for cancer at all
ages (males 59.1%, females 66.0%) (9). The treatment outcome was worse in the
15–19 years age group, probably due to the high percentage of soft
tissue sarcoma, osseous and trophoblastic neoplasms, and
leukemia/lymphoma. The 10-year relative survival in AYAs (15–29
years) (males 66.0%, females 75.3%) is worse than those in children
(0–14 years) (males 73.2%, females 79.3%) in Japan (21). The treatment outcomes for cancer in
AYAs is worse than that in pediatric patients with
leukemia.21 However, acute lymphoblastic leukemia in
AYAs has an improved prognosis with intensive chemotherapy
(22). The chemotherapy regimens used
for pediatric cancer should be applied to these cancers in AYAs
because such regimens have better prognoses than adult regimens for
cancer in AYAs (23). Among the
cancer types, carcinomas and germ cell and trophoblastic neoplasms
had better prognoses. None of the patients with thyroid carcinoma
or other carcinoma of head and neck died. In many countries, the
incidence of thyroid cancer has increased (24–26).
Although the reason for the increased incidence of thyroid cancer
in AYAs is unclear, improved screening technology, including
ultrasound, which can detect early thyroid cancer, might have led
to the increased detection of thyroid cancer (27). In AYAs, we might over-diagnose thyroid
cancer, which is not malignant and not lethal (28). Carcinoma of gastrointestinal tract and
breast cancers had poorer prognoses in AYAs than in older patients,
which suggests that these carcinomas have different biological
features in AYAs and need different approaches for detection and
treatment (29).
In terms of marital status, the proportion of
married patients at diagnosis increased with age group and was 0,
9.5, and 29.9%, respectively. These proportions are similar to
those in healthy individuals at similar ages (0.4, 6.6, and 31.5%)
based on the 2015 welfare survey in Japan (30). In addition, many patients married
after their diagnoses.
The percentage with children increased after
diagnosis in all three age groups, which suggests that fertility
was maintained in many cases. The high proportion of genitourinary
tract carcinoma patients who were married and raising children at
the date last confirmed could be due to fertility-sparing surgery
for cervical cancers. In this context, fertility preservation is
important for marriage and childbirth in AYA cancer survivors.
Employment is a very important issue in AYA cancer
patients. The proportion employed (full time or part time) at the
date last confirmed was 56.0% (196/350) at our institution. This is
similar to the results of Tai et al (31), who found that 49.4% of AYA cancer
survivors were employed for wages. Financial support and job
training are definitely needed for AYA cancer survivors.
The cancers in AYAs varied. There was a high
incidence of carcinoma, particularly genitourinary tract carcinomas
in females, and a low incidence of melanoma and skin carcinomas
among AYAs in Japan compared to the SEER data. Fertility-sparing
surgery was the most common method of fertility preservation.
Social and economic support is need for AYAs with cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KK drafted the manuscript. KK, YM and QZ contributed
to the collection and analysis of the data. MM and YS conceived and
designed the study, and edited the manuscript. All authors approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of the School of Medicine of Niigata University (Niigata,
Japan; no. 2611).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bleyer A, Barr R, Hayes-Lattin B, Thomas
D, Ellis C and Anderson B: Biology and Clinical Trials Subgroups of
the US National Cancer Institute Progress Review Group in
Adolescent and Young Adult Oncology: The distinctive biology of
cancer in adolescents and young adults. Nat Rev Cancer. 8:288–298.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nass SJ, Beaupin LK, Demark-Wahnefried W,
Fasciano K, Ganz PA, Hayes-Lattin B, Hudson MM, Nevidjon B,
Oeffinger KC, Rechis R, et al: Identifying and addressing the needs
of adolescents and young adults with cancer: Summary of an
institute of medicine workshop. Oncologist. 20:186–195. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bleyer A, Leary MO, Barr R and Ries LA:
Cancer epidemiology in older adolescents and young adults 15 to 29
years of age, including SEER incidence and survival: 1975–2000.
National Cancer Institute; Bethesda, MD: NIH Pub. No. 06–5767.
2006
|
|
4
|
Smith AW, Parsons HN, Kent EE, Bellizi K,
Zebrack BJ, Keel G, Lynch CF, Rubenstein MB and Keegan TH: AYA HOPE
Study Collaborative Group: Unmet support service needs and
health-related quality of life among adolescents and young adults
with cancer: The AYA HOPE study. Front Oncol. 3:752013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canadian Cancer and Society: Canadian
Cancer Statistics 2017. http://www.cancer.ca/statistics/March
25–2018
|
|
6
|
National Cancer institute: SEER Cancer
Statistics Review 1975–2014. https://seer.cancer.gov/csr/1975_2014/results_merged/sect_32_aya.pdf/March
25–2018
|
|
7
|
Katanoda K, Shibata A, Matsuda T, Hori M,
Nakata K, Narita Y, Ogawa C, Munakata W, Kawai A and Nishimoto H:
Childhood, adolescent and young adult cancer incidence in Japan in
2009–2011. Jpn J Clin Oncol. 47:762–771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue I, Nakamura F, Matsumoto K, Takimoto
T and Higashi T: Cancer in adolescents and young adults: National
incidence and characteristics in Japan. Cancer Epidemiol. 51:74–80.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cancer statistics in Japan: Center for
Cancer Registries, Center for Cancer Control and Information
Services. National Cancer Center; Japan: https://ganjoho.jp/reg_stat/statistics/stat/summary.htmlMarch
25–2018
|
|
10
|
National Cancer Institute: AYA Site
recode/WHO 2008 Definition. https://seer.cancer.gov/ayarecode/aya-who2008.htmlMarch
25–2018
|
|
11
|
Moon EK, Park HJ, Oh CM, Jung KW, Shin HY,
Park BK and Won YJ: Cancer incidence and survival among adolescents
and young adults in Korea. PLoS One. 9:e960882014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
FUTURE II Study Group: Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harper DM, Franco EL, Wheeler CM, Moscicki
AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa
Clemens SA and Dubin G; HPV Vaccine Study group, . Sustained
efficacy up to 4.5 years of a bivalent L1 virus-like particle
vaccine against human papillomavirus types 16 and 18: Follow-up
from a randomised control trial. Lancet. 367:1247–1255. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paavonen J, Jenkins D, Bosch FX, Naud P,
Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC,
Castellsague X, et al: Efficacy of a prophylactic adjuvanted
bivalent L1 virus-like-particle vaccine against infection with
human papillomavirus types 16 and 18 in young women: An interim
analysis of a phase III double-blind, randomised controlled trial.
Lancet. 369:2161–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sauvaget C, Nishino Y, Konno R, Tase T,
Morimoto T and Hisamichi S: Challenges in breast and cervical
cancer control in Japan. Lancet. 17:e305–e312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Ministry of Health, Labor and
Welfare: HPV, Hib, pediatric pneumococcal vaccines. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou28/March
25–2018
|
|
18
|
Neal MS, Nagel K, Duckworth J, Bissessar
H, Fischer MA, Portwine C, Tozer R and Barr RD: Effectiveness of
sperm banking in adolescents and young adults with cancer: A
regional experience. Cancer. 110:1125–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coccia PF, Pappo AS, Beaupin L, Borges VF,
Borinstein SC, Chugh R, Dinner S, Folbrecht J, Frazier AL, Goldsby
R, et al: Adolescent and young adult oncology, Version 2.2018, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
16:66–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loren AW, Mangu PB, Beck LN, Brennan L,
Magdalinski AJ, Partridge AH, Quinn G, Wallace WH and Oktay K:
American Society of Clinical Oncology: Fertility preservation for
patients with cancer: American Society of Clinical Oncology
clinical practice guideline update. J Clin Oncol. 31:2500–2510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito Y, Miyashiro I, Ito H, Hosono S,
Chihara D, Nakata-Yamada K, Nakayama M, Matsuzaka M, Hattori M,
Sugiyama H, et al: Long-term survival and conditional survival of
cancer patients in Japan using population-based cancer registry
data. Cancer Sci. 105:1480–1486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pui CH, Pei D, Campana D, Bowman WP,
Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Coustan-Smith E,
Jeha S, et al: Improved prognosis for older adolescents with acute
lymphoblastic leukemia. J Clin Oncol. 29:386–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayakawa F, Sakura T, Yujiri T, Kondo E,
Fujimaki K, Sasaki O, Miyatake J, Handa H, Ueda Y, Aoyama Y, et al:
Markedly improved outcomes and acceptable toxicity in adolescents
and young adults with acute lymphoblastic leukemia following
treatment with a pediatric protocol: A phase II study by the Japan
Adult Leukemia Study Group. Blood Cancer J. 4:e2522014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013National Cancer
Institute. Bethesda, MD: based on November 2015 SEER data
submission, posted to the SEER website. April. 2016, http://seer.cancer.gov/csr/1975_2013March
25–2018
|
|
25
|
Ahn HS, Kim HJ and Welch HG: Korea's
thyroid-cancer ‘epidemic’: Screening and overdiagnosis. N Engl J
Med. 371:1765–1767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn HS and Welch HG: South Korea's
thyroid-cancer ‘epidemic’: Turning the tide. N Engl J Med.
373:2389–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaccarella S, Franceschi S, Bray F, Wild
CP, Plummer M and Dal Maso L: Worldwide thyroid-cancer epidemic?
The increasing impact of overdiagnosis. N Engl J Med. 375:614–617.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takano T: Natural history of thyroid
cancer [Review]. Endocr J. 64:237–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adolescent and Young Adult Oncology
Progress Review Group: Closing the Gap: Research and care
imperatives for adolescents and young adults with cancer. Report of
the Adolescent and Young Adult Oncology Progress Review Group. U.S.
Department of Health and Human Services, National Institutes of
Health, . National Cancer Institutes and the LIVESTRONG Young
Adults Alliance. NIH Publication no. 06–6067. 2006.
|
|
30
|
Population census of Japan: Statistics
Bureau, Ministry of Internal Affairs and Communications. Japan:
http://www.stat.go.jp/data/kokusei/2015/kekka/pdf/gaiyou1.pdfMarch
25–2018
|
|
31
|
Tai E, Buchanan N, Townsend J, Fairley T,
Moore A and Richardson LC: Health status of adolescent and young
adult cancer survivors. Cancer. 118:4884–4891. 2012. View Article : Google Scholar : PubMed/NCBI
|