Introduction
In 2012, it was reported that primary liver cancer
(PLC) is one of the most common malignancy types and is a leading
cause of mortality globally (1,2).
Previously, a series of advanced surgical techniques and
pharmacotherapy, including liver transplantation, loco-regional
therapy and sorafenib, have been implemented in medical practice to
improve the survival time of patients with liver cancer (3–6). However,
in the United States between 1975 and 2012, the incidence and
mortality of this disease continued to increase and was
disproportionally distributed in the population (7).
Racial disparities in hepatocellular carcinoma (HCC)
survival time have been confirmed in a number of studies (8–11).
Numerous studies, between 2006 and 2012, have demonstrated that
Asian patients have the highest survival time, while
African-American patients have the lowest survival time (9,11–13). Furthermore, it has been reported that
between 1988 and 2012, Asian patients, who were treated in the
United States, have an increased probability of being treated via
liver resection, compared with other racial groups (11,12). There
are numerous contributing factors, including etiology, tumor stage
and social economic status, affecting the racial disparities
observed in PLC survival time (9,12,14). The mechanisms underlying factors
affecting HCC survival time and their relative importance in
disease progression remain undetermined.
The Asian population was reported as the fastest
growing and most heterogeneous ethnic group in the USA between 1992
and 2009 (15). There are numerous
differences in the etiology, genetic characteristics, cancer
susceptibility and living habits of the Asian subgroups (16,17). In
2012, it was reported that the Chinese population is one of the
largest Asian subgroups and account for a large proportion of
patients with HCC in the United States (1,18).
Furthermore, previous studies have demonstrated that Chinese
patients with colorectal and esophageal cancer have improved
survival time, compared with patients of other racial groups
(19,20). However, there are limited studies
examining differences in HCC outcomes between Chinese patients and
patients of other racial groups. Stewart et al (11) reported that compared with Caucasians,
Chinese patients had reduced HCC cause-specific mortality [hazard
ratio (HR)=0.81; 95% confidence interval (CI), 0.77–0.86], although
a detailed analysis with respect to clinical presentation,
treatment and survival time was not provided. Intrahepatic
cholangiocarcinoma (ICC) is the second most common PLC type and has
been increasing in incidence and mortality over the last 10 to 20
years (21,22); however, limited studies investigating
the racial disparities in ICC outcomes have been performed
(21,23).
Therefore, in the present study, racial disparities
in HCC and ICC survival time between Chinese patients and patients
of other racial groups were retrospectively analyzed with the
Surveillance, Epidemiology, and End Results (SEER) database between
January 1st 2004, and December 31st 2013 (24). Furthermore, the racial disparities
between Asian subgroups were analyzed.
Patients and methods
Database
Data from the SEER database, the largest public
cancer database and sponsored by the National Cancer Institute,
were retrospectively analyzed. The SEER program collects
demographics, including age, sex and race/ethnicity, as well as
clinical characteristics, including cancer stage, histology,
primary therapy and survival time, in the USA from 1973. It is a
population-based database that includes ~28% of the USA population
and multiple racial groups. The exact database used for the present
analysis was the Incidence-SEER 18 Registries Research Data +
Hurricane Katrina Impacted Louisiana Cases, November 2015 Sub
(1973–2013 varying) (25) using the
SEER*Stat 8.3.2 software (National Cancer Institute, Bethesda, MD,
USA). Permission was received for the use of these data (SEER ID:
13724-Nov 2015).
Patient selection
Patients who were histologically diagnosed as HCC or
ICC between January 1st 2004, and December 31st 2013 were included
in the present study. Patients were excluded if they were <18
years of age, had a history of a prior malignancy, the cause of
mortality was unknown (UNK), the follow-up period was <1 month,
or any key covariate data were incomplete or UNK, including
diagnosis confirmation methods, age, marital status, histological
stage, tumor size, residence and race/ethnicity.
Covariates
Key covariates with respect to baseline
characteristics, including year of diagnosis, age, sex,
race/ethnicity, residence, marital status, household income,
educational level, poverty, pathology, histological grade, tumor
size, vascular and adjacent invasion, metastasis, cancer stage,
serum α-fetoprotein (AFP) level, fibrosis score and primary therapy
were extracted from the database. Race/ethnicity was classified as
non-Hispanic White (NHW), Hispanic, African-American or Chinese, as
determined in the SEER database. Asian subgroups except Chinese
were merged into ‘Other Asian’ for the overall analysis and
subsequently categorized as Korean, Japanese, Vietnamese,
Philippines Filipino or Other Minority for subgroup analysis.
Treatment was categorized as radiation, resection, transplantation,
tumor destructive therapy (TDT), none or UNK. TDT included
radio-frequency ablation, laser therapy, photodynamic therapy,
electrocautery and cryosurgery, as described in the SEER database
(26). Data were extracted from the
dataset with the reference of the SEER Coding and Staging Manual
and Collaborative Staging System Manual (26).
Statistical analysis
Baseline demographic and clinical characteristics
data were summarized with descriptive statistics in Tables I and II. Continuous variables were presented as
mean ± standard deviation. Comparison of baseline characteristics
between races/ethnicities was calculated by one-way analysis of
variance, followed by Bonferroni's multiple comparison test, and
the χ2 test.
 | Table I.Baseline demographic and clinical
characteristics of patients with hepatocellular carcinoma from the
SEER database. |
Table I.
Baseline demographic and clinical
characteristics of patients with hepatocellular carcinoma from the
SEER database.
| Variables | Total | Chinese | NHW |
African-American | Other Asian | Hispanic | P-value |
|---|
| Total (%) | 27,767 (100) | 1,241 (4.47) | 13,867 (49.94) | 3,645 (13.13) | 3,437 (12.38) | 5,577 (20.08) |
|
| Age, years |
|
|
|
|
|
|
<0.001a |
| Mean ±
SD | 62±11 | 64±13 | 63±11 | 60±10 | 64±12 | 61±11 |
|
| Median
(IQR) | 61 (54–69) | 64 (55–73) | 61 (55–69) | 59 (54–64) | 64 (55–72) | 60 (53–68) |
|
|
P-valuec |
| Ref | 0.008 | <0.001 | 1.000 | 0.008 |
|
| Sex |
|
|
|
|
|
|
<0.001b |
| Female | 6,274 (22.59) | 307 (24.74) | 2,854 (20.58) | 815 (22.36) | 944 (27.47) | 1,354 (24.28) |
|
|
Male | 21,493 (77.41) | 934 (75.26) | 11,013 (79.42) | 2,830 (77.64) | 2,493 (72.53) | 4,223 (75.72) |
|
| Marital status, n
(%) |
|
|
|
|
|
|
<0.001b |
|
Married | 15,364 (55.33) | 985 (79.37) | 7,582 (54.68) | 1,336 (36.56) | 2,462 (71.63) | 2,999 (53.77) |
|
|
Single | 5,806 (20.91) | 96 (7.74) | 2,724 (19.64) | 1,334 (36.51) | 369 (10.74) | 1,283 (23.00) |
|
|
Divorced | 4,131 (14.88) | 54 (4.35) | 2,312 (16.67) | 690 (18.83) | 249 (7.24) | 826 (14.81) |
|
|
Widowed | 2,466 (8.88) | 106 (8.54) | 1,249 (9.01) | 285 (7.80) | 357 (10.39) | 469 (8.41) |
|
| Residence, n
(%) |
|
|
|
|
|
|
<0.001b |
|
Rural | 2,176 (7.84) | 5 (0.41) | 1,721 (12.41) | 188 (5.16) | 67 (1.95) | 195 (3.50) |
|
|
Urban | 25,591 (92.16) | 1,236 (99.59) | 12,146 (87.59) | 3,457 (94.84) | 3,370 (98.05) | 5,382 (96.50) |
|
| Household income
(USD) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 47,512±10,980 | 53,820±10,109 | 46,872±11,042 | 44,837±9,999 | 52,439±10,920 | 46,412±10,239 |
|
|
P-valuec |
| Ref | <0.001 | <0.001 | 0.001 | <0.001 |
|
|
Educationd (%) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 20.79±7.34 | 19.97±6.26 | 19.8±7.54 | 21.09±6.65 | 20.06±6.70 | 23.68±7.18 |
|
|
P-valuec |
| Ref | 1.000 | <0.001 | 1.000 | <0.001 |
|
|
Povertye
(%) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 12.86±5.17 | 11.83±4.05 | 12.15±5.29 | 14.35±5.46 | 11.92±4.31 | 14.48±4.79 |
|
|
P-valuec |
| Ref | 0.318 | <0.001 | 1.000 | <0.001 |
|
| Grade, n (%) |
|
|
|
|
|
|
<0.001b |
| I | 3,855 (13.88) | 146 (11.76) | 2,029 (14.63) | 491 (13.47) | 378 (10.99) | 811 (14.54) |
|
| II | 5,170 (18.62) | 249 (20.06) | 2,672 (19.27) | 675 (18.52) | 698 (20.31) | 876 (15.71) |
|
|
III | 2,198 (7.92) | 142 (11.44) | 1,051 (7.58) | 295 (8.09) | 347 (10.10) | 363 (6.51) |
|
| IV | 202 (0.73) | 3 (0.24) | 110 (0.80) | 24 (0.66) | 38 (1.11) | 27 (0.48) |
|
|
UNK | 16,342 (58.85) | 701 (56.49) | 8,005 (57.72) | 2,160 (59.26) | 1,976 (57.49) | 3,500 (62.76) |
|
| SEER stage (26), n
(%) |
|
|
|
|
|
|
<0.001b |
|
Localized | 16,435 (59.19) | 794 (63.98) | 8,202 (59.15) | 2,025 (55.56) | 1,985 (57.75) | 3,429 (61.48) |
|
|
Regional | 8,312 (29.93) | 336 (27.07) | 4,186 (30.19) | 1,151 (31.58) | 1,070 (31.13) | 1,569 (28.13) |
|
|
Distant | 3,020 (10.88) | 111 (8.94) | 1,479 (10.66) | 469 (12.87) | 382 (11.11) | 579 (10.38) |
|
| Tumor size diameter
(cm), n (%) |
|
|
|
|
|
|
<0.001b |
| 0–3 | 8,859 (31.90) | 369 (29.73) | 4,717 (34.02) | 1,034 (28.37) | 950 (27.64) | 1,789 (32.08) |
|
|
3–5 | 7,294 (26.27) | 314 (25.30) | 3,665 (26.43) | 922 (25.29) | 842 (24.50) | 1,551 (27.81) |
|
|
>5 | 11,614 (41.83) | 558 (44.96) | 5,485 (39.55) | 1,689 (46.34) | 1,645 (47.86) | 2,237 (40.11) |
|
| Vascular invasion,
n (%) |
|
|
|
|
|
|
<0.001b |
| No | 12,269 (44.19) | 593 (47.78) | 6,128 (44.19) | 1,489 (40.85) | 1,486 (43.24) | 2,573 (46.14) |
|
|
Yes | 10,363 (37.32) | 414 (33.36) | 5,344 (38.54) | 1,363 (37.39) | 1,239 (36.05) | 2,003 (35.92) |
|
|
UNK | 5,135 (18.49) | 234 (18.86) | 2,395 (17.27) | 793 (21.76) | 712 (20.71) | 1,001 (17.94) |
|
| Adjacent invasion,
n (%) |
|
|
|
|
|
|
<0.001b |
| No | 18,347 (66.07) | 860 (69.30) | 9,222 (66.46) | 2,281 (62.58) | 2,190 (63.72) | 3,794 (68.03) |
|
|
Yes | 6,881 (24.78) | 282 (22.72) | 3,349 (24.15) | 996 (27.33) | 906 (26.36) | 1,348 (24.17) |
|
|
UNK | 2,539 (9.14) | 99 (7.98) | 1,296 (9.35) | 368 (10.10) | 341 (9.92) | 435 (7.80) |
|
| Regional lymph
nodes, n (%) |
|
|
|
|
|
|
<0.001b |
| No | 24,358 (87.72) | 1,127 (90.81) | 12,078 (87.10) | 3,174 (87.08) | 3,072 (89.38) | 4,907 (87.99) |
|
|
Yes | 1,710 (6.16) | 34 (2.74) | 985 (7.103) | 268 (7.35) | 152 (4.42) | 271 (4.86) |
|
|
UNK | 1,699 (6.12) | 80 (6.45) | 804 (5.797) | 203 (5.57) | 213 (6.20) | 399 (7.15) |
|
| Metastasis, n
(%) |
|
|
|
|
|
| 0.001b |
| No | 24,060 (86.65) | 1,107 (89.20) | 12,051 (86.90) | 3,103 (85.13) | 2,961 (86.15) | 4,838 (86.75) |
|
|
Yes | 3,129 (11.27) | 111 (8.94) | 1,547 (11.16) | 474 (13.00) | 390 (11.35) | 607 (10.88) |
|
|
UNK | 578 (2.08) | 23 (1.85) | 269 (1.94) | 68 (1.87) | 86 (2.50) | 132 (2.37) |
|
| AFP, n (%) |
|
|
|
|
|
|
<0.001b |
| Negative | 5,889 (21.21) | 297 (23.93) | 3,239 (23.36) | 492 (13.50) | 640 (18.62) | 1,221 (21.89) |
|
|
Borderline | 71 (0.26) | 3 (0.24) | 47 (0.34) | 8 (0.22) | 4 (0.12) | 9 (0.16) |
|
|
Positive | 16,752 (60.33) | 780 (62.85) | 7,819 (56.39) | 2,547 (69.88) | 2,218 (64.53) | 3,388 (60.75) |
|
|
UNK | 5,055 (18.21) | 161 (12.97) | 2,762 (19.91) | 598 (16.41) | 575 (16.73) | 959 (17.20) |
|
| Fibrosis score, n
(%) |
|
|
|
|
|
|
<0.001b |
| No to moderate | 1,573 (5.66) | 167 (13.46) | 739 (5.33) | 205 (5.62) | 251 (7.30) | 211 (3.78) |
|
| Severe
to cirrhosis | 6,600 (23.77) | 266 (21.43) | 3,526 (25.43) | 755 (20.71) | 708 (20.60) | 1,345 (24.11) |
|
|
UNK | 19,594 (70.57) | 808 (65.11) | 9,602 (69.24) | 2,685 (73.67) | 2,478 (72.10) | 4,021 (72.10) |
|
| Treatment, n
(%) |
|
|
|
|
|
|
<0.001b |
| None/UNK | 16,963 (61.09) | 628 (50.60) | 8,143 (58.72) | 2,380 (65.29) | 1,997 (58.10) | 3,815 (68.41) |
|
|
Radiation | 1,513 (5.45) | 32 (2.58) | 878 (6.33) | 228 (6.26) | 150 (4.36) | 225 (4.03) |
|
|
Resection | 3,337 (12.02) | 309 (24.90) | 1,568 (11.3) | 419 (11.50) | 632 (18.39) | 409 (7.33) |
|
|
Transplant | 2,321 (8.36) | 75 (6.04) | 1,449 (10.45) | 192 (5.27) | 184 (5.35) | 421 (7.55) |
|
|
TDT | 3,633 (13.08) | 197 (15.87) | 1,829 (13.19) | 426 (11.69) | 474 (13.79) | 707 (12.68) |
|
 | Table II.Baseline demographic and clinical
characteristics of patients with intrahepatic cholangiocarcinoma in
the SEER database. |
Table II.
Baseline demographic and clinical
characteristics of patients with intrahepatic cholangiocarcinoma in
the SEER database.
| Variables | Total | Chinese | NHW |
African-American | Other Asian | Hispanic | P-value |
|---|
| Total (%) | 3,187 | 113 (3.55) | 2,093 (65.67) | 264 (8.28) | 280 (8.79) | 437 (13.71) |
|
| Age, years |
|
|
|
|
|
|
<0.001a |
| Mean ±
SD | 64±12 | 65±12 | 65±12 | 61±13 | 64±12 | 63±12 |
|
| Median
(IQR) | 64 (55–72) | 65 (57–73) | 65 (56–73) | 61 (52–69) | 65 (55–73) | 63 (54–71) |
|
|
P-valuec | Ref | 1.000 | 0.062 | 1.000 | 0.664 |
|
|
| Sex, n (%) |
|
|
|
|
|
| 0.069b |
| Female | 1,611 (50.55) | 59 (52.21) | 1,026 (49.02) | 150 (56.81) | 139 (49.64) | 237 (54.23) |
|
|
Male | 1,576 (49.45) | 54 (47.79) | 1,067 (50.98) | 114 (43.19) | 141 (50.36) | 200 (45.77) |
|
| Marital status, n
(%) |
|
|
|
|
|
|
<0.001b |
| Married | 1,962 (61.56) | 85 (75.22) | 1,316 (62.88) | 108 (40.91) | 198 (70.71) | 255 (58.35) |
|
|
Single | 409 (12.83) | 12 (10.62) | 221 (10.56) | 71 (26.89) | 27 (9.64) | 78 (17.85) |
|
|
Divorced | 364 (11.42) | 3 (2.65) | 247 (11.80) | 49 (18.56) | 18 (6.43) | 47 (10.76) |
|
|
Widowed | 452 (14.18) | 13 (11.51) | 309 (14.76) | 36 (13.64) | 37 (13.22) | 57 (13.04) |
|
| Residence, n
(%) |
|
|
|
|
|
|
<0.001b |
| Rural | 345 (10.83) | 1 (0.88) | 300 (14.33) | 21 (7.95) | 7 (2.50) | 16 (3.66) |
|
|
Urban | 2,842 (89.17) | 112 (99.12) | 1,793 (85.67) | 243 (92.05) | 273 (97.50) | 421 (96.34) |
|
| Household income
(USD) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 47,560±11,341 | 52,581±10,994 | 47,334±11,678 | 45,261±10,385 | 51,178±9,921 | 46,413±10,327 |
|
|
P-valuec |
| Ref | <0.001 | <0.001 | 1.000 | <0.001 |
|
|
Educationd (%) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 20.26±7.73 | 21.18±6.85 | 19.35±7.77 | 21.21±7.05 | 19.40±6.79 | 24.33±7.33 |
|
|
P-valuec |
| Ref | 0.121 | 1.000 | 0.338 | 0.001 |
|
|
Povertye
(%) |
|
|
|
|
|
|
<0.001a |
| Mean ± SD | 12.31±5.42 | 12.60±4.89 | 11.66±5.48 | 13.79±5.31 | 11.86±4.34 | 14.71±5.09 |
|
|
P-valuec |
| Ref | 0.670 | 0.467 | 1.000 | 0.002 |
|
| Grade, n (%) |
|
|
|
|
|
| 0.572b |
| I | 178 (5.59) | 8 (7.08) | 106 (5.06) | 21 (7.95) | 17 (6.07) | 26 (5.95) |
|
| II | 832 (26.11) | 26 (23.01) | 561 (26.80) | 72 (27.27) | 71 (25.36) | 102 (23.34) |
|
|
III | 734 (23.03) | 25 (22.12) | 470 (22.46) | 55 (20.83) | 65 (23.21) | 119 (27.23) |
|
| IV | 18 (0.56) | 1 (0.88) | 14 (0.67) | 0 (0.00) | 2 (0.71) | 1 (0.23) |
|
|
UNK | 1,425 (44.71) | 53 (46.89) | 942 (45.01) | 116 (43.94) | 125 (44.64) | 189 (43.25) |
|
| SEER stage (26), n
(%) |
|
|
|
|
|
| 0.084b |
|
Localized | 1,103 (34.61) | 38 (33.63) | 720 (34.40) | 102 (38.64) | 97 (34.64) | 146 (33.41) |
|
|
Regional | 1,133 (35.55) | 41 (36.28) | 781 (37.31) | 82 (31.06) | 86 (30.71) | 143 (32.72) |
|
|
Distant | 951 (29.84) | 34 (30.09) | 592 (28.28) | 80 (30.30) | 97 (34.64) | 148 (33.87) |
|
| Tumor size (cm), n
(%) |
|
|
|
|
|
| 0.163b |
| 0–3 | 472 (14.81) | 20 (17.70) | 315 (15.05) | 33 (12.50) | 48 (17.14) | 56 (12.81) |
|
|
3–5 | 636 (19.96) | 31 (27.43) | 420 (20.07) | 54 (20.45) | 54 (19.28) | 77 (17.62) |
|
|
>5 | 2,079 (65.23) | 62 (54.87) | 1,358 (64.88) | 177 (67.05) | 178 (63.57) | 304 (69.57) |
|
| Vascular invasion,
n (%) |
|
|
|
|
|
| 0.899b |
| No | 1,520 (47.69) | 48 (42.48) | 995 (47.55) | 122 (46.21) | 138 (49.29) | 217 (49.66) |
|
|
Yes | 720 (22.59) | 26 (23.01) | 477 (22.79) | 63 (23.86) | 64 (22.86) | 90 (20.59) |
|
|
UNK | 947 (29.71) | 39 (34.51) | 621 (29.67) | 79 (29.92) | 78 (27.85) | 130 (29.75) |
|
| Adjacent invasion,
n (%) |
|
|
|
|
|
| 0.001b |
| No | 2,324 (72.92) | 74 (65.49) | 1,538 (73.48) | 188 (71.21) | 210 (75.00) | 314 (71.85) |
|
|
Yes | 826 (25.92) | 35 (30.97) | 541 (25.85) | 67 (25.38) | 68 (24.29) | 115 (26.33) |
|
|
UNK | 37 (1.16) | 4 (3.54) | 14 (0.67) | 9 (3.41) | 2 (0.71) | 8 (1.83) |
|
| Regional iymph
nodes, n (%) |
|
|
|
|
|
| 0.264b |
| No | 2,091 (65.61) | 81 (71.68) | 1,357 (64.84) | 178 (67.42) | 190 (67.86) | 285 (65.22) |
|
|
Yes | 799 (25.07) | 21 (18.58) | 550 (26.28) | 65 (24.62) | 63 (22.50) | 100 (22.88) |
|
|
UNK | 297 (9.32) | 11 (9.74) | 186 (8.88) | 21 (7.95) | 27 (9.64) | 52 (11.90) |
|
| Metastasis, n
(%) |
|
|
|
|
|
| 0.054b |
| No | 2,102 (65.96) | 72 (63.72) | 1,420 (67.85) | 176 (66.67) | 172 (61.43) | 262 (59.95) |
|
|
Yes | 1,014 (31.91) | 38 (33.63) | 628 (30.00) | 84 (31.82) | 103 (36.79) | 161 (36.84) |
|
|
UNK | 71 (2.23) | 3 (2.65) | 45 (2.15) | 4 (1.51) | 5 (1.78) | 14 (3.21) |
|
| AFP |
|
|
|
|
|
|
<0.001b |
|
Negative | 1,151 (36.12) | 46 (40.71) | 752 (35.93) | 89 (33.71) | 124 (44.29) | 140 (32.04) |
|
|
Borderline | 4 (0.13) | 0 (0.00) | 0 (0.00) | 1 (0.38) | 1 (0.36) | 2 (0.46) |
|
|
Positive | 549 (17.22) | 14 (12.39) | 331 (15.81) | 68 (25.76) | 42 (15.00) | 94 (21.51) |
|
|
UNK | 1,483 (46.53) | 53 (46.90) | 1,010 (48.26) | 106 (40.15) | 113 (40.35) | 201 (46.59) |
|
| Fibrosis score |
|
|
|
|
|
| 0.001b |
| No to moderate | 239 (7.50) | 18 (15.93) | 150 (7.17) | 19 (7.20) | 32 (11.43) | 20 (4.58) |
|
| Severe
to cirrhosis | 148 (4.64) | 4 (3.54) | 103 (4.92) | 14 (5.30) | 8 (2.86) | 19 (4.35) |
|
|
UNK | 2,800 (87.86) | 91 (80.53) | 1,840 (87.91) | 231 (87.50) | 240 (85.71) | 398 (91.07) |
|
| Treatment |
|
|
|
|
|
| 0.001b |
| None/UNK | 1,851 (58.08) | 56 (49.56) | 1,156 (55.23) | 163 (61.74) | 177 (63.21) | 299 (68.42) |
|
|
Radiation | 379 (11.89) | 20 (17.70) | 264 (12.61) | 29 (10.98) | 23 (8.21) | 43 (9.84) |
|
|
Resection | 831 (26.07) | 32 (28.32) | 583 (27.85) | 60 (22.73) | 70 (25.00) | 86 (19.68) |
|
|
Transplant | 30 (0.94) | 1 (0.88) | 23 (1.10) | 2 (0.76) | 1 (0.36) | 3 (0.69) |
|
|
TDT | 96 (3.01) | 4 (3.54) | 67 (3.21) | 10 (3.79) | 9 (3.22) | 6 (1.37) |
|
The primary endpoints of the present study were the
overall survival (OS) time and cause-specific survival (CSS) time.
Survival time was calculated from the date of diagnosis to the date
of mortality. In the OS analysis, any cause of mortality was
treated as an event. In the CSS analysis, an event was defined as
mortality attributed to PLC. Survival time was estimated and
compared using Kaplan-Meier analysis and the log-rank test.
Multivariate analysis was performed to evaluate the
prognostic power of variables for survival time. Variables were
divided into three categories: i) Demographic factors, including
age, sex, residence, race, marital status, household income and
poverty; ii) tumor biological and clinical factors, including
pathology, histological grade, tumor size, vascular invasion,
regional lymph nodes, distant metastasis, SEER stage, AFP level and
fibrosis score; and iii) treatment-associated factors, including
radiation, resection, transplantation, TDT, and none or UNK
therapy. For the multivariate Cox model, for the process for
selecting a category of variables in SPSS software, race/ethnicity
was entered as block 1, the remaining demographic variables were
entered as block 2, tumor biological and clinical variables were
entered as block 3, and treatment-associated factors were entered
as block 4. Variables in blocks 2 and 3 were entered in a forward
stepwise method using the Likelihood Ratio test to determine their
impact on survival time (9). The HR
changes for each group following the stepwise adjustment are
depicted in forest plots.
There are a number of missing values for categorical
variables in the present study. Due to no significant differences
being determined from the analysis of all cases and cases of known
value, the missing values were separated into the subcategory ‘UNK’
and presented in Tables I and
II.
P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed by using
SPSS Statistics software, version 22 for Windows (IBM Corp.,
Armonk, NY, USA) and GraphPad Prism software, version 7 for Windows
(GraphPad Software Inc., La Jolla, CA, USA).
Results
Baseline characteristics
According to the inclusion and exclusion criteria of
the present study, 30,954 patients were identified in the SEER
database from 2004 to 2013. There were 27,767 (89.7%) patients
diagnosed with HCC and 3,187 (10.3%) diagnosed with ICC. Tables I and II show the baseline demographic and
clinical characteristics of the patients with HCC and ICC in the
present study.
In the HCC group (Table
I), 1,241 (4.47%) patients were Chinese, 13,867 (49.94%) were
NHW, 3,654 (13.13%) were African-American, 3,437 (12.38%) were
Other Asian and 5,577 (20.08%) were Hispanic. The mean age of Asian
patients (including Chinese) was increased, compared with the other
groups. African-American patients had the lowest mean age, compared
with the other groups. The sex ratio between the groups was not
identical, with the NHW group having the highest proportion of
males. Additionally, the Chinese group had the highest proportion
of married patients and the lowest proportion of divorced patients.
Of the Chinese patients, approximately 99.59% were living in rural
areas. The household income of the Chinese group was significantly
increased, compared with the other groups. Furthermore, the Chinese
patients had a notably increased education level and a reduced
poverty level. The proportion of localized stage, no vascular
invasion, no adjacent invasion, no regional lymph node metastasis
and no distant metastasis was increased in the Chinese group,
compared with the other groups. Chinese patients with HCC had a
significantly increased probability of exhibiting no to moderate
fibrosis, compared with the other groups. Additionally, Chinese
patients had an increased probability of receiving surgical
resection and had the lowest proportion of no or UNK treatment,
compared with the other groups.
In the ICC group (Table
II), there were 113 (3.55%) Chinese, 2,093 (65.67%) NHW, 264
(8.28%) African-American, 280 (8.79%) Other Asian and 437 (13.71%)
Hispanic patients. The mean age of the Chinese and other groups was
similar, although African-American patients had the lowest mean
age. The sex ratio was similar in each group at approximately 1:1,
which was different from the HCC group. The proportion of each
characteristic for marital status, residence, household income,
education level and poverty level in each group was similar to the
HCC distribution; however, there was no significant difference in
differentiation grade, stage, tumor size, vascular invasion and
metastasis among each group, which was different from the HCC data.
Finally, Chinese patients had the highest proportion of surgical
resection and the lowest proportion of no or UNK treatment.
Effect of race on PLC survival
time
At the time of analysis, 20,767 patients had
succumbed, with 17,413 succumbing due to cancer. The study cohort
was divided into the HCC and ICC groups for survival analysis.
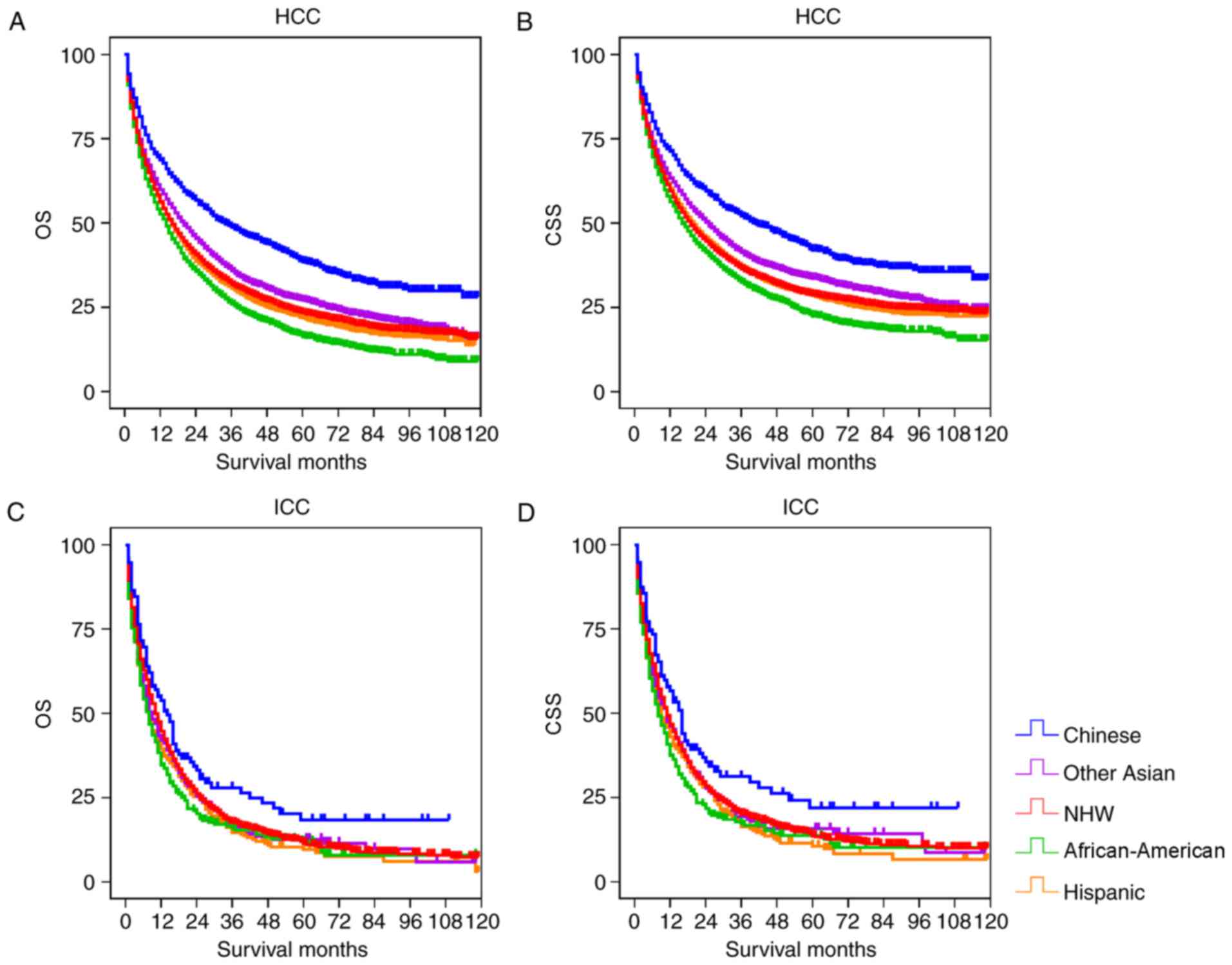
Fig. 1 depicts the OS and CSS rates
of different races in the two groups. In the HCC cohort, the
Chinese patients had significantly increased OS and CSS, compared
with the other groups (P<0.001; Table III). The OS and CSS of Other Asian
patients were significantly increased, compared with the NHW,
Hispanic and African-American groups (P<0.001), but was reduced,
compared with the Chinese group. African-American patients had the
lowest OS and CSS compared with other examined groups. In the ICC
cohort, the Chinese group had significantly increased OS and CSS,
compared with the other groups (P<0.05), but there was no
significant differences between the other races (Fig. 1; Table
IV).
 | Table III.Univariate analysis of prognostic
factors for OS and CSS of patients with hepatocellular carcinoma
with the log-rank test. |
Table III.
Univariate analysis of prognostic
factors for OS and CSS of patients with hepatocellular carcinoma
with the log-rank test.
|
|
| OS | CSS |
|---|
|
|
|
|
|
|---|
| Race/ethnicity | Total | Median
(months) |
Log-ranka | P-value | Median
(months) |
Log-ranka | P-value |
|---|
| Overall | 27,767 | 17 |
|
| 22 |
|
|
| Chinese | 1,241 | 34 |
|
| 46 |
|
|
| NHW | 13,867 | 16 | 114.253 | <0.001 | 21 | 74.836 | .000 |
|
African-American | 3,645 | 14 | 200.141 | <0.001 | 18 | 140.375 | .000 |
| Other Asian | 3,437 | 20 | 55.665 | <0.001 | 27 | 33.551 | .000 |
| Hispanic | 5,577 | 16 | 122.820 | <0.001 | 21 | 78.138 | .000 |
 | Table IV.Univariate analysis of prognostic
factors for overall and cause-specific survival of intrahepatic
cholangiocarcinoma by the log-rank test. |
Table IV.
Univariate analysis of prognostic
factors for overall and cause-specific survival of intrahepatic
cholangiocarcinoma by the log-rank test.
|
|
| OS | CSS |
|---|
|
|
|
|
|
|---|
| Race/ethnicity | Total | Median
(months) |
Log-ranka | P-value | Median
(months) |
Log-ranka | P-value |
|---|
| Overall | 3,187 | 10 |
|
| 11 |
|
|
| Chinese | 113 | 14 |
|
| 16 |
|
|
| NHW | 2,093 | 10 | 4.895 | 0.027 | 11 | 5.343 | 0.021 |
|
African-American | 264 | 8 | 9.490 | 0.002 | 8 | 10.069 | 0.002 |
| Other Asian | 280 | 9 | 5.017 | 0.025 | 11 | 4.331 | 0.037 |
| Hispanic | 437 | 8 | 8.345 | 0.004 | 9 | 8.667 | 0.003 |
Multivariate analysis of PLC CSS
To further determine the prognostic power of a
number of demographic-, socioeconomic-, tumor- and
treatment-associated factors on racial disparities observed in
survival time, forward stepwise multivariate analysis was performed
to identify the HR changes between the Chinese group and the other
groups. Figs. 2 and 3 show forest plots displaying the results
from the multivariate Cox regression model of CSS for all groups in
the present study (the Chinese group was set as the reference).
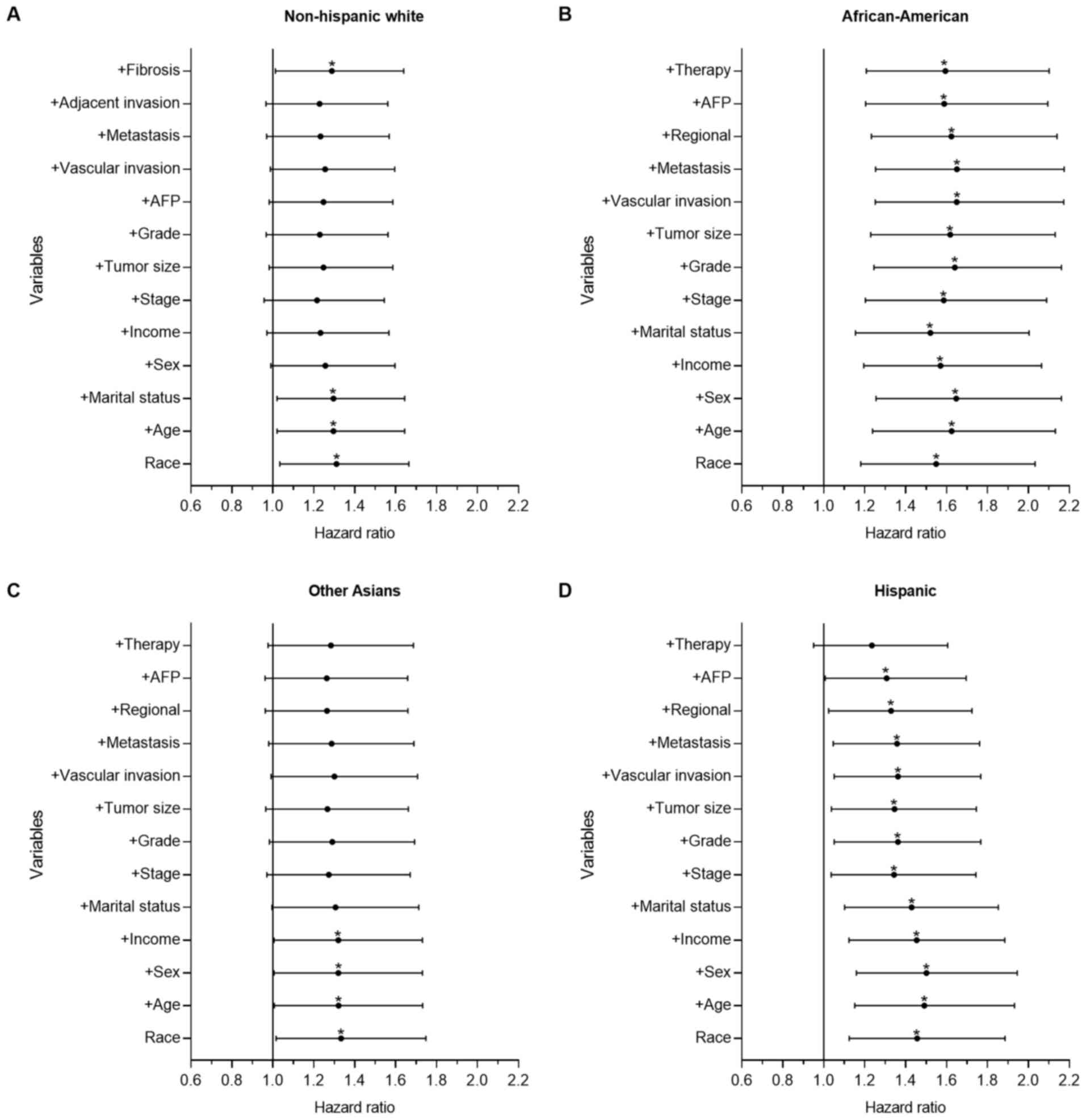 | Figure 3.Forest plot displaying the estimated
HR and 95% CI of ethnicity for the CSS of intrahepatic
cholangiocarcinoma from the multivariate Cox models of all groups.
The Chinese group was set as the reference. The first HR in the
bottom is the crude effect of race/ethnicity followed by HRs
following adjusting for contributing variables in a forward
stepwise method, in order to determine their impact on CSS. (A) HR
of NHW patients, where treatment was the only key factor. (B) HR of
African-American patients, where Chinese patients had significantly
improved survival time, which was demonstrated with univariate and
multivariate analysis. (C) HR of Other Asian patients, where
marital status, tumor biological and clinical variables were the
key factors. (D) HR of Hispanic patients, where treatment was the
only key factor. *P<0.05. HR, hazard ratio; CI, confidence
interval; CSS, cause-specific survival time; NHW, non-Hispanic
white; AFP, α fetoprotein. |
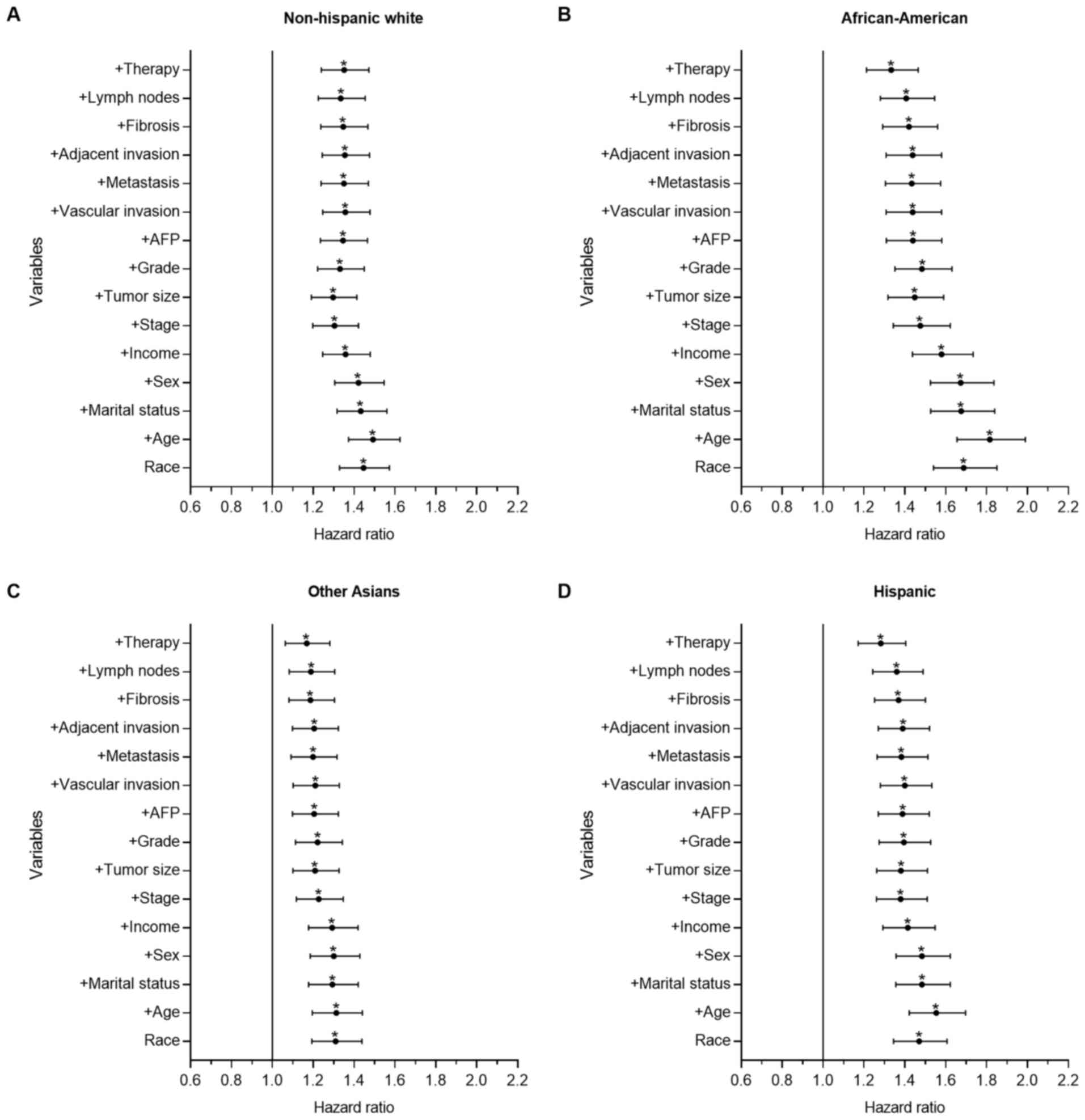
Fig. 2 depicts the
results for the HCC group. There were significant differences
between the Chinese group and the other groups, as demonstrated
with univariate and multivariate analysis. Compared with the
Chinese group, the mortality risk of the Other Asian (P<0.001;
HR=1.310; 95% CI, 1.193–1.438), NHW (P<0.001; HR=1.446; 95% CI,
1.329–1.573), Hispanic (P<0.001; HR=1.470; 95% CI, 1.345–1.607)
and African-American groups (P<0.001; HR=1.688; 95% CI,
1.540–1.850) increased by 31.0, 44.6, 47.0 and 68.8%, respectively.
Following adjusting for all other contributing factors, the
mortality risk of the Other Asian (P=0.001; HR=1.168; 95% CI.
1.063–1.282), NHW (P<0.001; HR=1.351; 95% CI, 1.240–1.472),
Hispanic (P<0.001; HR=1.283; 95% CI, 1.172–1.404) and
African-American groups (P<0.001; HR=1.333; 95% CI, 1.213–1.465)
increased by 16.8, 35.1, 28.3 and 33.3%, respectively. In the HCC
cohort, age, tumor size, stage and treatment served important roles
affecting the observed racial disparities in survival time.
Fig. 3 shows the
results of the ICC group. There were significant differences in the
univariate analysis between the Chinese and the other groups. In
the multivariate analysis, there was no significant difference
between the Chinese group and the Other Asian (P=0.073; HR=1.284;
95% CI, 0.977–1.687) and Hispanic groups (P=0.113; HR=1.235; 95%
CI, 0.951–1.605), although significant differences remained between
the Chinese group and the African-American (P=0.001; HR=1.594; 95%
CI, 1.208–2.101) and NHW groups (P=0.039; HR=1.288; 95% CI,
1.013–1.639). Compared with the Chinese group, the mortality risk
of the NHW and African-American groups increased by 28.8 and 59.4%,
respectively. Treatment served an important role in racial survival
disparities between the Chinese and the other groups.
Racial disparities of CSS in patients
that received surgical resection
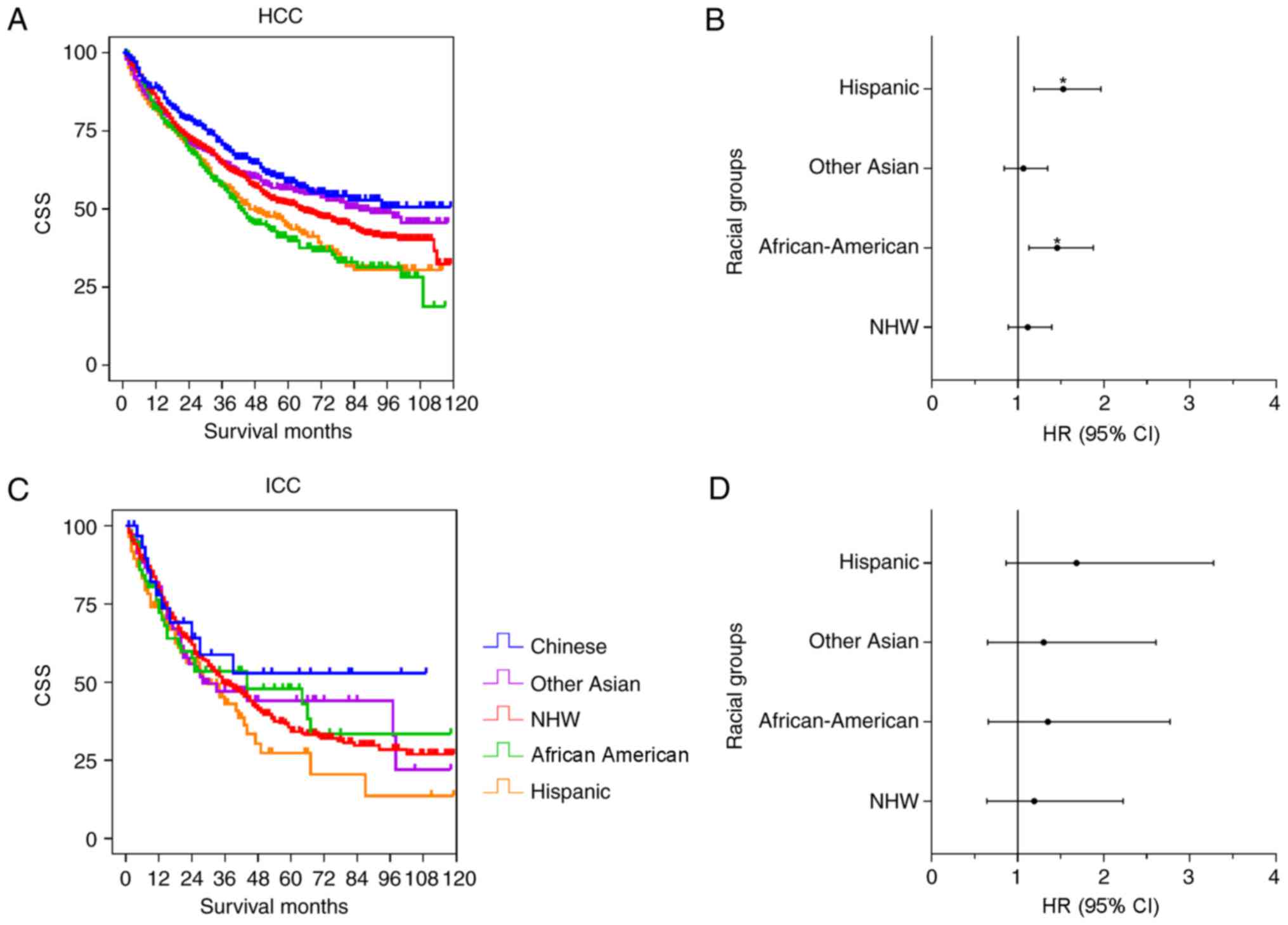
Surgery was the optimal method for curative therapy.
The racial disparities among patients who received surgical
resection was further analyzed. Fig.
4 shows the results from the univariate and multivariate
analysis of the HCC and ICC cohorts. In the HCC cohort (Fig. 4A and B), Chinese, Other Asian and NHW
patients demonstrated improved survival time, compared with the
Hispanic and African-American patients. There were significant
differences between the Chinese group and the NHW (P=0.018),
Hispanic (P<0.001) and African-American (P<0.001) groups,
although no significant difference was observed between the Chinese
group and Other Asian group (P=0.149). Following adjustment for
contributing factors, there was no significant difference between
the Chinese group and the Other Asian (P=0.605; HR=1.064; 95% CI,
0.841–1.345) and NHW groups (P=0.350; HR=1.113; 95% CI,
0.889–1.393), whereas significant differences were observed between
the Chinese group and the Hispanic (P=0.001; HR=1.525; 95% CI,
1.186–1.962) and African-American (P=0.004; HR=1.455; 95% CI,
1.128–1.876) groups (Table V).
Compared with the Chinese group, the mortality risk of the Hispanic
and African American groups increased by 45.5 and 52.5%,
respectively. Fig. 4C and D shows the
ICC data distribution, and there were no significant differences
between the Chinese group and the other groups in the univariate
and multivariate analysis (Table
V).
 | Table V.Multivariate analysis of survival
time in HCC and ICC with liver resection. |
Table V.
Multivariate analysis of survival
time in HCC and ICC with liver resection.
|
| HCC | ICC |
|---|
|
|
|
|
|---|
| Race/ethnicity | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Chinese |
| 1 |
|
| 1 |
|
| NHW | 0.350 | 1.113 | 0.889–1.393 | 0.577 | 1.194 | 0.641–2.226 |
|
African-American | 0.004 | 1.455 | 1.128–1.876 | 0.409 | 1.352 | 0.660–2.770 |
| Other Asian | 0.605 | 1.064 | 0.841–1.345 | 0.457 | 1.302 | 0.650–2.608 |
| Hispanic | 0.001 | 1.525 | 1.186–1.962 | 0.125 | 1.683 | 0.865–3.276 |
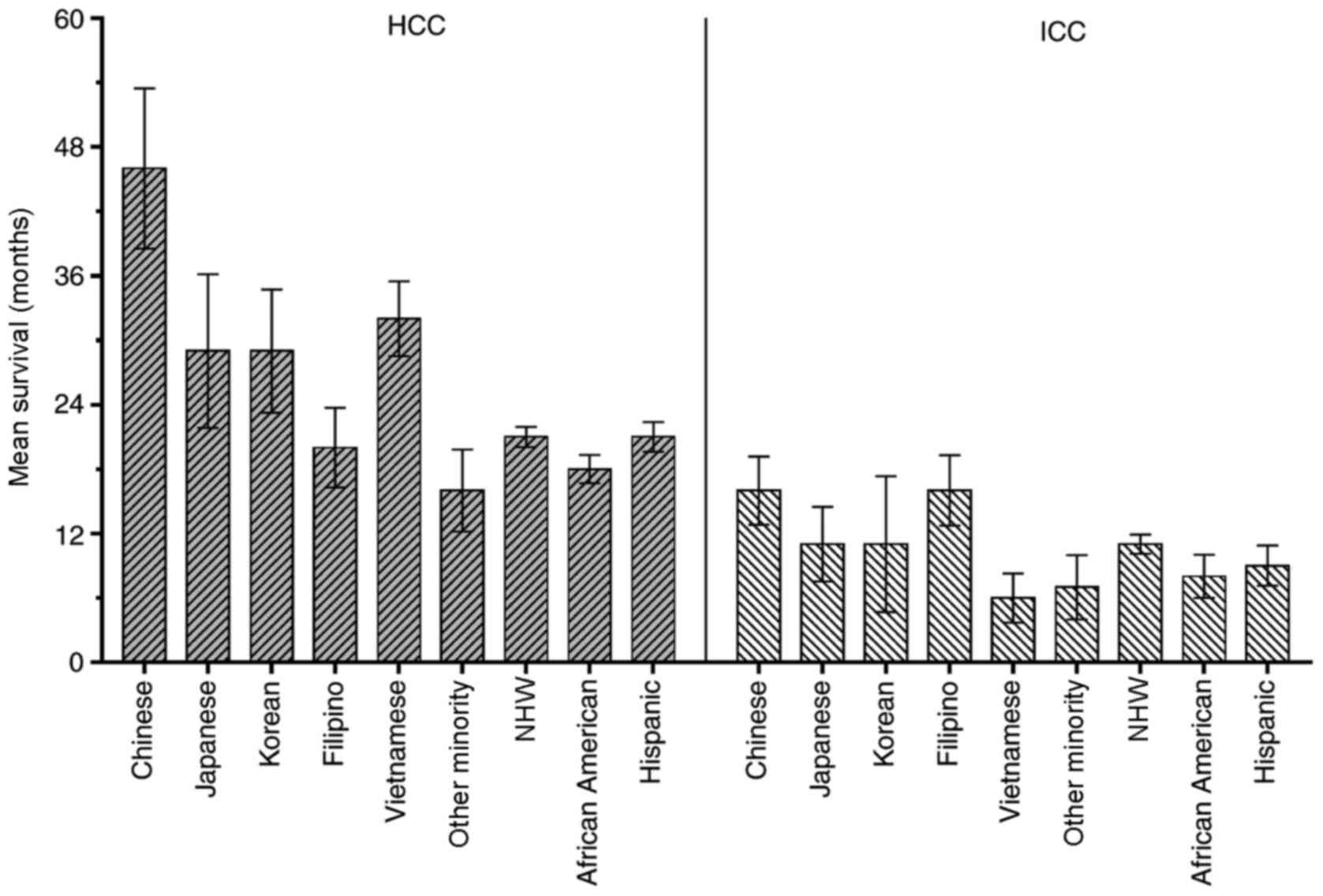
Racial disparities in CSS among Asian
subgroups
The racial disparities for survival time among Asian
subgroups in the present study cohort was further analyzed. In the
HCC group, there were 1,241 (26.5%) Chinese, 434 (9.3%) Japanese,
589 (12.6%) Korean, 759 (16.2%) Filipino, 1,193 (25.5%) Vietnamese
and 462 (9.9%) ‘Other Minority’, including Indian, Pakistani,
Kampuchean and Thai patients. In the ICC group, there were 113
(28.8%) Chinese, 36 (9.2%) Japanese, 53 (13.5%) Korean, 96 (24.4%)
Filipino, 37 (9.4%) Vietnamese and 58 (14.8%) Other Minority
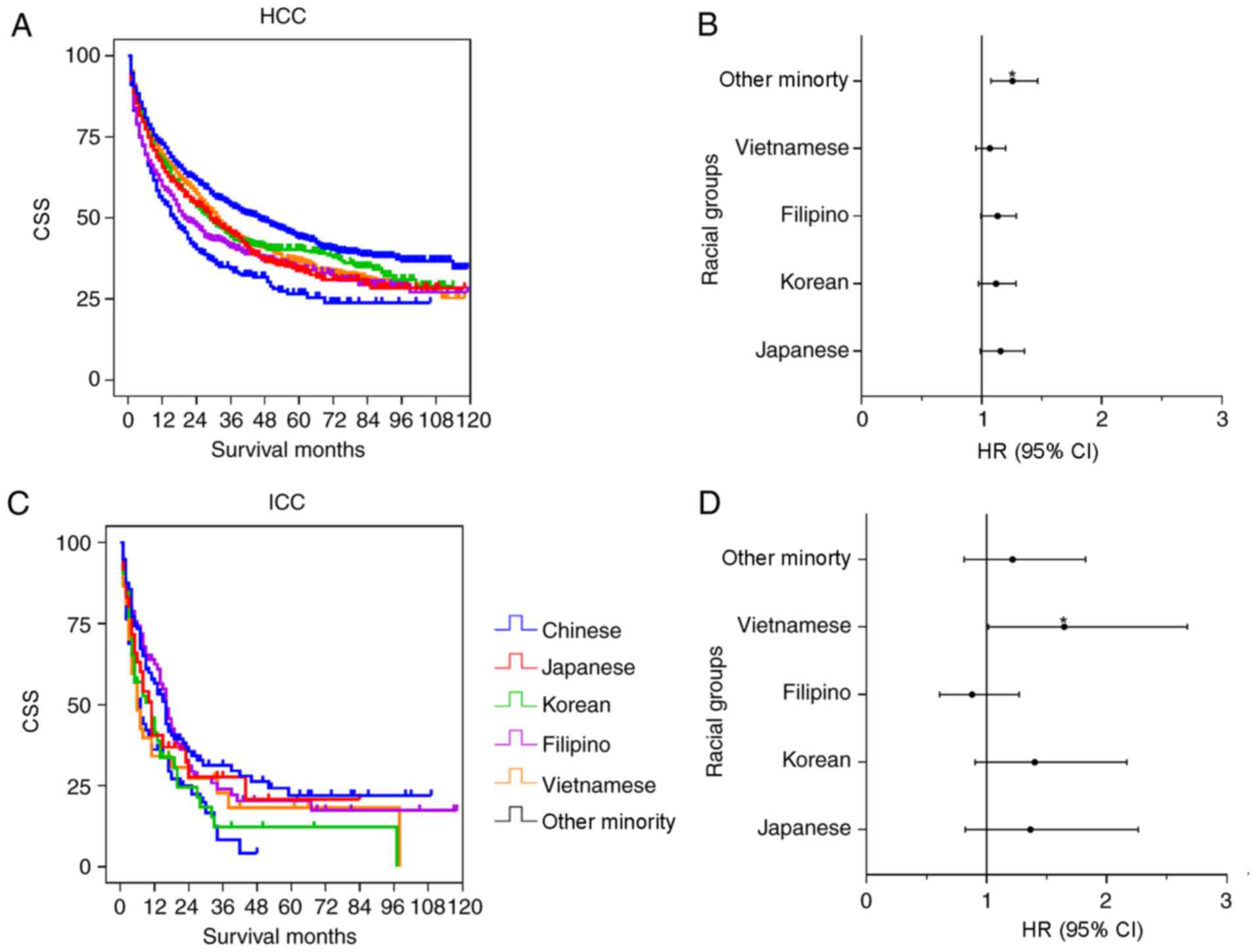
patients. Fig. 5 shows the mean CSS
of the different ethnic groups in the present study. Primarily, the
mean CSS of the HCC group was improved, compared with the ICC
group.
Fig. 6 shows the
results of the univariate and multivariate analysis in the Asian
subgroups. The Chinese group had significantly improved survival
time, compared with the Vietnamese (P=0.002), Japanese (P=0.001),
Korean (P=0.009), Filipino (P<0.001) and Other Minority groups
(P<0.001) in the HCC univariate analysis. However, no
significant differences were observed among the Chinese group and
the other subgroups except for the Other Minority group (P=0.004;
HR=1.257; 95% CI, 1.077–1.466) in the multivariate analysis
(Table VI). For the ICC cohort, the
univariate analysis indicated that the survival time of Chinese
patients was significantly increased, compared with the Korean
(P=0.029) and Other Minority (P=0.004) groups, whereas there were
no significant differences between the Chinese group and the
Japanese (P=0.377), Filipino (P=0.767) and Vietnamese (P=0.062)
groups. In the multivariate analysis, there was a significant
difference between the Chinese group and the Vietnamese group
(P=0.043; HR=1.647; 95% CI, 1.016–2.670). No significant
differences between the Chinese group and the other subgroups were
determined (Table VI).
 | Table VI.Multivariate analysis of survival
time among Asian subgroups. |
Table VI.
Multivariate analysis of survival
time among Asian subgroups.
|
| HCC | ICC |
|---|
|
|
|
|
|---|
| Race/ethnicity | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Chinese |
| Japanese | 0.070 | 1.158 | 0.988–1.356 | 0.229 | 1.364 | 0.822–2.263 |
| Korean | 0.119 | 1.118 | 0.972–1.286 | 0.130 | 1.401 | 0.905–2.167 |
| Filipino | 0.061 | 1.132 | 0.994–1.288 | 0.495 | 0.879 | 0.608–1.272 |
| Vietnamese | 0.263 | 1.068 | 0.951–1.200 | 0.043 | 1.647 | 1.016–2.670 |
| Other minority | 0.004 | 1.257 | 1.077–1.466 | 0.340 | 1.217 | 0.813–1.823 |
Discussion
PLC is one of the cancer types increasing in
incidence and mortality in the USA over the past three decades
(1,2,7).
Race/ethnicity has been confirmed as one of the independent risk
factors affecting liver cancer survival time and thus has attracted
the attention of numerous population-based studies (14,19,27).
Primarily, for PLC, Asian patients have an increased
survival rate, compared with other groups, and African-American
patients have the lowest survival rate (4,27);
however, the way this racial disparity is caused and the factors
that contribute the most important roles remains controversial. A
number of reports demonstrated that Chinese patients have improved
survival outcomes for colorectal and esophageal cancer types
(19,20), but whether this phenomenon occurs for
PLC is UNK. Due to the high prevalence of PLC among the Asian
population (1,28), it was necessary to investigate
prognostic factors in Chinese and other Asian patients.
In the present study, the racial disparities in PLC
were retrospectively analyzed between a Chinese group and other
racial groups for the clinical presentation, treatment and survival
time from a 10-year cohort in the SEER database. It was
demonstrated that the Chinese group had improved survival time,
compared with the other groups in the HCC cohort, following
univariate and multivariate analysis, and analyzed the significance
of the contributing factors generating the racial disparities. The
racial disparities in patients with ICC was also investigated and
it was demonstrated that the Chinese group also had improved
survival time, compared with the other groups, following univariate
analysis, but following multivariate analysis this survival time
benefit was not observed. It was confirmed that a synergistic
effect of contributing factors, including demographic,
socioeconomic, tumor biology and treatment, caused the racial
survival disparity for PLC types.
The association between tumor biological factors and
survival time has been widely investigated (29–31). HCC
and ICC are the two most common types of PLC. Primarily, tumor
size, invasion, differentiation and stage are the dominant
contributing factors for prognosis in HCC and ICC (29,30).
However, due to the differences in etiology and carcinogenesis,
there are significant differences in the tumor biological factors
characteristics of HCC and ICC (21).
Vascular invasion has been confirmed as a key factor
for tumor stage and survival time. Lee et al (32) reported that vascular invasion was
associated with a younger age, aggressive tumor behavior, poor
liver function reserve and poor performance in daily activity,
which negatively impacts HCC survival time. Patients with HCC
vascular invasion are frequently diagnosed with an advanced stage
in the Barcelona Clinic for Liver Cancer staging system (6). In the present study, Chinese patients
had a reduced percentage of vascular invasion and adjacent HCC
invasion, which may partially explain why Chinese patients had
improved survival time. It was also determined that Chinese
patients in the HCC group had an increased probability to exhibit
poor differentiation and increased tumor size, which are associated
with poor survival time (29);
however, there was no significant difference in ICC vascular
invasion among the Chinese and the other groups, although Chinese
patients demonstrated an increased proportion of adjacent invasion.
These results were consistent with the survival analysis that
indicated no significant differences among these groups following
adjusting for contributing factors.
Demographic and socioeconomic factors also
contribute to cancer survival (17,33). Aizer
et al (34) reported that
marriage was an important socioeconomic factor affecting cancer
survival time. He et al (35)
demonstrated that for PLC, married patients had the highest
survival time, and widowed patients had the lowest. In the present
study, the Chinese group had the highest proportion of being
married, compared with the other ethnic groups in the HCC and ICC
cohorts; therefore, marriage may have a positive effect on cancer
prognosis. Married patients have an increased probability to
receive timely detection and are more compliant with proper therapy
recommendations, including surgery, chemotherapy and medications.
Additionally, their spouses provide encouragement and sufficient
social and economic support for medical intervention and curative
therapy. Marriage also has a positive impact on cardiovascular and
endocrine function, cortisol level and immune function, which may
improve the effects of cancer treatment and management (34).
Treatment was another dominant factor affecting
survival time (3,36). In the present study, Chinese patients
had the highest proportion of resection and the lowest proportion
of no or UNK treatment for the HCC and ICC cohorts. Surgical
resection has been recognized as the optimal curative therapy
method for early-stage liver tumor types. The racial disparities
among the Chinese patients who received surgical resections and
patients of other races who received surgical resection were
further analyzed. The results indicated that Chinese patients had
improved survival time, compared with the other groups, in the HCC
cohort following univariate analysis; however, following
multivariate analysis, Chinese patients only had improved survival
time when compared with African-American and Hispanic patients.
Additionally, there was no significant difference between the
Chinese group and the NHW and Other Asian groups. Furthermore,
there were no significant differences between the Chinese group and
the other groups in the ICC cohort. Factors associated with racial
disparities affecting liver cancer survival time following surgical
resection are complex (3,37,38). For
example, the majority of Asian patients with HCC had a hepatitis B
virus (HBV) infection (5). Following
virus screening and antiviral therapy, Chinese patients and other
patients with HBV-associated HCC frequently have an improved liver
function, daily activity and exhibit less comorbidity, reducing the
difficulty of surgical resection and management (37,39);
however, NHW, Hispanic and African-American patients frequently
exhibit hepatitis C virus, non-alcohol fatty liver disease and
alcohol abuse, which are frequently accompanied by cirrhosis,
diabetes and obesity, resulting in disease management difficulties.
Additionally, race was significantly associated with treatment
selection. Hoehn et al (37)
reported that Asian and NHW patients with HCC had an increased
probability of receiving curative therapy, including surgical
resection and transplantation, whereas African-American patients
had a reduced probability of receiving curative therapy. Stewart
et al (11) reported that
Chinese, Korean and Japanese patients with HCC had an increased
probability of receiving surgical treatment, compared with other
Asian populations (11). Selecting an
appropriate treatment is complex. Factors, including the disease
itself, socioeconomic status, insurance status and social support,
serve an important role in treatment selection; however, in the
present study, these data were not available. Furthermore, although
surgical resection was the only curative therapy method for liver
tumor types, patients with ICC still had a poor prognosis, due to
its low early detection rate and higher rate of mortality (22). No other studies reporting racial
disparities in the survival time of patients with ICC have been
reported previously. It is necessary to further investigate the
risk and prognostic factors in the ICC cohort and their effect on
different races.
Previous studies on racial disparities in liver
cancer usually grouped Asian populations into one category and
demonstrated that Asian patients had improved survival time,
compared with other groups (12,37);
however, the term ‘Asian’ encompasses numerous subgroups with
different genetic characteristics, geographic origins and living
habits, each of which has a different etiology, susceptibility and
clinical presentation (16). It was
necessary to investigate the racial disparities in survival time
between Asian subgroups, which assisted in providing treatment
strategies with increased precision for ethnic groups with
different characteristics; therefore, the racial disparities among
Asian subgroups were further analyzed. It was demonstrated that the
Chinese group had improved survival time, compared with other Asian
subgroups, following univariate analysis for the HCC and ICC
cohorts, but the difference was not significant following adjusting
for contributing factors. This result requires further
confirmation, due to the small size of the Other Minority group in
the present study.
There were a number of limitations to the present
study. Firstly, this was a retrospective study, and a number of the
contributing factors associated with prognosis were not included,
due to the limited data in the SEER database. Therefore, the
effects of etiology, comorbidity and chemotherapy on PLC survival
time could not be evaluated, which introduces information bias and
reduces the validity of multifactorial analysis. Secondly, not all
patients in the present study had full data for each covariate, due
to the characteristics of the SEER database. The SEER database is
the only public database that provides large samples for analysis,
which allowed beneficial information to be obtained. Finally, the
underlying mechanisms at the genetic and carcinogenic level could
not be obtained from the SEER database. Nevertheless, to the best
of our knowledge, the present study is the first to analyze racial
disparities between Chinese patients and patients of other racial
groups for PLC, and it provided beneficial information for clinical
practice and future oncological studies.
In conclusion, the racial disparities in clinical
presentation, treatment and survival time of PLC between a Chinese
group and other racial groups was analyzed. It was demonstrated
that Chinese patients had improved survival time, compared with
other patients in the HCC cohort. Furthermore, the prognostic power
of demographic, socioeconomic, tumor biological and
treatment-associated factors on PLC survival time was investigated.
Further study is required with a focus on the large-scale analysis
of genetic characteristics from different ethnic groups, in order
to investigate the underlying mechanisms. Additionally,
personalized therapy based on demographic and socioeconomic status
is beneficial for improving the survival time of patients with
PLC.
Acknowledgements
The authors would like to thank the National Cancer
Institute and the Surveillance, Epidemiology and End Results
Program for the large-scale population-based cancer data, and Dr
Baibing Mi, Department of Epidemiology, Xi'an Jiaotong University
for statistical analysis support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81727802).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FR, XC, LH, RW and YL designed the study. FR, JZ,
ZG, WL, WG and LH acquired and analyzed the data. FR, JZ, ZG, HZ,
ZX and LH interpreted the data. FR, JZ, ZG, XC and LH drafted the
manuscript. LH, RW and YL revised the manuscript critically. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The SEER database does not include any human or
demographic identifying information, and the data used for analysis
were de-identified. Therefore, ethics approval and formal informed
consent to participate was not required.
Patient consent for publication
All identifying information is removed in the
present study; therefore, formal consent for publication was not
required.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PLC
|
primary liver cancer
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
AFP
|
α fetoprotein
|
|
NHW
|
non-Hispanic white
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
SEER
|
Surveillance, Epidemiology, and End
Results
|
|
UNK
|
unknown
|
|
TDT
|
tumor destructive therapy
|
|
OS
|
overall survival
|
|
CSS
|
cause-specific survival
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zak Y, Rhoads KF and Visser BC: Predictors
of surgical intervention for hepatocellular carcinoma: Race,
socioeconomic status, and hospital type. Arch Surg. 146:778–784.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Artinyan A, Mailey B, Sanchez-Luege N,
Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD and
Kim J: Race, ethnicity, and socioeconomic status influence the
survival of patients with hepatocellular carcinoma in the United
States. Cancer. 116:1367–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Q, Li N, Zeng X, Han Q, Li F, Yang C,
Lv Y, Zhou Z and Liu Z: Hepatocellular carcinoma in a large medical
center of China over a 10-year period: Evolving therapeutic option
and improving survival. Oncotarget. 6:4440–4450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Reig ME, Rodriguez De Lope C and
Bruix J: Current strategy for staging and treatment: The BCLC
update and future prospects. Semin Liver Dis. 30:61–74. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975-2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen MH, Garcia RT, Simpson PW, Wright
TL and Keeffe EB: Racial differences in effectiveness of
alpha-fetoprotein for diagnosis of hepatocellular carcinoma in
hepatitis C virus cirrhosis. Hepatology. 36:410–417. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Hansen BE, Peppelenbosch MP, De Man
RA, Pan Q and Sprengers D: Factors associated with ethnical
disparity in overall survival for patients with hepatocellular
carcinoma. Oncotarget. 8:15193–15204. 2017.PubMed/NCBI
|
|
10
|
El-Serag HB, Kramer J, Duan Z and Kanwal
F: Racial differences in the progression to cirrhosis and
hepatocellular carcinoma in HCV-infected veterans. Am J
Gastroenterol. 109:1427–1435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart SL, Kwong SL, Bowlus CL, Nguyen
TT, Maxwell AE, Bastani R, Chak EW and Chen MS Jr: Racial/ethnic
disparities in hepatocellular carcinoma treatment and survival in
California, 1988-2012. World J Gastroenterol. 22:8584–8595. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alawadi ZM, Phatak UR, Kao LS, Ko TC and
Wray CJ: Race not rural residency is predictive of surgical
treatment for hepatocellular carcinoma: Analysis of the texas
cancer registry. J Surg Oncol. 113:84–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sloane D, Chen H and Howell C: Racial
disparity in primary hepatocellular carcinoma: Tumor stage at
presentation, surgical treatment and survival. J Natl Med Assoc.
98:1934–1939. 2006.PubMed/NCBI
|
|
14
|
Welzel TM, Graubard BI, Quraishi S, Zeuzem
S, Davila JA, El-Serag HB and McGlynn KA: Population-attributable
fractions of risk factors for hepatocellular carcinoma in the
United States. Am J Gastroenterol. 108:1314–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong LL, Hernandez B, Kwee S, Albright CL,
Okimoto G and Tsai N: Healthcare disparities in Asians and Pacific
Islanders with hepatocellular cancer. Am J Surg. 203:726–732. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sawai H, Nishida N, Mbarek H, Matsuda K,
Mawatari Y, Yamaoka M, Hige S, Kang JH, Abe K, Mochida S, et al: No
association for Chinese HBV-related hepatocellular carcinoma
susceptibility SNP in other East Asian populations. BMC Med Genet.
13:472012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makarova-Rusher OV, Altekruse SF, McNeel
TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF and McGlynn KA:
Population attributable fractions of risk factors for
hepatocellular carcinoma in the United States. Cancer.
122:1757–1765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin J, Qiu M, Xu R and Sandra Dobs A:
Comparison of survival and clinicopathologic features in colorectal
cancer among African American, Caucasian and Chinese patients
treated in the United States: Results from the surveillance
epidemiology and end results (SEER) database. Oncotarget.
6:33935–33943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin MQ, Li YP, Wu SG, Sun JY, Lin HX,
Zhang SY and He ZY: Differences in esophageal cancer
characteristics and survival between Chinese and Caucasian patients
in the SEER database. Onco Targets Ther. 9:6435–6444. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esnaola NF, Meyer JE, Karachristos A,
Maranki JL, Camp ER and Denlinger CS: Evaluation and management of
intrahepatic and extrahepatic cholangiocarcinoma. Cancer.
122:1349–1369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Yang T, Wu M and Shen F:
Intrahepatic cholangiocarcinoma: Epidemiology, risk factors,
diagnosis and surgical management. Cancer Lett. 379:198–205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antwi SO, Mousa OY and Patel T: Racial,
ethnic, and age disparities in incidence and survival of
intrahepatic cholangiocarcinoma in the united states; 1995-2014.
Ann Hepatol. 17:604–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Cancer Institute. Overview of the
SEER Program. https://seer.cancer.gov/about/overview.html
|
|
25
|
National Cancer Institute. SEER*Stat
Databases. November. 2015, Submission. https://seer.cancer.gov/data-software/documentation/seerstat/nov2015/.
|
|
26
|
National Cancer Institute. SEER Program
Coding and Staging Manual. 2018, https://seer.cancer.gov/tools/codingmanuals/index.html
|
|
27
|
Davila JA and El-Serag HB: Racial
differences in survival of hepatocellular carcinoma in the United
States: A population-based study. Clin Gastroenterol Hepatol.
4:104–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: Past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou
YY, Su CW, Lin HC and Huo TI: Vascular invasion in hepatocellular
carcinoma: Prevalence, determinants and prognostic impact. J Clin
Gastroenterol. 48:734–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang D, Hanna DL, Usher J, LoCoco J,
Chaudhari P, Lenz HJ, Setiawan VW and El-Khoueiry A: Impact of sex
on the survival of patients with hepatocellular carcinoma: A
surveillance, epidemiology and end results analysis. Cancer.
120:3707–3716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aizer AA, Chen MH, McCarthy EP, Mendu ML,
Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE,
et al: Marital status and survival in patients with cancer. J Clin
Oncol. 31:3869–3876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He XK, Lin ZH, Qian Y, Xia D, Jin P and
Sun LM: Marital status and survival in patients with primary liver
cancer. Oncotarget. 8:64954–64963. 2016.PubMed/NCBI
|
|
36
|
Teo EK, Han SH, Terrault N, Luketic V,
Jensen D, Keeffe EB and Lok AS; HBV-OLT Study Group, . Liver
transplantation in patients with hepatitis B virus infection:
Outcome in Asian versus white patients. Hepatology. 34:126–132.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoehn RS, Hanseman DJ, Wima K, Ertel AE,
Paquette IM, Abbott DE and Shah SA: Does race affect management and
survival in hepatocellular carcinoma in the United States? Surg.
158:1244–1251. 2015. View Article : Google Scholar
|
|
38
|
Couto CA, Gelape CL, Calmet F, Martin P
and Levy C: Effect of ethnicity on liver transplant for
hepatocellular carcinoma. Exp Clin Transplant. 11:339–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mathur AK, Osborne NH, Lynch RJ, Ghaferi
AA, Dimick JB and Sonnenday CJ: Racial/ethnic disparities in access
to care and survival for patients with early-stage hepatocellular
carcinoma. Arch Surg. 145:1158–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|