Introduction
In recent years, bladder cancer has become the most
common urogenital neoplasm in China. The incidence of bladder
cancer ranks first in the genitourinary cancers and it has a high
recurrence rate after treatment. Therefore, it is imperative to
obtain an effective method to treat this disease (1). However, ideal prognosis indicators are
not yet specified (2–4) for the pathogenesis of bladder cancer and
specific prognostic information is scarce, although many
histological and biological indicators, such as staging and grading
of cancer cells, infiltrated degree of blood vessels, size of
tumor, single or multiple and deoxyribose nucleic acid (DNA) ploidy
are used to evaluate the development tendency of bladder
cancer.
With the development of molecular biology,
biologists have studied the occurrence and developmental change
rules of bladder cancer at the genetic level, which has become the
study focus in recent years. The genes related to bladder cancer
include the cancer gene, cancer suppressor gene and DNA mismatching
repair gene (5,6). Wilms tumor 1-associated protein (WTAP)
is a gene closely related to occurrence and developmental change
and is involved in the formation of multiple tumors, thus playing
an important role. On the contrary, there are no reports on its
effects in bladder cancer thus far.
Therefore, the aim of the study was to investigate
the relationship between WTAP expression in bladder cancer and the
recurrence and prognosis of bladder cancer, providing theoretical
bases for the diagnosis, treatment and prognosis of bladder
cancer.
Materials and methods
Collection of tumor tissues
Specimens were obtained from the patients who
underwent surgery in the Zhongnan Hospital of Wuhan University
(Wuhan, China) and had complete hospitalization data. A total of 62
fresh specimens of bladder transitional cell cancer tissues were
collected as the bladder cancer group (48 males and 14 females),
while 20 normal bladder mucosa specimens were selected as the
control group (14 males and 6 females). The average age in the
control group was 57±19 years and that in the bladder cancer group
was 52±13 years. Through comparisons, there were no statistically
significant differences in the sex and age between the two groups
(P>0.05).
The study was approved by the Ethics Committee of
Zhongnan Hospital of Wuhan University and informed consent was
signed by the patients or guardians.
Main reagents
The main reagents used were: bicinchoninic acid
(BCA) protein assay kit (Beyotime, Shanghai, China); TRIzol total
ribose nucleic acid (RNA) extraction kit (Tiangen Biotech, Beijing,
China); reverse transcription-polymerase chain reaction (RT-PCR)
kit (Tiangen Biotech); rabbit anti-human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) and WTAP polyclonal antibodies (cat. nos.
2118 and 56501, respectively; Cell Signaling Technology, Inc.;
Danvers, MA, USA).
Hematoxylin and eosin (H&E)
staining
Bladder tissues in the two groups were embedded into
paraffin wax and made into paraffin blocks which were cut into 5-µm
sections as the blank sections. Then, H&E staining was
conducted using the routine histopathological method. The stained
specimens were observed under light microscope (×200, Olympus
Corporation, Tokyo, Japan) and then histopathological analysis was
carried out.
Immunohistochemistry
Prepared tissue paraffin sections of two groups were
soaked in xylene (10 min each time, 2 times), and then in the
gradient ethanol for 5 min. After the antigen repair,
phosphate-buffered saline (PBS) was used to wash the resulting
products (3 min each time, 3 times). Streptavidin peroxidase (SP)
staining was followed by PBS washing (3 min each time, 3 times).
PBS was then absorbed and normal goat serum for blocking was
dripped into the sections for 15 min incubation at room
temperature. The rabbit anti-human WTAP polyclonal antibody (1:200)
were added by dripping and the wet boxes were placed into the
refrigerator at 4°C overnight. After PBS washing (5 min each time,
3 times), SignalStain® Boost IHC Detection Reagent
secondary antibody (1:600; cat. no. 8114; Cell Signaling
Technology, Inc.) was added by dripping for 15 min incubation at
37°C. Then, the working fluid was dripped into the foregoing
product for incubation under the same conditions, after PBS washing
again (5 min each time, 3 times). Then, the freshly-prepared
diaminobenzidine (DAB) solution was dripped into the incubated
sections and the color development degree was observed and
controlled under the microscope. In addition, hematoxylin was used
to re-stain the sections which were differentiated for 20 sec by 1%
hydrochloric acid alcohol to restore the blue color. Neutral gum
was used to seal the section after the dehydration by gradient
ethanol. All the stained specimens were observed under an Eclipse
TE2000-U light microscope (×200) (Nikon Corp., Tokyo, Japan) and
the analysis was performed.
RT-PCR testing
The tissues of the control group and the bladder
cancer group were transferred into the Eppendorf (EP) tubes
containing the RNAiso Plus extraction solution, respectively. They
were settled at room temperature for 5 min to fully disintegrate
the specimens. The specimens were then centrifuged at 12,000 × g at
4°C. The supernatant was obtained and was added with 0.2 ml
chloroform. The supernatant was mixed evenly and settled under the
same conditions as above. Afterwards, the mixed solution was
centrifuged at 12,000 × g at 4°C for 15 min and the supernatant was
absorbed which was added with the same volume of isopropanol. After
mixing evenly, the solution was settled for 10 min at room
temperature. Then, the solution was centrifuged under the same
conditions as above for 10 min and the sediments were reserved with
the supernatant carefully removed, and added with 1 ml 75% ethanol.
After mixing evenly, centrifugation was conducted under the same
conditions for 5 min. Similarly, the supernatant was removed and
the centrifugation was repeated once. After RNA sediments were
washed and the liquid was removed with the RNase-free water added.
Part of total RNA solution was diluted into the 1 µg/µl solution
and the reverse transcription reaction solution was prepared
according to the requirements in the instructions of
PrimeScript® RT reagent kit with genomic DNA (gDNA)
eraser and was added with the corresponding RNA specimens to
conduct the reverse transcription and further obtain the
complementary DNA (cDNA). The resulting cDNA was stored under
−20°C. Subsequently, the level of mRNA was determined as indicated
in the instructions of teh SYBR® Premix Ex Taq™ II (Tli
RNaseH Plus) kit. The primer sequences of WTAP were: 5′-3′ CAACCT
CTTTAGCCAAACAAGAA and 3′-5′ ATTCCTGAGTGC AACAGC.
Western blot detection
The tissues of the control and bladder cancer groups
were washed with the iced normal saline and the proteins were
extracted according to the protocol of the total protein extraction
kit. The immunoprecipitation (IP) lysis buffer containing
phenylmethanesulfonyl fluoride (PMSF) and protease inhibitor were
added into the ice and the tissues were fully ground. The tissue
homogenate was centrifuged at 12,000 × g at 4°C for 10 min and the
supernatant was taken to be centrifuged under the same conditions
for 20 min. The resulting supernatant was taken. The protein
content was then tested according to the protocol of the protein
kit and the protein sample containing the same content of total
proteins was added for sampling to conduct the sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under
constant voltage of 220 V, until the bromophenol blue reached the
bottom of gel. The gel was cut according to the molecular weight of
the target protein and the separated proteins were
electro-transferred onto the polyvinylidene fluoride (PVDF)
membrane. The PVDF membrane attached with the proteins was placed
in 5% skim milk and blocked in the shaking incubator at room
temperature for 3 h. Rabbit anti-human GAPDH and WTAP polyclonal
antibodies (1:1,000) were added for incubating and were placed at
4°C overnight. The next day, the membranes were fully washed using
Tween-Tris buffered saline (TTBS) (10 min each time, 3 times) and
the goat anti-rabbit secondary polyclonal antibody (1:2,000; cat.
no. 7074; Cell Signaling Technology, Inc.) were added to incubate
at room temperature for 1 h. After TTBS washing (10 min each time,
3 times), the enhanced chemiluminescence (ECL) developing solution
was dripped to develop the color and then imaged.
Statistical processing
Statistical Product and Service Solutions 17.0 (SPSS
17.0) statistical software was used for the statistical analyses
and the single factor analyses for the related data were conducted
by Kaplan-Meier method. The significance of the differences was
compared by the log-rank test. The effects of multiple clinical and
pathological factors on the recurrence time were studied using Cox
proportional hazard model. P<0.05 was considered to indicate a
statistically significant difference.
Results
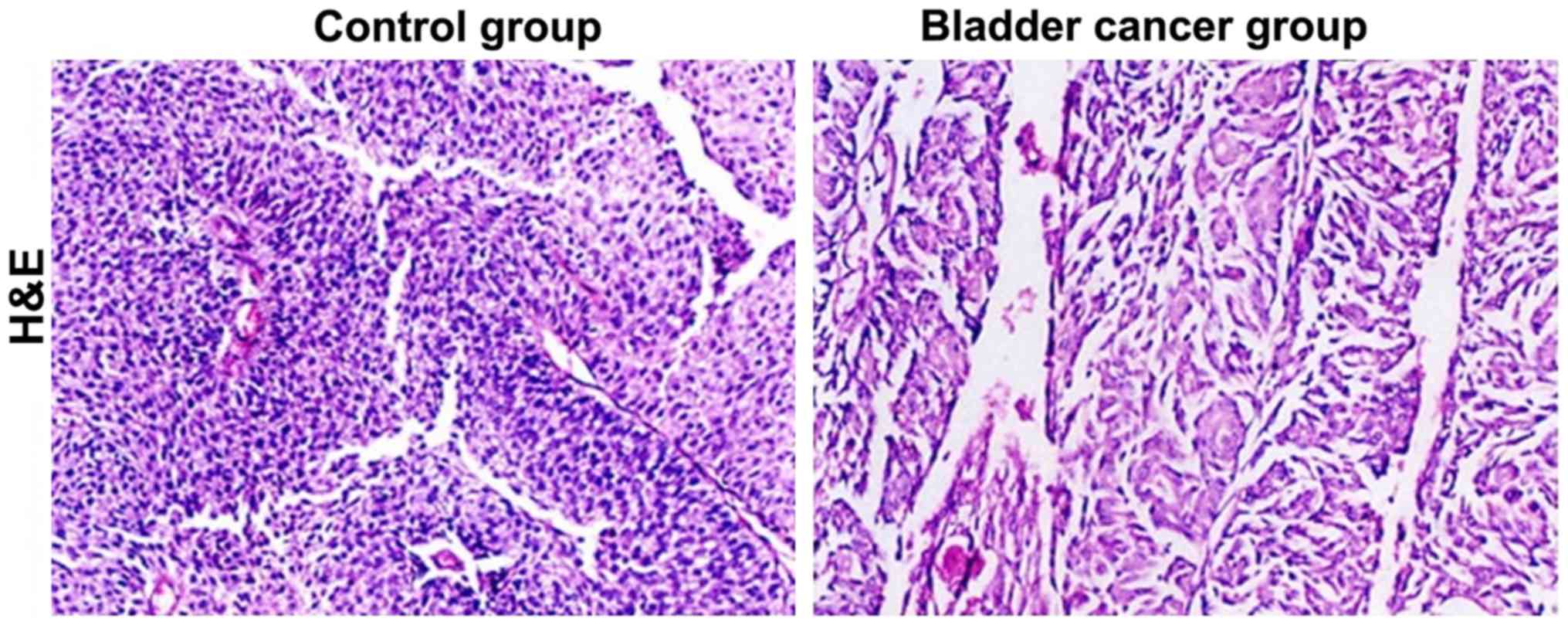
Pathological conditions observed by
H&E staining method
The H&E staining sections of bladder tissues in
the control and bladder cancer groups were used to specify the
pathological morphology and the cell growth features of bladder
cancer. Compared with tissues of the control group, those of the
bladder cancer group were destroyed and the cell nuclei were
damaged (Fig. 1). Moreover, there
were a large number of infiltrating inflammatory cells with
significant histopathology.
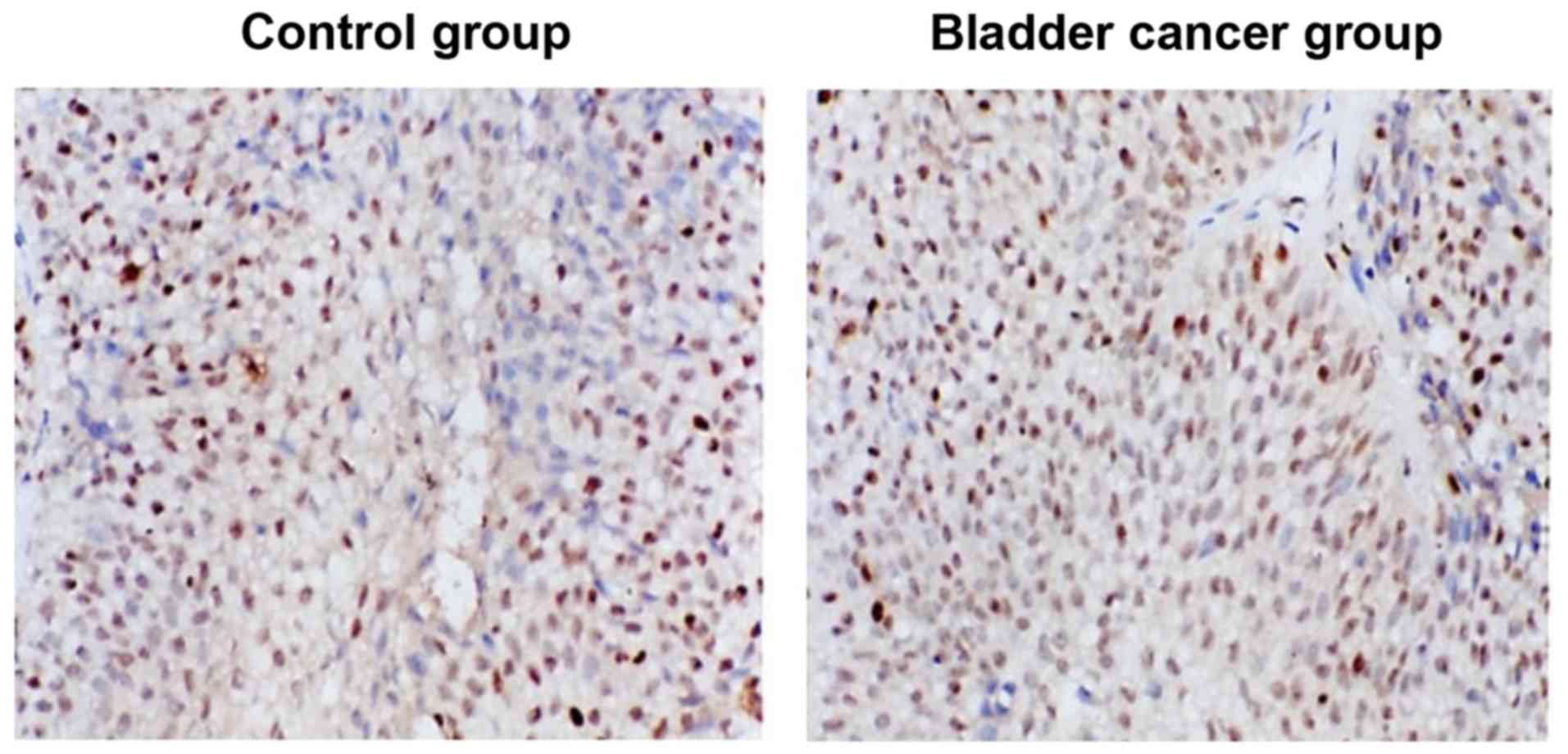
Immunohistochemical staining
As shown in Fig. 2,
WTAP was low-expressed in the control group and brownish-yellow and
tan cell nuclei were generated in the tissue sections after WTAP
immunohistochemical staining. Compared with WTAP expression of the
control group, that in the bladder cancer group was significantly
increased.
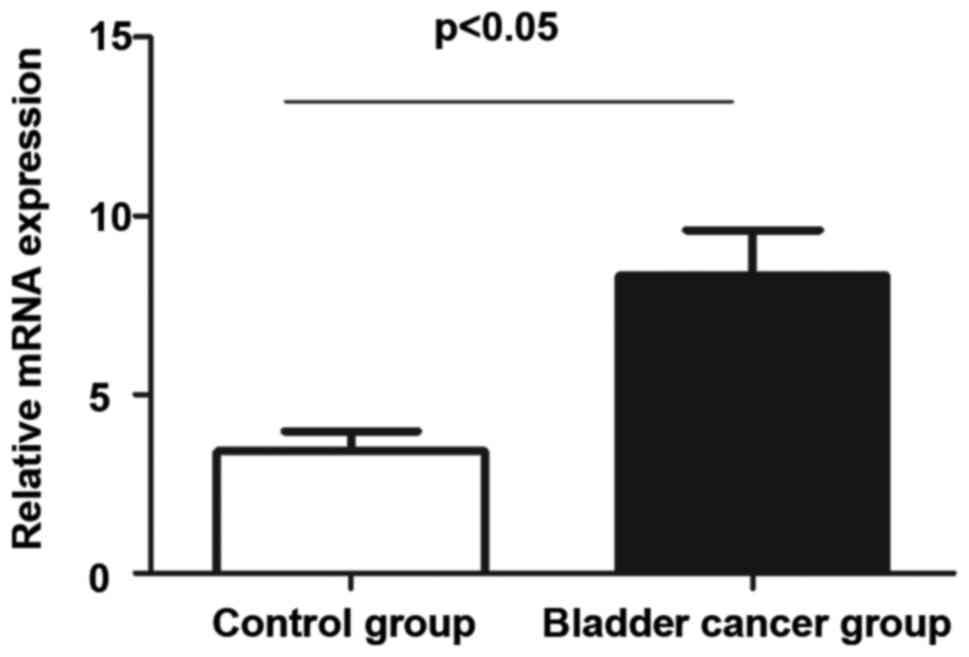
WTAP mRNA RT-PCR results in the
control group and the bladder cancer group
The tissue specimens of the control and bladder
cancer groups were extracted, respectively. Then, RT-PCR was
conducted for the total RNA extracted for the two groups. According
to results, the expression of WTAP in the control group was
significantly lower than that in the bladder cancer group and this
indicated that there were a large number of WTAP mRNAs transcribed
in the bladder cancer group (Fig.
3).
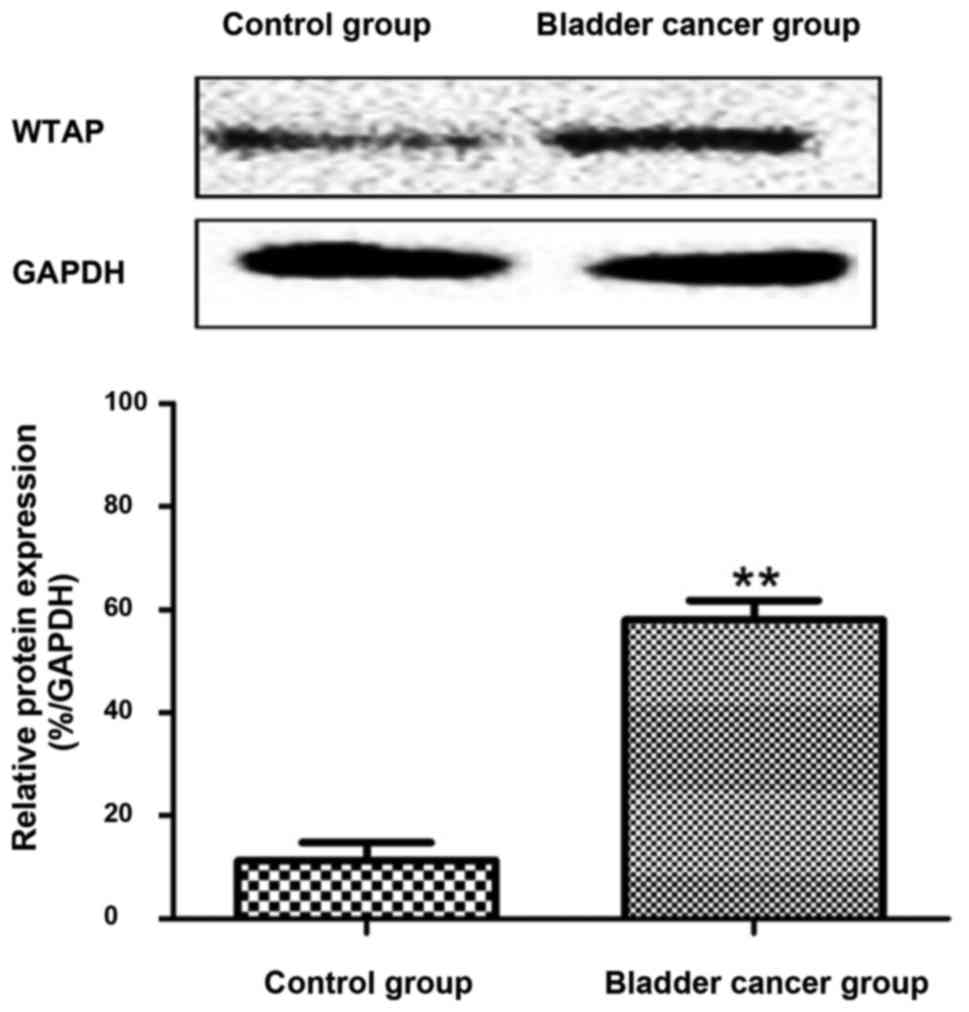
Western blot analysis of WTAP proteins
in the control and bladder cancer groups
According to western blot results, WTAP proteins
were poorly expressed in the bladder tissues of the control group,
while the expression was high in the tissues of the bladder cancer
group (Fig. 4).
Relationship between WTAP and the
prognosis of bladder cancer patients
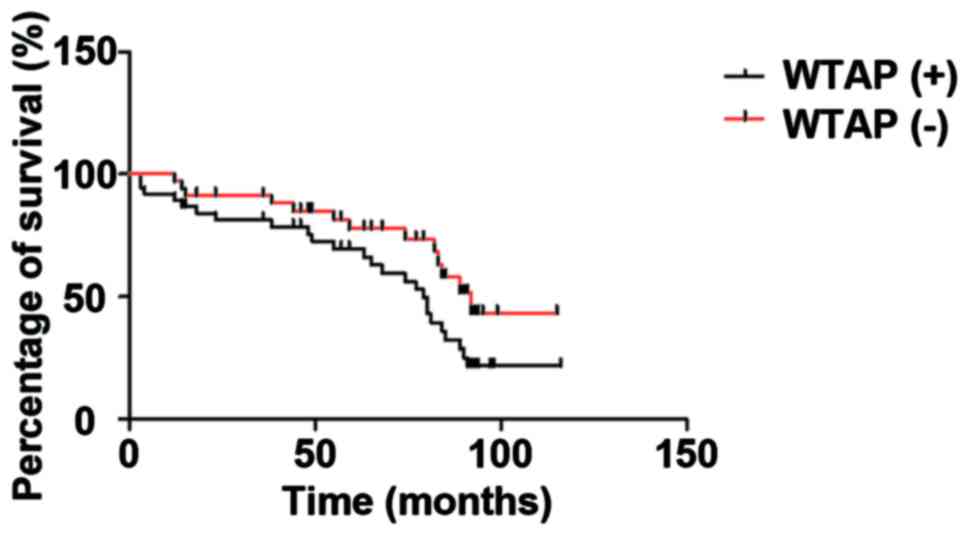
The Kaplan-Meier survival analyses were conducted to
show the effects of both the negative and positive WTAP expression
levels on the prognosis of patients. Fig.
5 shows that the total survival period in the negative WTAP
expression group was significantly higher than that in the positive
WTAP expression group (P<0.05).
Discussion
The occurrence and development of bladder cancer are
the results of multiple cancer genes and cancer suppressor genes
simultaneously or sequentially acting in the same environment. In
recent years, the incidence of bladder cancer is rising year by
year world-wide affecting both adult and young people (7,8). Bladder
cancer refers to a common malignant tumor that generally occurs in
the bladder mucosa, and is the most common tumor in the
genitourinary system (9–11). People at different ages, both female
and male, can be affected by bladder cancer, but males and the
elderly are more vulnerable to this disease which severely harms
human health (12–14). Therefore, it is crucial to identify an
effective method to treat bladder cancer. A number of studies have
previously been carried out to determine the diagnosis and
treatment of bladder cancer (15–17). WTAP
is a key gene involving many tumor diseases. Previous findings have
shgown that WTAP is involved and plays an important role in the
occurrence and development of tumors (18–20).
However, the effects of WTAP in bladder cancer should be studied
further and its molecular mechanisms should be also verified.
WTAP, which is a key regulatory factor, is thought
to be closely related to the occurrence and development of many
kinds of malignant tumors (21). WTAP
can promote the occurrence and development of malignant tumor by
stabilizing C-Myc, promoting cell proliferation and anchoring
independent growth, blocking senescence and differentiation
(22). In this study, we conducted a
preliminary study on whether WTAP can be used as a diagnostic
marker for bladder cancer. Our results showed that the cell
structure was destroyed and the nucleus shrank in the bladder
cancer group, while the tissue in the control group was intact.
Immunohistochemical staining showed that the expression of WTAP in
bladder cancer group was significantly higher than that in control
group. This suggests that the expression of WTAP in bladder tumors
is more specific. Therefore, WTAP may be used as a new tumor marker
in the diagnosis of bladder tumors. At the same time, as a
therapeutic target, further research on WTAP is needed. The results
of RT-PCR and Western blot analysis showed that WTAP mRNA and
protein were highly expressed in bladder cancer group.
In conclusion, the present study proved that WTAP
expression in the bladder cancer group was significantly increased
compared with that in the control group, indicating that WTAP may
play an important role in the occurrence and development of bladder
cancer. This provides a new thought and direction for the diagnosis
and treatment of bladder cancer and is expected to be a new
approach for the treatment of bladder cancer patients in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC analyzed HE staining result. XW performed
immunohistochemistry. LC wrote and XW revised the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhongnan Hospital of Wuhan University (Wuhan, China) and informed
consent was signed by the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information (optional)
Not applicable.
References
|
1
|
Gupta S, Hau AM, Beach JR, Harwalker J,
Mantuano E, Gonias SL, Egelhoff TT and Hansel DE: Mammalian target
of rapamycin complex 2 (mTORC2) is a critical determinant of
bladder cancer invasion. PLoS One. 8:e810812013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cairns P, Evron E, Okami K, Halachmi N,
Esteller M, Herman JG, Bose S, Wang SI, Parsons R and Sidransky D:
Point mutation and homozygous deletion of PTEN/MMAC1 in primary
bladder cancers. Oncogene. 16:3215–3218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellmunt J, Albiol S and Kataja V; ESMO
Guidelines Working Group, . Invasive bladder cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 Suppl 4:79–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiebig HH, Dengler AW and Roth T: Human
tumor xenografts: predictivity, characterization and discovery of
new anticancer agents. Contributions to Oncology. Relevance of
Tumor Models for Anticancer Drug Development. Fiebig HH and Burger
AM: 54. Contr Oncol Basel; Karger: pp. 29–50. 1999
|
|
5
|
El-Kott AF, Khalil AM and El-Kenawy A-M:
Immunohistochemical expressions of uPA and its receptor uPAR and
their prognostic significant in urinary bladder carcinoma. Int Urol
Nephrol. 36:417–423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Span PN, Witjes JA, Grebenchtchikov N,
Geurts-Moespot A, Moonen PM, Aalders TW, Vriesema JL, Kiemeney LA,
Schalken JA and Sweep FC: Components of the plasminogen activator
system and their complexes in renal cell and bladder cancer:
Comparison between normal and matched cancerous tissues. BJU Int.
102:177–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richter J, Beffa L, Wagner U, Schraml P,
Gasser TC, Moch H, Mihatsch MJ and Sauter G: Patterns of
chromosomal imbalances in advanced urinary bladder cancer detected
by comparative genomic hybridization. Am J Pathol. 153:1615–1621.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruch J, Wöhr G, Hautmann R, Mattfeldt T,
Brüderlein S, Möller P, Sauter S, Hameister H, Vogel W and Paiss T:
Chromosomal changes during progression of transitional cell
carcinoma of the bladder and delineation of the amplified interval
on chromosome arm 8q. Genes Chromosomes Cancer. 23:167–174. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lindblad-Toh K, Tanenbaum DM, Daly MJ,
Winchester E, Lui WO, Villapakkam A, Stanton SE, Larsson C, Hudson
TJ, Johnson BE, et al: Loss-of-heterozygosity analysis of
small-cell lung carcinomas using single-nucleotide polymorphism
arrays. Nat Biotechnol. 18:1001–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jänne PA, Li C, Zhao X, Girard L, Chen TH,
Minna J, Christiani DC, Johnson BE and Meyerson M: High-resolution
single-nucleotide polymorphism array and clustering analysis of
loss of heterozygosity in human lung cancer cell lines. Oncogene.
23:2716–2726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suraweera N, Duval A, Reperant M, Vaury C,
Furlan D, Leroy K, Seruca R, Iacopetta B and Hamelin R: Evaluation
of tumor microsatellite instability using five quasimonomorphic
mononucleotide repeats and pentaplex PCR. Gastroenterology.
123:1804–1811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonnal C, Ravery V, Toublanc M, Bertrand
G, Boccon-Gibod L, Hénin D and Grandchamp B: Absence of
microsatellite instability in transitional cell carcinoma of the
bladder. Urology. 55:287–291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iyer G, Al-Ahmadie H, Schultz N, Hanrahan
AJ, Ostrovnaya I, Balar AV, Kim PH, Lin O, Weinhold N, Sander C, et
al: Prevalence and co-occurrence of actionable genomic alterations
in high-grade bladder cancer. J Clin Oncol. 31:3133–3140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hudson MA and McReynolds LM: Urokinase and
the urokinase receptor: Association with in vitro invasiveness of
human bladder cancer cell lines. J Natl Cancer Inst. 89:709–717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russell PJ, Raghavan D, Gregory P, Philips
J, Wills EJ, Jelbart M, Wass J, Zbroja RA and Vincent PC: Bladder
cancer xenografts: A model of tumor cell heterogeneity. Cancer Res.
46:2035–2040. 1986.PubMed/NCBI
|
|
16
|
Russell PJ, Raghavan D, Philips J and
Gregory P: Applications of the xenograft as a model of invasive
transitional cell carcinoma of the bladder. Prog Clin Biol Res.
260:167–181. 1988.PubMed/NCBI
|
|
17
|
Kovnat A, Armitage M and Tannock I:
Xenografts of human bladder cancer in immune-deprived mice. Cancer
Res. 42:3696–3703. 1982.PubMed/NCBI
|
|
18
|
Kovnat A, Buick RN, Connolly JG, Jewett
MA, Keresteci AG and Tannock IF: Comparison of growth of human
bladder cancer in tissue culture or as xenografts with clinical and
pathological characteristics. Cancer Res. 44:2530–2533.
1984.PubMed/NCBI
|
|
19
|
Zhang H, Aina OH, Lam KS, de Vere White R,
Evans C, Henderson P, Lara PN, Wang X, Bassuk JA and Pan CX:
Identification of a bladder cancer-specific ligand using a
combinatorial chemistry approach. Urol Oncol. 30:635–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin TY, Li YP, Zhang H, Luo J, Goodwin N,
Gao T, White RV, Lam KS and Pan CX: Tumor-targeting multifunctional
micelles for imaging and chemotherapy of advanced bladder cancer.
Nanomedicine (Lond). 8:1239–1251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson AM, Weasner BP, Weasner BM and
Kumar JP: The Drosophila Wilms' Tumor 1-Associating Protein (WTAP)
homolog is required for eye development. Dev Biol. 390:170–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horiuchi K, Kawamura T, Iwanari H, Ohashi
R, Naito M, Kodama T and Hamakubo T: Identification of Wilms' tumor
1-associating protein complex and its role in alternative splicing
and the cell cycle. J Biol Chem. 288:33292–33302. 2013. View Article : Google Scholar : PubMed/NCBI
|