Introduction
Anaplastic thyroid cancer (ATC) is rare,
representing only 1–2% of all thyroid cancer cases (1); however, it accounts for up to 50% of all
thyroid cancer-related mortalities (2). ATC is more common in the elderly, and it
almost always develops from a pre-existing well-differentiated
thyroid cancer (3,4). The prognosis of ATC is extremely poor,
with a recorded median survival time from diagnosis of ~4 months
and a 1-year survival rate of ≤20% (5,6);
furthermore, an analysis of 516 cases using the US Surveillance,
Epidemiology and End Results database showed the median survival
time to be 3 months and the 1-year survival rate to be 19.3%
(3). Even in patients treated with a
combined modality of surgery and chemotherapy, the 2-year survival
rate is poor (7). The survival rate
of patients with ATC has not changed over the past 20 years
(8). Furthermore, it is unclear
whether a comprehensive therapy, including chemotherapy and
radiotherapy, followed by palliative surgery, will improve the
prognosis in all patients. Sorafenib was approved for clinical use
in 2014 for treating Iodine-131 refractory differentiated thyroid
cancer (DTC), but not for anaplastic cancer; certain investigators
supported regulatory approval of lenvatinib for treating
unresectable thyroid cancer in Japan (9). In 2015, the Japanese Ministry of Health,
Labor and Welfare approved the clinical use of lenvatinib for
treating patients with ATC and DTC; this drug is now commercially
available there for the same purpose. Lenvatinib is a
multi-tyrosine kinase inhibitor (TKI) that targets vascular
endothelial growth factor (VEGF) receptors 1–3, fibroblast growth
factor (FGF) receptors 1–4, platelet-derived growth factor
receptor-α, and the ret proto-oncogene and KIT proto-oncogene
receptor tyrosine kinase (10). In a
preclinical study, lenvatinib demonstrated antitumor activity in
mouse ATC xenograft models (11). In
addition, the aforementioned phase 2 study reported the safety and
efficacy of lenvatinib in 17 patients with ATC, with a median
progression-free survival time of 7.4 months [95% confidence
interval (CI), 1.7–12], a median OS time of 10.6 months (95% CI,
3.8–19.8) and an objective response rate of 24 (9). We previously reported a minor series
study on lenvatinib for 7 patients with ATC in 2017 (12). We reported that the response rate was
43%, and the disease control rate was 57% under the limitation of a
short follow-up period and evaluating only the highest response of
lenvatinib for ATC.
The present study reports the response rate of 23
patients with ATC to lenvatinib. The aim of the study was to assess
the safety and efficacy of lenvatinib in patients with stage IVC
ATC (Tumor-Node-Metastasis staging system 8th edition) (13). Furthermore, the management of severe
adverse events (AEs) associated with lenvatinib use in order to
maximize the benefits obtained from this TKI treatment is
discussed.
Materials and methods
Patients
The present study was a retrospective study
analyzing the clinical data from 23 patients with unresectable and
pathologically confirmed ATC who were treated at Kanagawa Cancer
Center (Yokohama, Kanagawa, Japan) between April 2015 and March
2017. Patients diagnosed with stage IVC ATC and treated with
lenvatinib were included, while those who had been treated with
other anticancer agents prior to using lenvatinib were excluded.
Patients who could not take oral lenvatinib due to ATC-related
dysphagia were also excluded. A total of 2 patients were initially
diagnosed with stage IVB disease, but once distant metastasis was
confirmed during surgery, IVC was diagnosed, and the patients were
registered in the study. No stage IVA patients were encountered
during the enrollment period. A total of 8 patients underwent
surgical resection for the primary tumor prior to lenvatinib
treatment in order to prevent extensive spread to the nearby
important organs, including the air tract, esophagus and common
carotid artery, and 2 patients underwent tumor volume reduction and
prophylactic tracheotomy. The remaining 13 patients were not
eligible to undergo any surgical treatment. A study was thus
performed on whether lenvatinib can be considered a novel orphan
drug for patients with ATC that cannot be otherwise treated.
Overall, 19 patients received lenvatinib at a daily
dose of 24 mg/day, while the remaining 4 patients started it at
daily doses of 20 mg (1 patient), 14 mg (2 patients) and 10 mg (1
patient) due to low body weight (BW) and poor performance status.
The median duration of treatment was 5.4 months (range 0.4–27.9
months). Dose interruptions and incremental reductions in the dose
(to 20, 14 or 10 mg/day) were permitted in case of toxic effects.
The chemotherapy committee of the hospital approved the lenvatinib
regimen, and the patients individually signed consent forms
following adequate explanation of the treatment.
The study population comprised 15 women and 8 men
(median age, 77.0 years; age range, 42–89 years). The tumor size
was 44.2±17.8 mm (median ± standard deviation), with a 25th
percentile of 29.0 mm and a 75th percentile of 58.5 mm. The median
± SD of BW was 55.6±12.2 kg, with a 25th percentile of 46.6 kg and
a 75th percentile of 59.3 kg. Patient characteristics are
summarized in Table I.
 | Table I.Patient baseline clinical
characteristics (n=23). |
Table I.
Patient baseline clinical
characteristics (n=23).
| Characteristics | Value |
|---|
| Median age (range),
years | 77.0 (42–89) |
| Gender, n (%) |
|
| Male | 7 (30.4) |
|
Female | 16 (69.6) |
| PS, n (%) |
|
| 0 | 11 (47.8) |
| 1 | 7 (30.4) |
| 2 | 5 (21.7) |
| Site of metastasis, n
(%) |
|
| Lung | 19 (82.6) |
|
Bone | 3 (13.0) |
|
Others | 9 (39.1) |
| Median size of
tumor, mma | 44.2±17.8
(29.0–58.5) |
| BW, kga | 55.6±12.2
(46.6–59.3) |
| Median initial
dose, mga | 21.9±4.3
(24–24) |
| Median ongoing
dose, mga | 11.5±3.0
(9.5–14) |
Evaluation
The radiological response to the TKI therapy was
classified on the basis of the Response Evaluation Criteria In
Solid Tumors (RECIST) version 1.1 criteria (14) as follows: Complete remission (CR),
partial response (PR), stable disease (SD) and progressive disease
(PD). Safety was assessed according to the National Cancer
Institute Common Toxicity Criteria version 3.0 (15). In order to evaluate safety, the
occurrence of any AE (grade 3–5) and the time for treatment
discontinuation were recorded. Disease control rate (DCR) was
defined as the percentage of patients who had CR, PR or SD. The
assessment of the response evaluation was based on the RECIST
criteria evaluated 1, 2 and 3 months post-treatment, with ≥4 weeks
of response persistence. This continued until the final follow-up
date or mortality without an end point or best response. The
administration period for patients in whom the treatment was
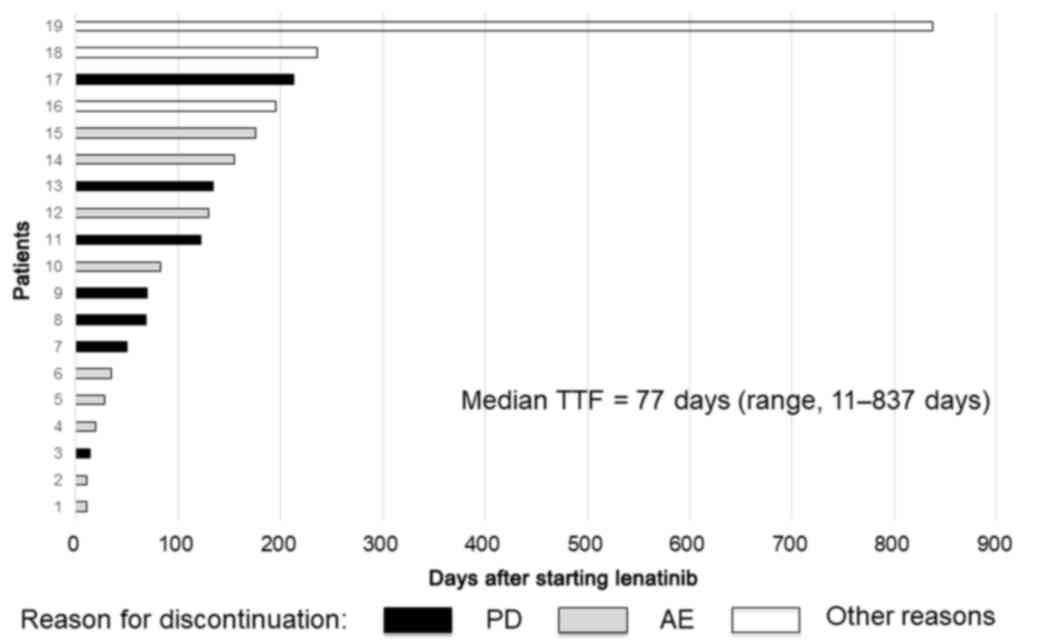
discontinued is graphically shown in Fig.
1 as the time to treatment failure (TTF) together with the
reason for discontinuation.
Statistical analysis
Overall survival (OS) was defined as the time
elapsed between the dates of the first treatment and mortality.
Kaplan-Meier estimator on the SPSS software (version 24; IBM Corp.,
Armonk, NY, USA) was used to calculate OS and applied the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. To verify the efficacy of lenvatinib, the
OS times of patients who were treated with surgery first and of
those who were treated with lenvatinib only were also calculated.
Efficacy analysis of OS was summarized by the Kaplan-Meier method
using median time with 95% confidence interval (CI).
Results
Patients
The proportion of patients who received lenvatinib
and experienced treatment-related AEs was 100%. The most common AE
was hypertension (21/23, 91%). Other common AEs were general
fatigue and anorexia (15/23, 65%), proteinuria (14/23, 61%) and
tumor-skin fistulas (6/23, 26%). The majority of the AEs, with the
exception of the tumor fistulas, could be controlled with
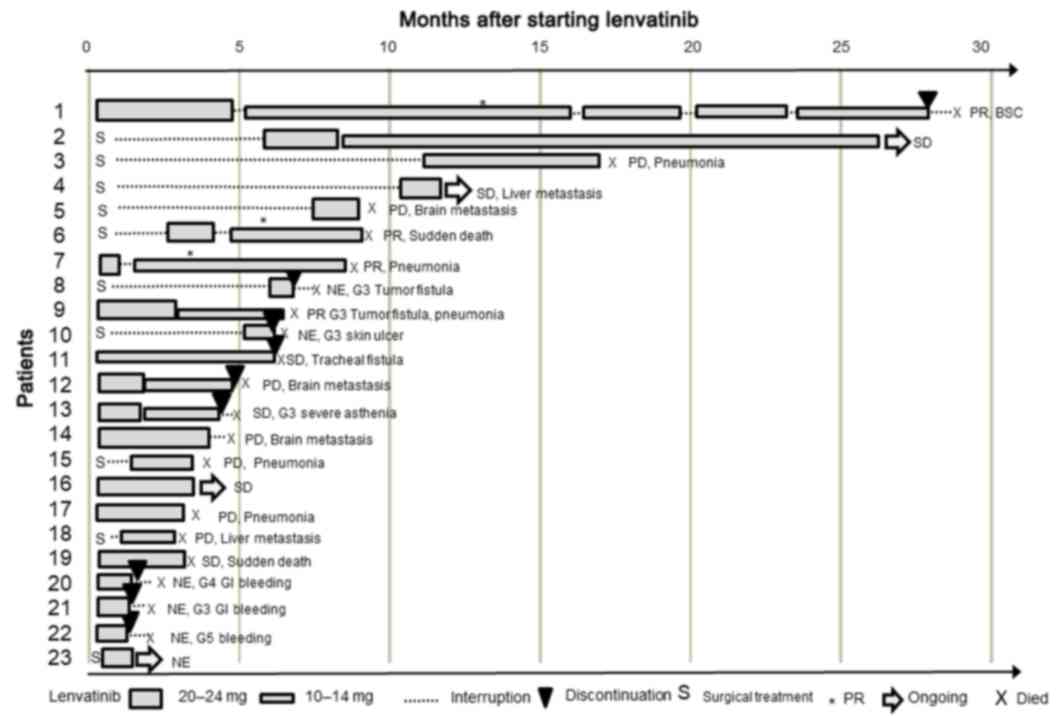
medication. The individual progress of the patients has been
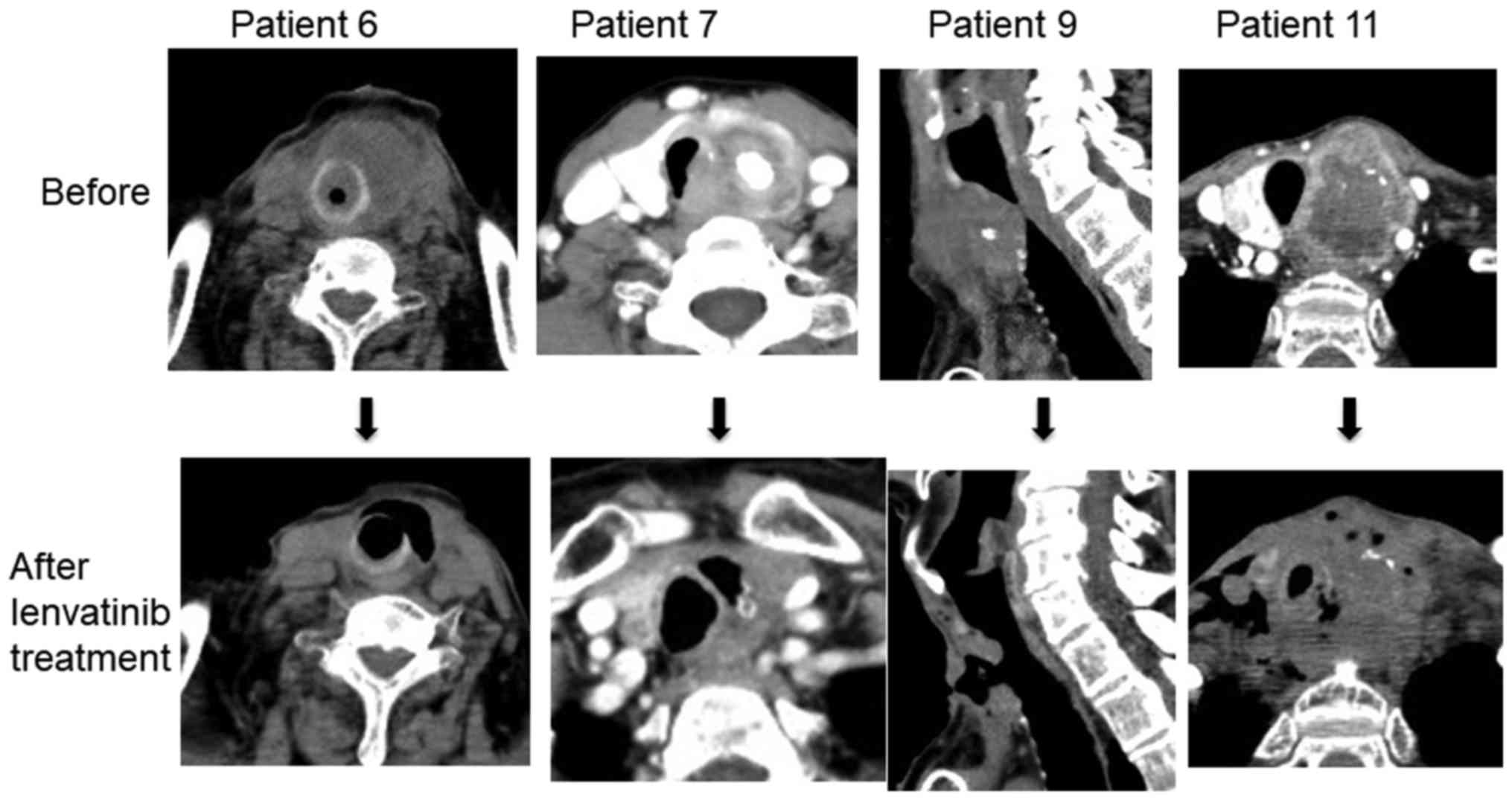
graphically presented with a swimmer plot in Fig. 2. Patients with tracheal fistulas are
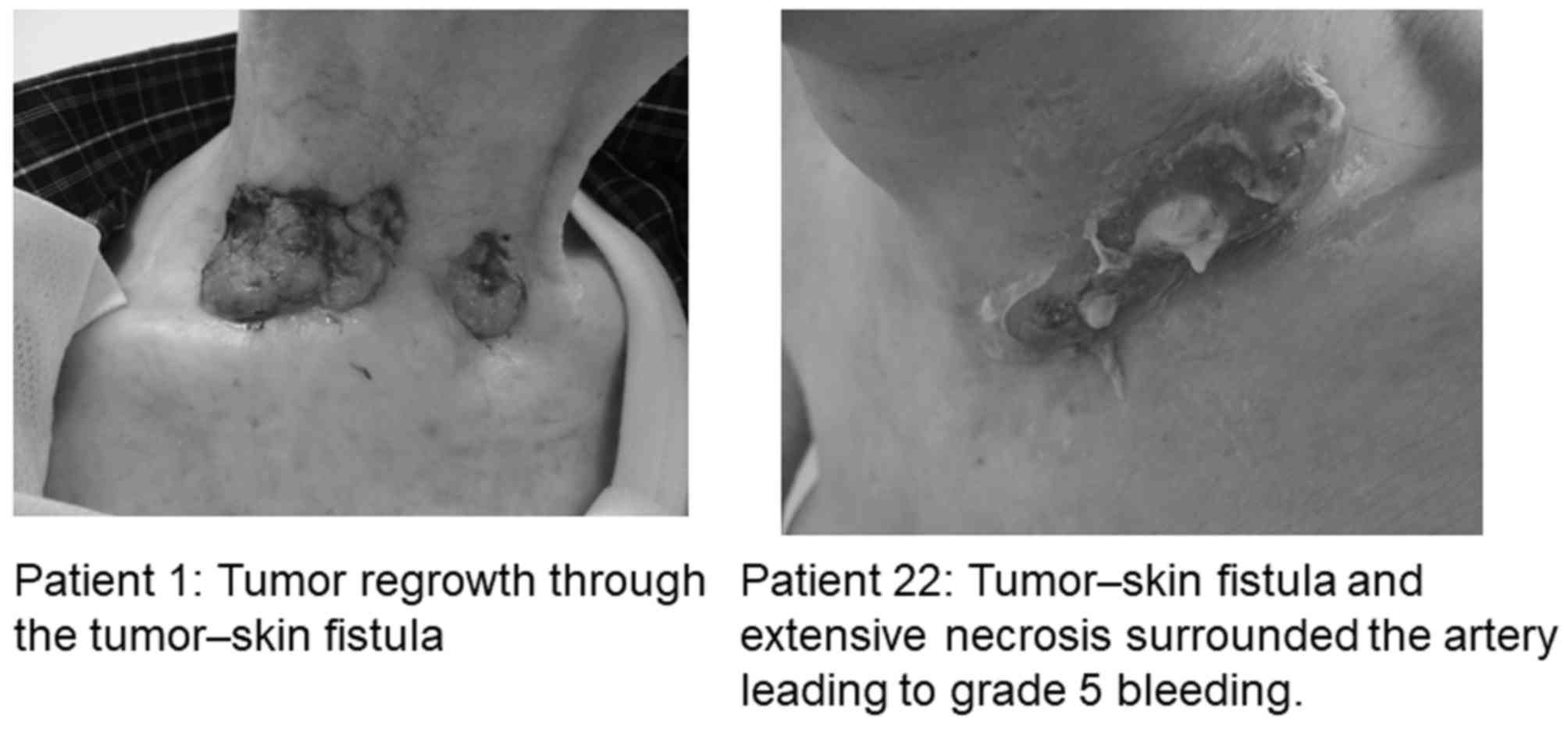
shown in Fig. 3, and representative
images of the fistulas are shown in Fig.
4.
A total of patients (patient nos. 1, 6, 7 and 9)
exhibited a PR, 6 patients (patient nos. 2, 4, 11, 13, 16 and 19)
experienced SD and 4 patients (patient nos. 5, 12, 14 and 18)
developed new lesions (3 brain metastases and 1 liver metastasis)
that were eventually considered PD. In addition, 3 patients
(patients 3, 15 and 17) exhibited PD. The remaining 6 patients
(patient nos. 8, 10 and 20–23) were not evaluated, as they could
not undergo examinations at 1-month intervals and were designated
as non-evaluable. A total of 9 (39%) patients discontinued the
treatment due to treatment-related AEs that were grade 3 or higher.
These 9 patients succumbed within 1 month of the cessation of the
lenvatinib, an indication of the aggressiveness of the ATC. The
treatment in 2 patients was resumed following healing of the tumor
fistula or cavitation, and they exhibited a PR. A total of 19
patients discontinued the treatment, and they all succumbed; their
TTFs are shown in Fig. 4. The reasons
for treatment discontinuation were as follows: AEs, 9 patients; PD,
7 patients; and other reasons (sudden mortality, aspiration
pneumonia or treatment rejection by the patient), 3 patients. The
median TTF was 77 days (range, 11–837 days).
Efficacy
The overall response rate (ORR) was 17.4% and the
DCR was 43.5% (Table II). The median
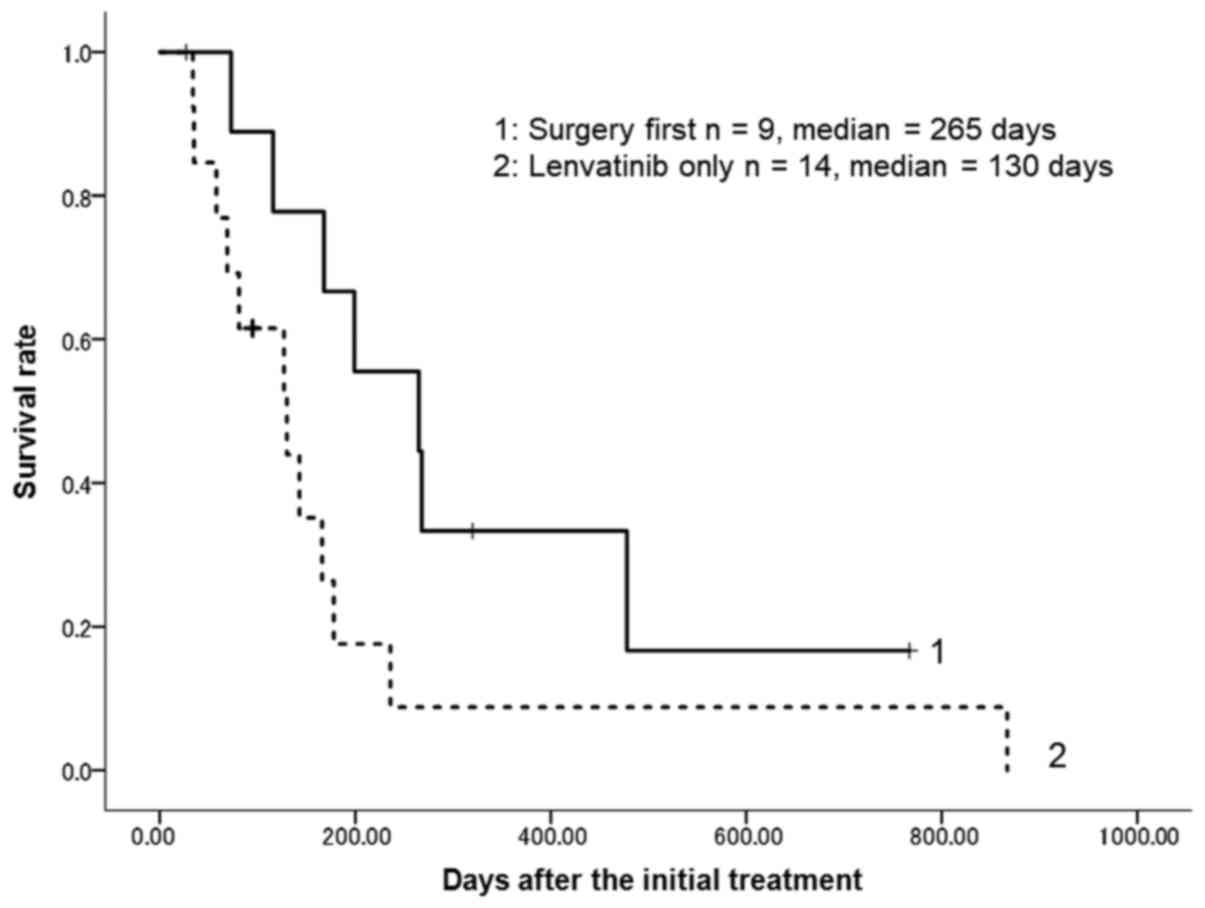
OS time was 166 days. The OS time of the patients treated with
surgery first was greater than that of the patients treated with
lenvatinib only. The median OS time was 130 days (95% CI, 58–178)
for patients treated with lenvatinib only, whereas it was 265 days
(95% confidence interval, 73–478) for those treated with surgery
first (Fig. 5). Although the survival
curves for the two groups appeared to be different, there was no
statistically significant difference between the groups (log-rank
test, P=0.07).
 | Table II.Antitumor effectiveness of lenvatinib
in patients with anaplastic thyroid carcinoma (n=23). |
Table II.
Antitumor effectiveness of lenvatinib
in patients with anaplastic thyroid carcinoma (n=23).
| Response to
lenvatinib | Patients, n
(%) |
|---|
| Complete
response | 0 (0.0) |
| Partial
response | 4 (17.4) |
| Stable disease | 6 (26.1) |
| Progressive
disease | 7 (30.4) |
| Not evaluable | 6 (26.1) |
| Overall response
rate | 4 (17.4) |
| Disease control
rate | 10 (43.5) |
Discussion
Current therapies for ATC have limited efficacy;
when combined with other chemotherapies, a higher response rate
(50%) can be achieved, but the duration of the response is often
short (2–5 months) (16). A phase 2
trial (17) of paclitaxel in patients
with ATC reported an ORR of 53%; another trial of carboplatin and
paclitaxel in combination with fosbretabulin reported a
non-significant increase in OS time (18). The median OS time was 5.2 months (95%
CI, 3.1–9.0) for the CP/fosbretabulin arm [n=55; hazard ratio 0.73
(95% CI, 0.44–1.21)] and 4.0 months (95% CI, 2.8–6.2) for the CP
arm (n=25; P=0.22 (log-rank test)]. A phase 2 trial targeted ATC
cases with recurrent masses observed following surgery or
subsequent to any additional external irradiation (16); by contrast, the present study was
fundamentally different from the aforementioned study, as it was a
single-arm study for patients treated with lenvatinib alone. In
addition, the researchers of the paclitaxel trial had to modify the
response criteria by decreasing the requirement of response
persistence from 4 to 2 weeks due to quick disease progression.
Although the ORR was 53%, the median survival of all patients
following the diagnosis (25 weeks) was almost identical to that of
patients in the present study (24 weeks). Given the poor prognosis,
the American Joint Committee on Cancer staging system considers all
patients diagnosed with ATC to have stage IV disease;
subcategorization into stages IVA, IVB and IVC is based on whether
the cancer is confined to the thyroid gland, has extra thyroidal
extension or has metastasized to distant sites, respectively
(19). Hence, as unresected ATC is
almost certainly lethal, the American Thyroid Association (ATA)
guidelines recommend that surgery be performed if technically
possible and if not likely to cause any unacceptable morbidity
(20). Stage IVA tumors are
resectable, as intrathyroidal anaplastic tumors are classified as
stage IVA disease, while the presence of lymph node involvement or
gross extrathyroidal extension without distant metastasis is
classified as stage IVB. (21) Stage
IVB tumors can be either resectable or unresectable depending on
the expertise of the surgeon. However, Haigh et al (22) reported aggressive surgery to be
worthwhile in selective cases when combined with chemotherapy
and/or radiotherapy, even if some macroscopic disease was left
behind to preserve organ function. The median survival rates in
patients with stage IVA, IVB and IVC disease have been reported as
9.00, 4.80 and 3.02 months, respectively (23). Among the present study patients, 9
underwent surgery prior to lenvatinib treatment. Surgery provides
the advantage of securing the airway and preventing fistula
formation when there is no residual tumor around the airway
(22). OS time was compared in
patients initially treated with surgery and in those treated with
lenvatinib only. The surgery-first patients experienced a prognosis
extension of 135 days compared with the median OS value; however,
there was no significant difference between the surgery-first and
lenvatinib-only groups. In patients who did not undergo surgery,
fistula formation occurred in 6/14 patients (42.9%), and the
fistula was difficult to treat. According to studies that reported
tumor fistulas in 2010 and 2011, frequent dose interruptions are
required for patients receiving oral TKIs (sorafenib, sunitinib
and/or lenvatinib) (24,25). Stage IVC ATCs were typically treated
with systemic chemotherapy and/or radiation therapy or palliative
care options prior to lenvatinib approval for clinical use in 2015
(2). Certain multicenter
collaborative studies and trials on TKI treatment for ATCs have
been published since then (9,26,27).
Tahara et al (9) reported a
phase 2 clinical trial and included only 6 (35%) patients with
stage IVC ATC; the remaining patient population was comprised of 4
(24%), 5 (29%) and 2 (12%) patients with stage IVA, stage IVB and
unknown stage ATC, respectively. Moreover, the patients previously
underwent treatments, including surgery (n=14; 82%), chemotherapy
(n=7; 41%) and radiation (n=9; 53%). The present study only
targeted patients with stage IVC ATCs with extremely poor
prognoses, and was designed as a single-arm study with lenvatinib
as the first-line anticancer drug.
There are certain limitations to the present study,
including the small sample size, the retrospective design,
including bias and confounding factors, the lack of a comparator
group, and the inability to generalize the results to other
populations. Nevertheless, to the best of our knowledge, this is
the first report on 23 patients treated with lenvatinib only for
stage IVC ATC, and the results will provide useful data for future
treatment studies. Based on these findings, it may be concluded
that lenvatinib is a potential candidate for clinical trials with
concomitant medicines in the future; for example, using a v-raf
murine sarcoma viral oncogene homolog B1 (BRAF) inhibitor for
treating a tumor with BRAF mutation should clearly provide good
results (28). Also, to the best of
our knowledge, the present study is the first to compare the
results of lenvatinib and surgery among patients with stage IVC ATC
in the same institution. Future studies should focus on
demonstrating the benefits of lenvatinib as the standard treatment
for stage IVC ATC, on how to decrease the incidence of AEs and on
how to manage the severe AEs associated with lenvatinib use.
Regarding the most frequent AEs, hypertension,
gastrointestinal symptoms and hand-foot syndrome may respond to
oral medicines or Hirudoid Soft® Ointment 0.3% for skin
disorders. In the present study, patients required a short-term
interruption of lenvatinib for recovering from AEs, including
proteinuria, fatigue, anorexia and thrombocytopenia. For managing
more serious AEs, the present study describes our experience with
tumor fistulas and lenvatinib-induced bleeding.
In the treatment of anaplastic carcinoma, the
biggest problem is that the majority of patients present with
unresectable primary tumors, and this may explain the development
of necrosis or fistulas during lenvatinib treatment. Once a fistula
develops, frequent dose interruptions are required to prevent the
spread of the fistula (29). Recent
guidelines published by the ATA discuss the management of the
compromised airway in such patients, and suggest that a tracheotomy
should be performed to secure the airway in circumstances of
life-threatening airway obstruction (30). Airway compromise with a thyroid mass
may be a presenting feature of ATC, or it may occur in a patient
with a pre-existing diagnosis of the disease (30). Notably, patient nos. 8 and 10 in the
present study (Fig. 2) underwent
prophylactic tracheotomies to prevent later respiratory
complications. However, their follow-up showed no benefit from the
lenvatinib treatment. Each of these patients suffered from local
wound healing complications, thus, treatment was discontinued and
their OS times were significantly lower than those of other
patients. The patients in the present study were ineligible for
chemotherapy or radiation therapy, and their survival was estimated
to be ~3 months (23) without
lenvatinib treatment. The present study demonstrates that
lenvatinib is of value as an orphan drug for stage IVC ATC.
Although surgical resections of primary tumors lead to longer OS
times even in the presence of distant metastasis, volume reduction
surgeries and prophylactic tracheotomies complicate matters and
make lenvatinib treatment difficult.
Patient no. 20 in the present study (Fig. 2) developed grade 4 gastrointestinal
bleeding, and patient no. 22 developed grade 5 tumor bleeding
during lenvatinib treatment. Tumor-skin fistulas were observed in 6
patients, and 1 patient (patient no. 22) experienced direct
exposure of the carotid artery to the fistula cavity that likely
contributed to the subsequent carotid blowout syndrome with
torrential hemorrhage (24). The
complete encasement of the artery whether by the tumor or necrotic
tissues requires careful drug administration or dose interruptions.
Vascular disruption by inhibition of existing VEGF/VEGF
receptor-dependent tumor blood vessels often leads to tumor
necrosis and cavitation, and the same mechanism is likely to
explain the protracted wound healing observed with lenvatinib
use.
In conclusion, the present study assessed the
treatment outcomes in 23 patients with unresectable ATC at a single
institute and discussed the management of AEs associated with
lenvatinib use. Generally, a DTC, even subsequent to recurrences
and with distant metastases, is slowly progressive, whereas ATC
exhibits an extremely poor prognosis, with chemotherapy overall
being ineffective against it. Although lenvatinib showed a limited
positive effect, it is associated with a high incidence of AEs, and
this must be weighed against the benefits of the treatment. The
successful treatment of fistulas developed due to necrosis is
crucial for improving the treatment outcomes. Future investigations
on VEGF or FGF expression in ATC should assist in optimizing the
analyses for lenvatinib efficacy and for preventing
treatment-related fatal AEs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HI, HY and NS designed the study. HT analyzed the
data. HN checked analysis and interpretation data, especially
statistical analysis. NS, HN, ST and KM contributed by performing
the surgery and caring for the patients. ST and KM contributed to
data acquisition. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The chemotherapy committee of Kanagawa Cancer Center
(Yokohama, Kanagawa, Japan) approved this regimen of lenvatinib for
use in patients with ATC. The cancer board of the hospital also
approved lenvatinib treatment, including surgery, for patients with
ATC. The study was approved by the Institutional Review Board of
Kanagawa Cancer Center.
Patient consent for publication
All patients provided a comprehensive consent form
stating that personal data could be used for academic presentation
or paper presentation while ensuring complete anonymity prior to
receiving the treatment.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HI is an endocrine surgeon working at the Kanagawa
Cancer Center and has an extensive experience of several surgeries
for ATC, as well as ATC treatment.
Glossary
Abbreviations
Abbreviations:
|
AE
|
adverse event
|
|
ATA
|
American Thyroid Association
|
|
ATC
|
anaplastic thyroid cancer
|
|
BW
|
body weight
|
|
DCR
|
disease control rate
|
|
DTC
|
differentiated thyroid cancer
|
|
FGF
|
fibroblast growth factor
|
|
ORR
|
overall response rate
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PR
|
partial response
|
|
RECIST
|
Response Evaluation Criteria In Solid
Tumors
|
|
SD
|
stable disease
|
|
TKI
|
tyrosine kinase inhibitor
|
|
TTF
|
time to treatment failure
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Kilfoy BA, Devesa SS, Ward MH, Zhang Y,
Rosenberg PS, Holford TR and Anderson WF: Gender is an age-specific
effect modifier for papillary cancers of the thyroid gland. Cancer
Epidemiol Biomarkers Prev. 18:1092–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowe NM, Loughran S, Slevin NJ and Yap BK:
Anaplastic thyroid cancer: The addition of systemic chemotherapy to
radiotherapy led to an observed improvement in survival-a single
centre experience and review of the literature. In:
ScientificWorldJournal. 2014:6745832014.
|
|
3
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venkatesh YS, Ordonez NG, Schultz PN,
Hickey RC, Goepfert H and Samaan NA: Anaplastic carcinoma of the
thyroid. A clinicopathologic study of 121 cases. Cancer.
66:321–330. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smallridge RC and Copland JA: Anaplastic
thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol
(R Coll Radiol). 22:486–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neff RL, Farrar WB, Kloos RT and Burman
KD: Anaplastic thyroid cancer. Endocrinol Metab Clin North Am.
37:525–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh EM, Lee KE, Kwon H, Kim EY, Bae DS and
Youn YK: Analysis of patients with anaplastic thyroid cancer
expected to have curative surgery. J Korean Surg Soc. 83:123–129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bisof V, Rakusic Z and Despot M: Treatment
of patients with anaplastic thyroid cancer during the last 20
years: Whether any progress has been made? Eur Arch
Otorhinolaryngol. 272:1553–1567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for anaplastic thyroid cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui J and Funahashi Y: Preclinical
biomarker research and patient stratification of molecular target
agents: The anti-angiogenic inhibitor Lenvatinib mesylate (E7080)
(Japanese). Nihon Yakurigaku Zasshi. 142:162–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamazaki H, Shimizu S, Iwasaki H, Yoshida
T, Suganuma N, Yamanaka T, Kojima I, Masudo K, Toda S, Nakayama H
and Masuda M: Efficacy and safety of lenvatinib for unresectable
anaplastic thyroid cancer. Gan To Kagaku Ryoho. 44:695–697.
2017.PubMed/NCBI
|
|
13
|
Tuttle RM, Haugen B and Perrier ND:
Updated american joint committee on cancer/tumor-node-metastasis
staging system for differentiated and anaplastic thyroid cancer
(Eighth edition): What changed and why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Japanese translation of common terminology
criteria for adverse events (CTCAE), and instructions and
guidelines (Japanese). Int J Clin Oncol. 9 (Suppl):1–82. 2004.
|
|
16
|
Derbel O, Limem S, Ségura-Ferlay C,
Lifante JC, Carrie C, Peix JL, Borson-Chazot F, Bournaud C, Droz JP
and de la Fouchardière C: Results of combined treatment of
anaplastic thyroid carcinoma (ATC). BMC Cancer. 11:4692011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ain KB, Egorin MJ and DeSimone PA:
Treatment of anaplastic thyroid carcinoma with paclitaxel: Phase 2
trial using ninety-six-hour infusion. Collaborative anaplastic
thyroid cancer health intervention trials (CATCHIT) group. Thyroid.
10:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sosa JA, Elisei R, Jarzab B, Balkissoon J,
Lu SP, Bal C, Marur S, Gramza A, Yosef RB, Gitlitz B, et al:
Randomized safety and efficacy study of fosbretabulin with
paclitaxel/carboplatin against anaplastic thyroid carcinoma.
Thyroid. 24:232–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goffredo P, Thomas SM, Adam MA, Sosa JA
and Roman SA: Impact of timeliness of resection and thyroidectomy
margin status on survival for patients with anaplastic thyroid
cancer: An analysis of 335 cases. Ann Surg Oncol. 22:4166–4174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American thyroid association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haigh PI, Ituarte PH, Wu HS, Treseler PA,
Posner MD, Quivey JM, Duh QY and Clark OH: Completely resected
anaplastic thyroid carcinoma combined with adjuvant chemotherapy
and irradiation is associated with prolonged survival. Cancer.
91:2335–2342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hui EP, Ma BB, King AD, Mo F, Chan SL, Kam
MK, Loong HH, Ahuja AT, Zee BC and Chan AT: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Machiels JP, Henry S, Zanetta S, Kaminsky
MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S,
et al: Phase II study of sunitinib in recurrent ormetastatic
squamous cell carcinoma of the head and neck: GORTEC 2006-01. J
Clin Oncol. 28:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Savvides P, Nagaiah G, Lavertu P, Fu P,
Wright JJ, Chapman R, Wasman J, Dowlati A and Remick SC: Phase II
trial of sorafenib in patients with advanced anaplastic carcinoma
of the thyroid. Thyroid. 23:600–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weitzman SP and Cabanillas ME: The
treatment landscape in thyroid cancer: A focus on cabozantinib.
Cancer Manag Res. 7:265–278. 2015.PubMed/NCBI
|
|
28
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blevins DP, Dadu R, Hu M, Baik C,
Balachandran D, Ross W, Gunn B and Cabanillas ME: Aerodigestive
fistula formation as a rare side effect of antiangiogenic tyrosine
kinase inhibitor therapy for thyroid cancer. Thyroid. 24:918–922.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mani N, McNamara K, Lowe N, Loughran S and
Yap BK: Management of the compromised airway and role of
tracheotomy in anaplastic thyroid carcinoma. Head Neck. 38:85–88.
2016. View Article : Google Scholar : PubMed/NCBI
|