Introduction
Laryngeal carcinoma (LC), the second most common
type of head and neck cancer, is currently a serious public health
problem in China (1,2). Among its subtypes, laryngeal squamous
cell carcinoma (LSCC) accounts for 95–98% of LC cases, signifying
that LSCC has become a public health problem globally (1–4).
Additionally, the American Cancer Society demonstrated that the
incidence and mortality rate of LSCC are gradually increasing
annually in USA (5). Therefore, it is
of notable importance to develop more effective treatment
strategies for this disease. The traditional and standard therapy
for LSCC is a combination of surgery and chemotherapy or
radiotherapy, but the clinical outcome has not demonstrated notable
improvement in patient survival rates, despite advancements in
treatment methods (6,7). The low survival rate may be due to the
strong migration ability of LSCC cells, which can result in
recurrence and metastasis (8).
Numerous previous studies have demonstrated that
reactive oxygen species (ROS) serve important roles in the
development of a number of cancer types, including colorectal,
breast, oral, gastric, colon, cervical and prostate cancer, and
particularly in LSCC (9–15). However, the excessive accumulation of
ROS can cause cancer cell apoptosis and necrosis, resulting in
antitumor effects in these cancer types (9,12,14). Therefore, it may be possible to
utilize the dual effects of ROS to develop an effective therapeutic
method for LSCC. For example, our research group reported that the
excessive production of ROS triggered by 9-hydroxypheophorbide
(9-HPbD) causes endoplasmic reticulum stress and oxidative stress,
which promotes the apoptosis of human LSCC HN-3 cells (16). Additionally, it was determined that
the combined treatment of CBDCA and 9-HPbD with reducing effective
dosage of the anticancer drugs exhibited significant cytotoxicity
in LSCC cells with a limited number of side effects and low
toxicity in normal cells (17). A
study by Baek et al (18)
demonstrated that ROS induced by ascorbic acid resulted in the
activation of the protein kinase C signaling pathway and cytosolic
calcium signaling, causing the necrosis of Hep2 LSCC cells.
Carboplatin (CBDCA) is a well-known
second-generation platinum anticancer drug that has been frequently
used to treat various cancer types, including bladder cancer and
malignant melanoma, due to its broad-spectrum anticancer properties
and low cost (19). Additionally, it
has reduced toxicity in the ear, kidney and nervous system compared
with first-generation drugs, including cisplatin. Due to its
toxicity to the kidney and ear, and numerous side effects,
including emesis, nausea and cumulative myelosupression, as well as
its low tolerance for long-term use (20,21), it is
commonly used in combination with radiotherapy or surgery for
cancer treatment (22,23). The molecular mechanisms underlying its
antitumor effects depend upon binding to DNA in the nucleus, which
blocks DNA synthesis, causing apoptosis or necrosis (24). However, Cheng et al (25) reported that CBDCA could also induce
oxidative stress to produce ROS, which in turn promoted apoptosis
in cardiomyocytes. Based on these results, it was postulated that
CBDCA is cytotoxic in LSCC cells due to the oxidative stress caused
by ROS, which promotes cancer cell apoptosis. Additionally, one
previous study demonstrated that the generation of ROS upon the
treatment of LSCC cells with CBDCA to some extent, but further
direct evidence is required (26).
In the present study, it was determined if CBDCA had
antitumor effects in LSCC cells and the underlying molecular
mechanisms were assessed. Therefore, the viability, expression of
ROS, and degree of apoptosis or necrosis in HN-3 cells were
evaluated following treatment with various doses of CBDCA. HN-3
cells were also treated with CBDCA combined with glutathione (GSH)
or H2O2, following which their viability,
migration ability and apoptosis were evaluated.
Materials and methods
Materials
The HN-3 cell line was obtained from the Asan
Medical Center (Seoul, South Korea) (27). Hoechst 33342 dye, MTT and propidium
iodide (PI) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). CBDCA was purchased from Selleck Chemicals
(Houston, TX, USA). Antibodies against cleaved caspase-3 (rabbit,
cat. no. AB3623), cleaved poly(ADP-ribose) polymerase (PARP;
rabbit, cat. AB3620) were purchased from Merck KGaA (Darmstadt,
Germany); goat anti-epidermal growth factor receptor (EGFR; cat.
no. sc-03-G; dilution 1:200) and phosphorylated c-Jun (p-c-Jun;
cat. no. sc-99) were from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA); rabbit anti-GAPDH polyclonal antibody (cat. no. ab9485;
dilution 1:2,000) was supplied by Abcam (Cambridge, UK).
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
D399) was purchased from Molecular Probes (Thermo Fisher Scientific
Inc., Waltham, MA, USA).
Cell culture
The HN-3 human LSCC cell line was derived from a
63-year-old male patient with previously untreated LSCC (27). Cells were cultured in a cell incubator
at 37°C in an atmosphere containing 5% CO2 in RPMI-1640
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) and 1% penicillin/streptomycin. The culture medium
(RPMI-1640) was replaced every 3 days.
Cell viability assay
An MTT assay was performed to analyze cell
viability. Briefly, when HN-3 cell confluence reached 90%, the
cells were seeded into 96-well plates at a density of
5×103 cells/well and further cultured in a cell
incubator overnight at 37°C. Subsequently, the indicated doses of
CBDCA (0–5.0 mg/ml), GSH (5 mM) or H2O2 (0.2
mM) were added to the cells, followed by further incubation in a
cell incubator in an atmosphere containing 5% CO2 at
37°C for 0, 24 or 48 h. Following this, 50 µl MTT solution (2
mg/ml) was added to the wells and the plates were further incubated
for 2 h at 37°C. Lastly, the Thermo Scientific Varioskan Flash
Multimode Reader was used to measure the absorbance at 540 nm. Cell
viability was calculated according to the following equation: Cell
viability (%)=(mean absorbance in the treatment group/mean
absorbance in the control group) ×100.
Detection of ROS
For ROS detection by confocal microscopy as
previously described (26), HN-3
cells were cultured on glass-bottom 35-mm dishes. Subsequently, 24
h after treatment with CBDCA, GSH or H2O2, as
aforementioned, the cells were incubated with 2 µM
H2DCFDA in culture medium at 37°C for 30 min. Following
this, the cells were gently washed twice with Dulbecco's PBS.
Images were collected using a 488 nm excitation light from the
argon laser, a 560 nm dichroic mirror and a 505–550 nm band pass
barrier filter. A total of 3 random fields were selected for
imaging and conducting statistical analyses to measure the ROS
concentration.
Hoechst 33342 and PI double
staining
The HN-3 cells (cell confluence, 80–90%) were seeded
into 3.5-cm dishes, and then treated with CBDCA, GSH or
H2O2, as aforementioned. After 24 h at 37°C,
the cells were incubated with Hoechst 33342 (2 µg/ml) for 30 min at
37°C, following which the old medium was replaced with fresh
RPMI-1640 medium. Subsequently, the cells were incubated with PI (2
µg/ml) for 10 min at 37°C. Finally, the stained cells were observed
using a laser scanning confocal microscope (Laser Microdissection
Systems Leica LMD6500 and LMD7000; Leica Microsystems GmbH,
Wetzlar, Germany) to determine the apoptosis and necrosis levels of
HN-3 cells. A total of 3 random fields were selected for imaging
and conducting statistical analyses to confirm the relative
apoptosis/necrosis rate.
Cell migration assay
In brief, the HN-3 cells were seeded (cell
confluence, 80–90%) into 24-well plates and reference points near
the wound were marked to ensure the use of the same area for image
acquisition on the plates. After a 24 h incubation at 37°C,
confluent monolayers of the HN-3 cells were wounded using a 200 ml
pipette tip to create a straight line. Following 2 washes with PBS,
indicated doses of CBDCA (0.04 or 0.08 mg/ml), GSH (5 mM) or
H2O2 (0.2 mM) were added to the cells, and
then the cells were incubated in a cell incubator in an atmosphere
containing 5% CO2 at 37°C for 36 h. The wound width
(initial 1.2 mm) was measured at four redefined locations when the
wound was produced and after 12, 24 and 36 h. The distances between
the two edges of the wound were measured at the reference points
and statistically analyzed.
Western blot analysis
The HN-3 cells were seeded (cell confluence, 80–90%)
into 6-cm dishes. Following cells reaching 90% confluence,
indicated doses of CBDCA (0.04 or 0.08 mg/ml), GSH (5 mM) or
H2O2 (0.2 mM) were added, and then the cells
were incubated in an incubator for 24 h at 37°C. Subsequently, the
cells were collected and lysed in radioimmunoprecipitation assay
buffer (Sigma-Aldrich; Merck KGaA), and total protein was obtained
following centrifugation at 4°C at 14,000 × g for 30 min. Following
the protein concentrations being measured using a BCA kit (Beyotime
Institute of Biotechnology, Jiangsu, China), the proteins were
separated using 10% SDS-PAGE and then electrophoretically
transferred onto polyvinylidene difluoride membranes. Subsequently,
the membranes were incubated overnight at 4°C with 1:1,000 diluted
primary antibodies conjugated with horseradish peroxidase (HRP),
subsequent to being blocked in 5% non-fat milk in Tris-buffered
saline with Tween 20 (0.5%) (TBST) for 1 h at room temperature,
washed 3 times with TBST, and then incubated with HRP-conjugated
goat anti-rabbit IgG polyclonal secondary antibody (1:2,000; Abcam,
Cambridge, UK, ab97051) at room temperature for 2 h. The proteins
were visualized using SuperSignal West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. Membranes were scanned with ImageJ (k
1.45; National Institutes of Health, Bethesda, MD, USA) to quantify
the band intensity.
Statistical analysis
All of the experiments in the present study were
repeated at least three times, and the final results were expressed
as the mean ± standard deviation. Significant differences among
experimental groups were analyzed by one-way analysis of variance
followed by the Least Significant Difference post-hoc test.
Statistical analyses were performed using SPSS 11.5 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
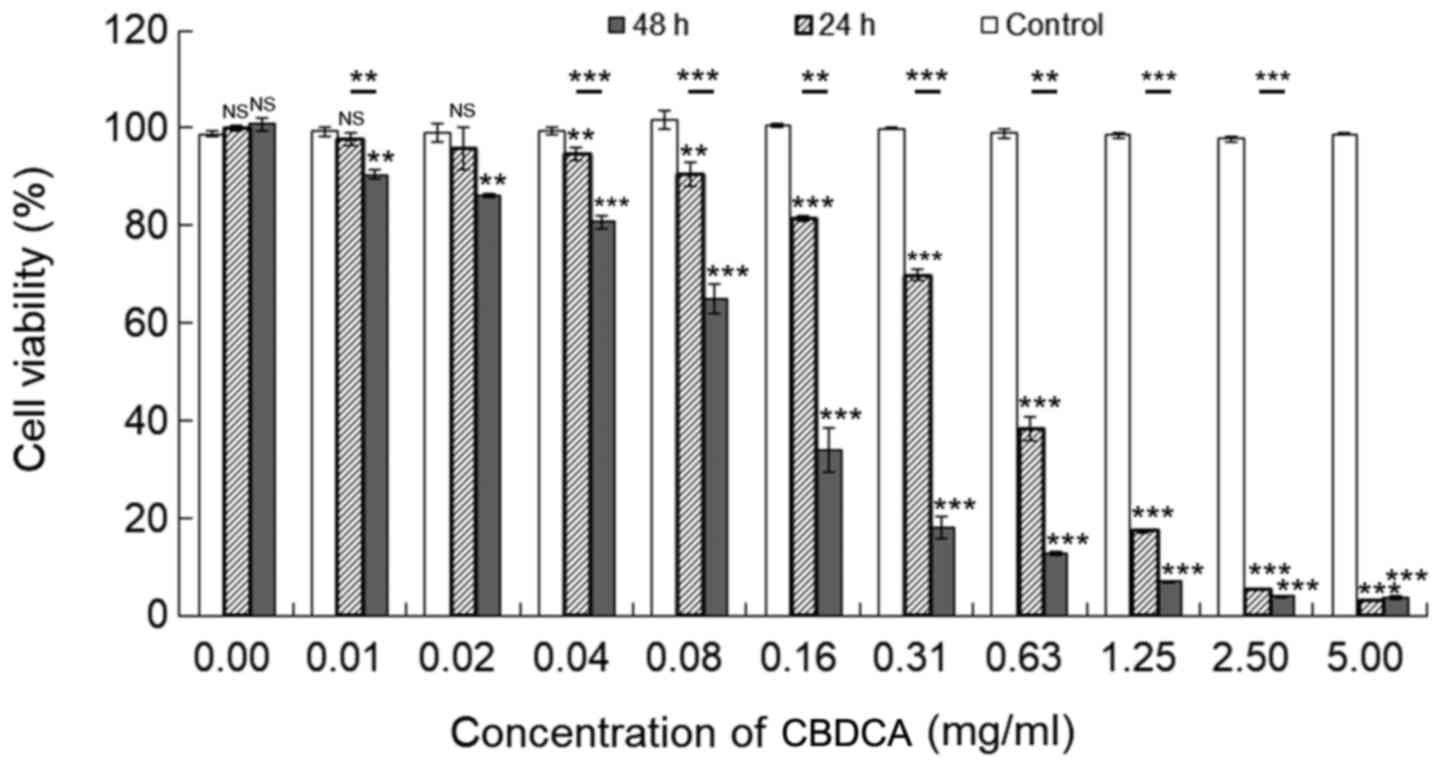
CBDCA inhibits the viability of HN-3
cells in a dose- and time-dependent manner
To determine if CBDCA has antitumor effects in LSCC,
an MTT assay was conducted to detect the viability of HN-3 human
LSCC cells following treatment with different doses of CBDCA for 0,
24 or 48 h. As depicted in Fig. 1,
the viability of HN-3 cells significantly decreased as the
concentration of CBDCA increased from 0–5 mg/ml (P<0.05,
excluding a number of cases when the concentration of CBDCA was
0.01 and 0.02 mg/ml). Furthermore, treatment of CBDCA for 48 h
resulted in increased inhibitory effects on cell viability,
compared with treatment for 24 h. Therefore, CBDCA suppressed the
viability of HN-3 cells in dose- and time-dependent manners.
CBDCA promotes the production of ROS
and apoptosis of HN-3 cells
Numerous studies have reported that CBDCA exerts
antitumor effects in a number of malignant tumor types, including
lung, non-small cell lung, cervical and ovarian cancer, by inducing
the apoptosis of these cancer cells, which is mediated by the
accumulation of ROS (19,28–32).
Therefore, whether this molecular mechanism also applies to HN-3
cells was investigated. Firstly, H2DCFDA imaging was
used to detect the expression level of ROS following treatment with
CBDCA for 24 h. The treatment time was selected because as the
upstream molecule of the function of CBDCA, the production of ROS
occurred relatively early which could be observed distinctly at the
time point of 24 h. As depicted in Fig.
2A, it was determined that the intensity of green fluorescence
released by HN-3 cells was increased as the concentration of CBDCA
increased from 0–0.64 mg/ml, and then declined as the concentration
of CBDCA further increased from 0.64-5 mg/ml. This effect was
confirmed by statistical analysis (P<0.01 for 0.16, 0.32, 0.64
and 1.25 mg/ml) (Fig. 2B).
Subsequently, Hoechst 33342/PI double staining was performed to
evaluate the apoptosis and necrosis levels in HN-3 cells following
treatment with CBDCA. Similar to the aforementioned results, the
apoptosis of HN-3 cells first increased with CBDCA concentrations
ranging from 0–0.64 mg/ml, and then decreased with CBDCA
concentrations ranging from 0.64-5 mg/ml, while the necrosis level
of HN-3 cells increased during this period (Fig. 2C and D). Thus, these data demonstrated
that CBDCA promoted the production of ROS and subsequent apoptosis
of HN-3 cells in a dose-dependent manner within a certain
concentration range.
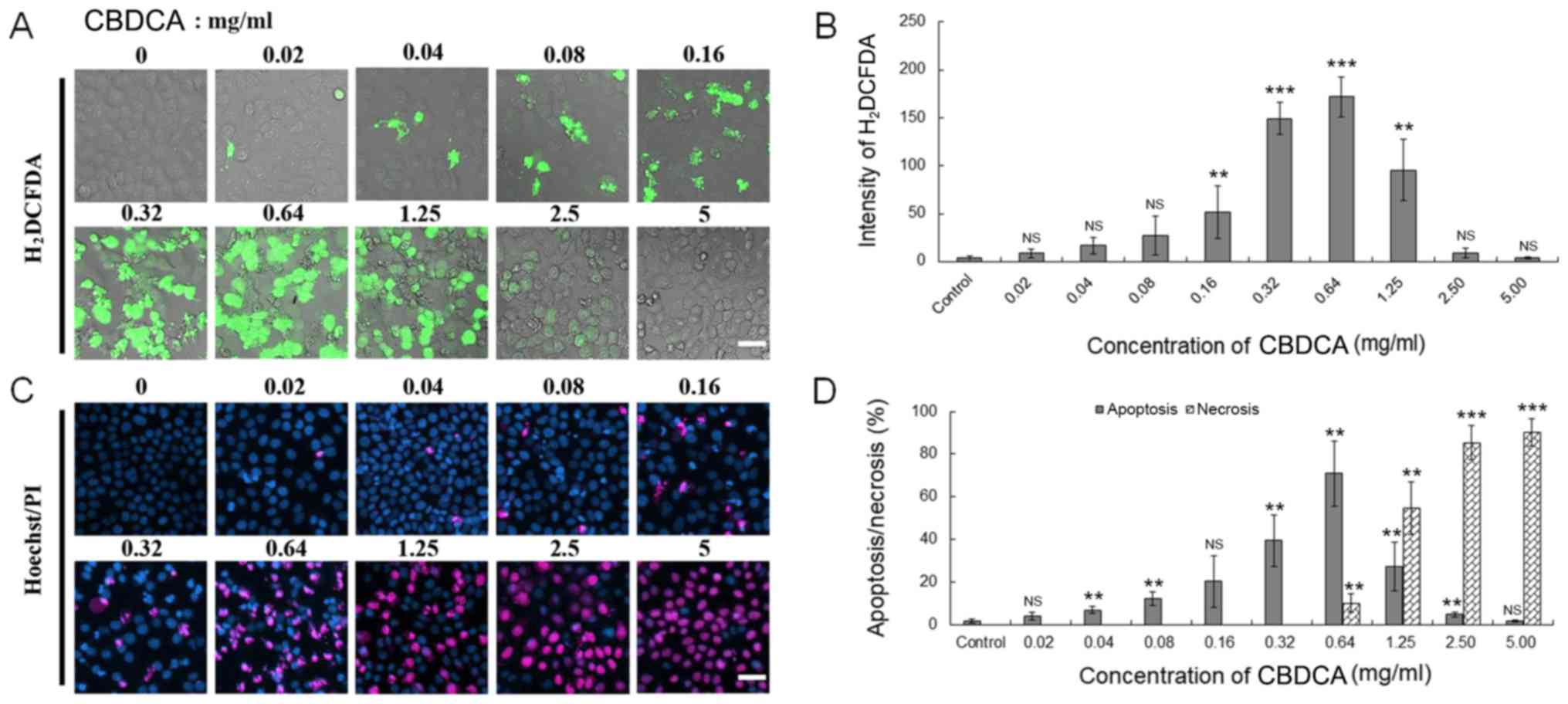 | Figure 2.CBDCA may promote the production of
ROS and apoptosis in HN-3 cells. (A) The HN-3 cells were treated
with indicated doses of dimethyl sulfoxide (control) or CBDCA, and
2 µM H2DCFDA successively. Subsequently, a laser
scanning confocal microscope was used to observe the green
intensity of H2DCFDA to inspect the expression level of
ROS. Scale bar, 50 µm. (B) Quantified expression of ROS in the HN-3
cells, which were analyzed by fluorescent staining using
H2DCFDA. (C) The aforementioned HN-3 cells were also
used to conduct cell fluorescent staining to detect the ratio
between apoptosis and necrosis through staining with Hoechst 33342
(2 µg/ml; 30 min) and PI (2 µg/ml; 10 min). Scale bar, 20 µm. (D)
Quantified ratio of apoptosis/necrosis in HN-3 cells, which were
analyzed by fluorescent staining using Hoechst/PI. The data were
expressed as mean ± standard deviation, **P<0.01 and
***P<0.001 vs. control. ROS, reactive oxygen species; CBDCA,
carboplatin; PI, propidium iodide; NS, not significant;
H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate. |
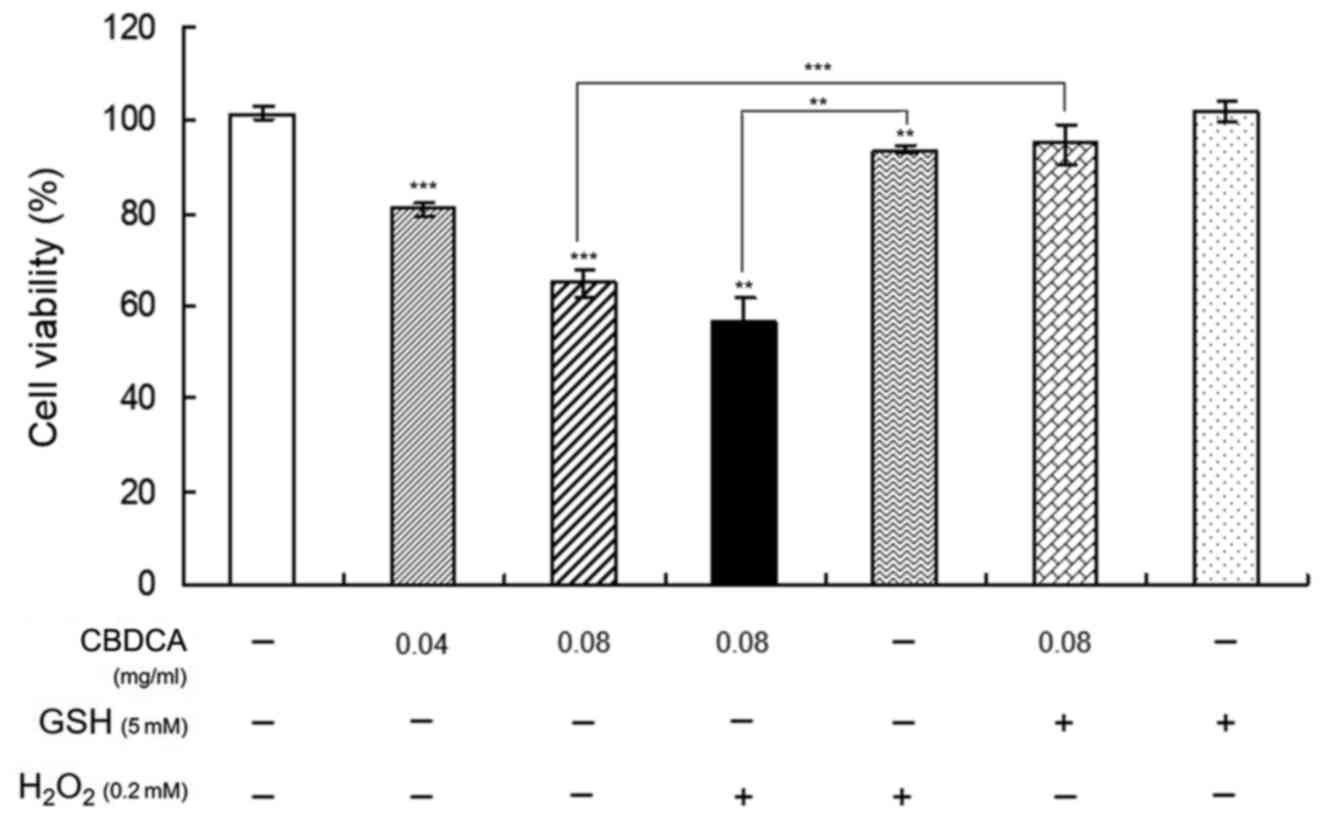
Combination treatment with GSH
inhibits the suppressive effects of CBDCA on HN-3 cell
proliferation
To confirm the aforementioned results demonstrating
that CBDCA may increase the accumulation of ROS, causing the
apoptosis of HN-3 cells, HN-3 cells were treated with CBDCA and GSH
or H2O2 for 48 h prior to testing the cell
viability with an MTT assay. Additionally, incubation time was
selected as it was the most beneficial for observing the change
induced by the regulation of ROS production whilst maintaining a
~60% cell viability post-treatment. Furthermore, the application of
0.08 mg/ml CBDCA resulted in the positive and negative regulation
of production of ROS by H2O2 and GSH,
respectively, thus benefitting the observation of the change in the
following experiments. In addition, based on the concentration
screening experiments (data not shown), the concentrations of
H2O2 and GSH were selected as 0.2 and 5 mM,
respectively, guaranteeing a notable effect on cell viability. The
results in Fig. 3 demonstrate that
treatment with GSH or H2O2 alone had
negligible effects on the viability of HN-3 cells, but the
combination treatment of CBDCA with GSH almost completely blocked
the inhibitory effects of CBDCA on cell viability compared with the
treatment of CBDCA (0.08 mg/ml) alone (P<0.01), whereas the
inhibitory effects were significantly promoted by the combination
treatment of CBDCA with H2O2 (P<0.01).
These data indicated that the ROS levels were increased by the
oxidant H2O2 and promoted HN-3 cell apoptosis
induced by CBDCA, whereas the deoxidizer GSH had the opposite
effects. These results confirmed that CBDCA induces the apoptosis
of HN-3 cells by increasing the production of ROS.
Combination treatment of CBDCA with
GSH blocks the inhibitory role of CBDCA on the migration of HN-3
cells
The antitumor effects of CBDCA not only reflected
suppression of cancer cell viability, but also their migration
ability. Therefore, following combined treatment of HN-3 cells with
CBDCA and GSH or H2O2, their cell migration
ability was assessed using a wound-healing assay. Although
statistically significant at 24 h treatment, GSH treatment alone
could only slightly affect the cell migration ability, whereas
treatment of CBDCA or H2O2 (except
H2O2 treatment for 12 h) alone significantly
inhibited the migration ability compared with the cells without any
treatment (Fig. 4A and B). However,
when HN-3 cells were treated with a combination of CBDCA and GSH,
the inhibitory effects of CBDCA on cell migration ability were
blocked by GSH, whereas the combined treatment of CBDCA and
H2O2 promoted migration.
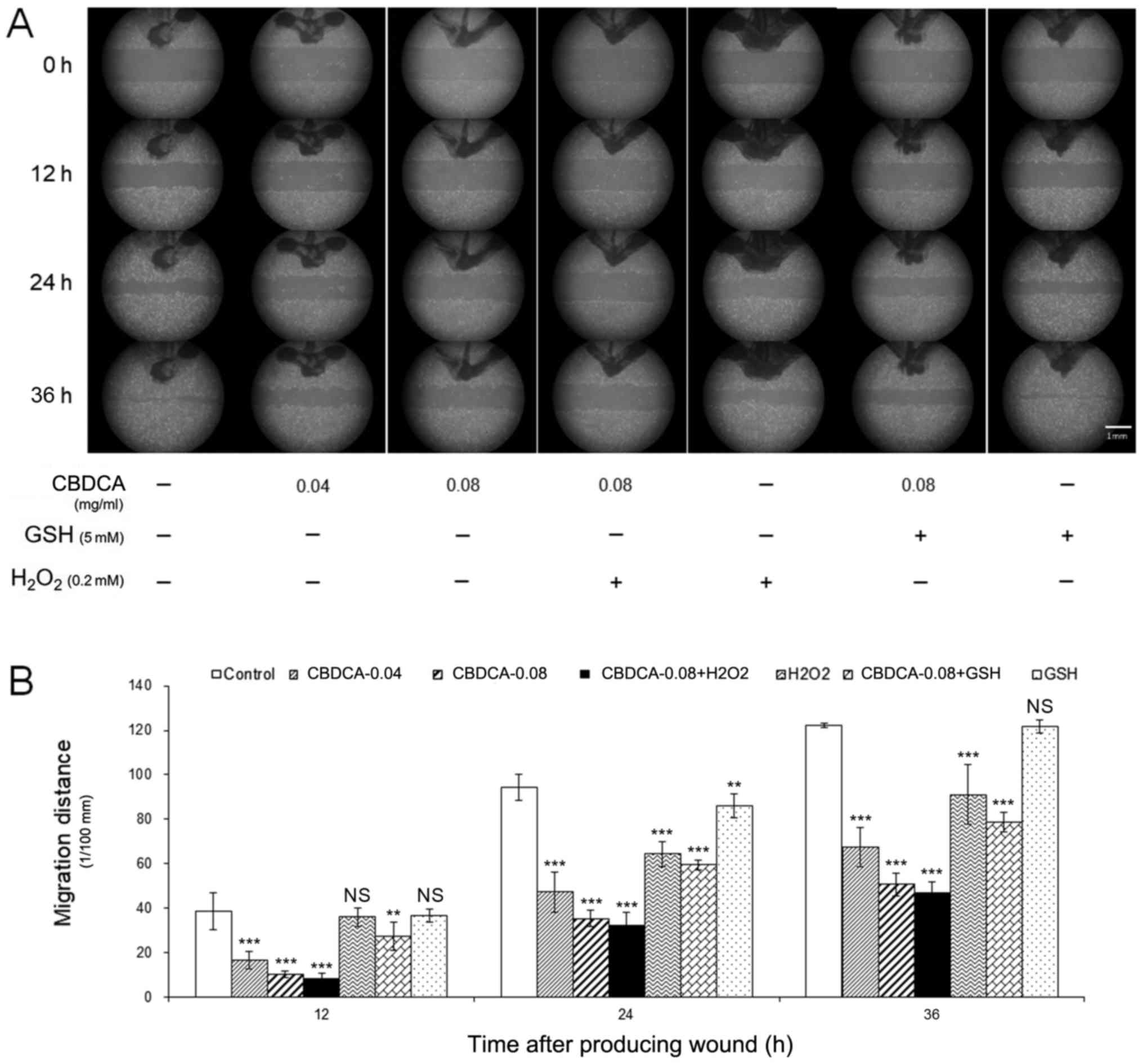 | Figure 4.Combined treatment of GSH or
H2O2 may block or promote the inhibitory role
of CBDCA on the migration of HN-3 cells. (A) The HN-3 cells were
seeded into six-well plates, and then indicated doses of CBDCA, GSH
or H2O2 were added, and further cultured in a
cell incubator at 37°C in an atmosphere containing 5%
CO2 for 0, 24 or 48 h. Subsequently, a wound-healing
assay was performed to investigate the effect of CBDCA, GSH or
H2O2 on the migration ability of HN-3 cells.
(B) The statistical analysis for the migration ability of the
aforementioned HN-3 cells. The data were expressed as mean ±
standard deviation, **P<0.01 and ***P<0.001 vs. control.
CBDCA, carboplatin; GSH, glutathione; NS, not significant. |
Combination treatment of CBDCA and GSH
inhibits the apoptosis induced by CBDCA in HN-3 cells
Up to this point, the present results indirectly
demonstrated that CBDCA caused the apoptosis of HN-3 cells by
increasing the release of ROS. Subsequently, the present study
aimed to directly verify this phenomenon. Therefore, HN-3 cells
treated as aforementioned were collected and lysed to obtain total
proteins, which were subsequently analyzed by western blotting. The
results demonstrated that, compared with the non-treatment group,
CBDCA and H2O2 treatment alone significantly
(P<0.05) increased the expression levels of p-c-Jun, caspase-3
and PARP, which represented the degree of apoptosis. Additionally,
CBDCA (0.08 mg/ml) treatment inhibited the expression of EGFR which
reflects the proliferation ability (P<0.05; Fig. 5). The combination treatment of CBDCA
and H2O2 exacerbated this trend, compared
with CBDCA treatment alone, whereas the combination treatment of
CBDCA and GSH impaired this effect (Fig.
5). These data demonstrated that the combined application of
CBDCA and H2O2 enhanced the levels of
apoptosis induced by CBDCA in HN-3 cells, whereas GSH blocked these
effects. These results support the previous observations that CBDCA
increases the production of ROS in HN-3 cells, causing
apoptosis.
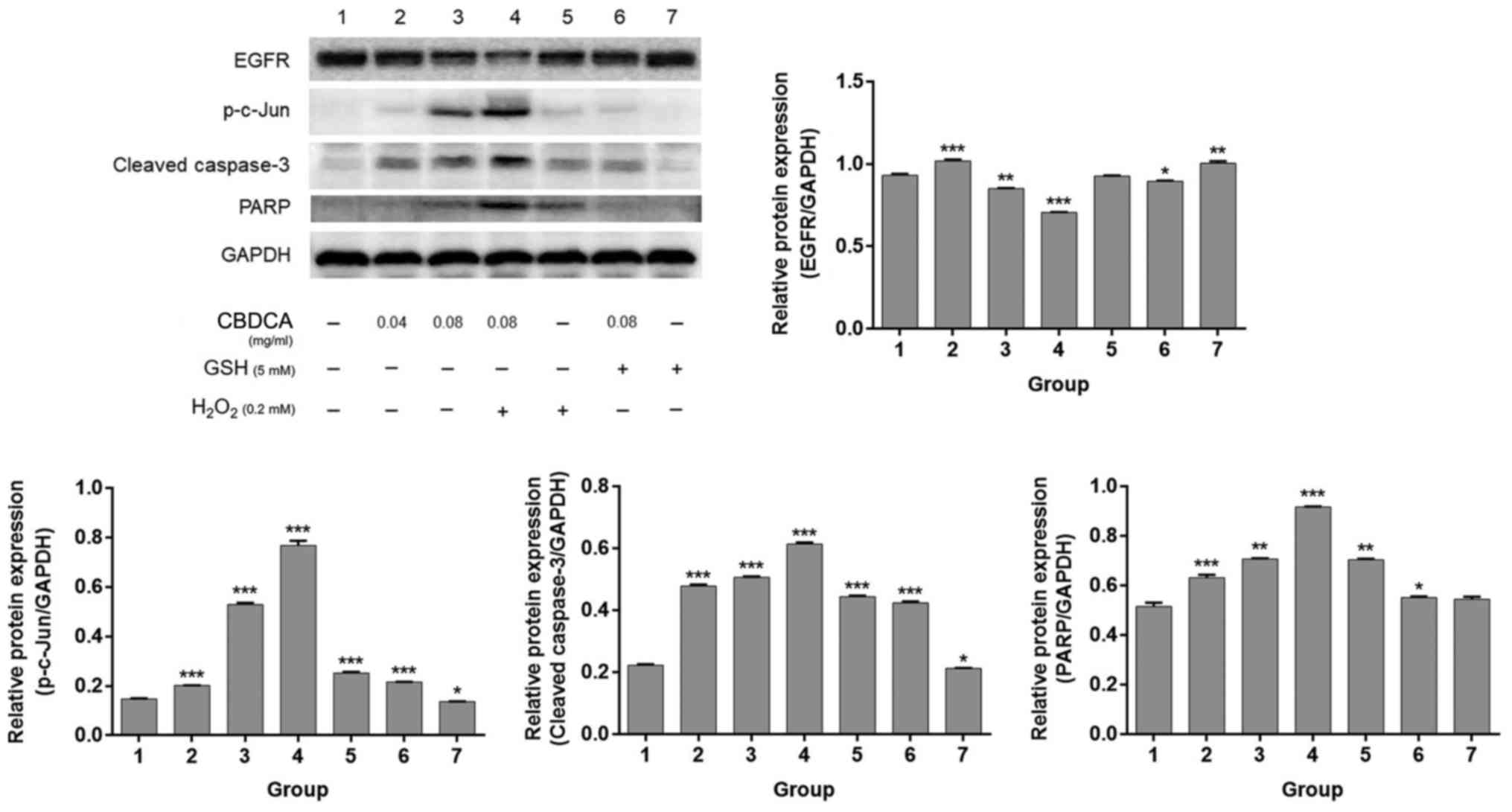 | Figure 5.Combined treatment of GSH or
H2O2 may inhibit or promote the apoptosis
induced by CBDCA in HN-3 cells. The indicated doses of CBDCA, GSH
or H2O2 were added to HN-3 cells and further
cultured in a cell incubator at 37°C in an atmosphere containing 5%
CO2 for 24 h. These cells were collected and lysed to
acquire total proteins to inspect the protein expression levels of
EGFR, p-c-Jun, PARP and cleaved-caspase-3 using western blotting.
GAPDH was used to confirm the equal amount of proteins loaded in
each lane. Membranes were scanned with ImageJ to quantify band
intensity. The data were expressed as mean ± standard deviation,
*P<0.05, **P<0.01 and ***P<0.001 vs. lane 1. EGFR,
epidermal growth factor receptor; PARP, poly(ADP-ribose)
polymerase; CBDCA, carboplatin; GSH, glutathione; p-c-Jun,
phosphorylated c-Jun. |
Discussion
Platinum anticancer agents, including cisplatin and
CBDCA, have been frequently used as chemotherapies to treat various
cancer types including bladder cancer and malignant melanoma
(33). They enter cells with the
assistance of copper transporter 1, and are converted into their
corresponding aqua complexes, thereby crosslinking DNA to exhibit
antitumor activity (34,35). Among these types of agents, CBDCA, a
second-generation platinum anti-cancer agent, was developed to
overcome the side effects of cisplatin, including nephrotoxicity
and neurotoxicity (36). CBDCA has
notable antitumor activity against a variety of cancer types,
including ovarian and small-cell lung cancer (31,32).
Previous studies have demonstrated that the oxidative stress caused
by ROS promoted the apoptosis of LSCC cells through a number of
functional mechanisms (16,17,37). For
example, in 2009 it was reported that the combined treatment of
9-HPbD and CBDCA caused increased cytotoxicity and resulted in
apoptosis of a greater number of LSCC cells (17). Additionally, by reducing their dosage,
reduced toxicity was observed in normal cells (17). In 2010, one study demonstrated that
9-HPbD caused oxidative stress, which resulted in the increased
production of ROS and apoptosis of HN-3 cells through the extrinsic
apoptotic pathway, and endoplasmic reticulum stress was also
involved in this process (16). In
addition, in 2016, a study demonstrated that 9-HPbDα-based
photodynamic therapy promoted apoptosis and necrosis, and
suppressed cell migration, which was directly mediated by ROS
accumulation (37). In light of these
results, the present study focused on understanding the molecular
mechanisms involved in the antitumor effects of a number of
traditional drugs including CBDCA for the treatment of LSCC, with
the goal of determining novel treatment strategies for this
disease. In the present study, the aim was to determine if the
mechanism underlying HN-3 cell death was high expression of ROS due
to CBDCA.
Although CBDCA has been used to treat various
malignant tumor types including non-small cell lung cancer and
ovarian cancer for ~30 years (20,21,24), only
a limited number of studies have been conducted on its use in the
treatment of LSCC (17,26,38). To
the best of our knowledge, our research group was the first to
investigate the antitumor effects and mechanisms of CBDCA in LSCC
(17,26). Therefore, the present study would be
of notable significance for the development of novel LSCC
treatments.
In the present study, it was verified that CBDCA may
exert antitumor effects in LSCC. The results revealed its
anticancer effects against HN-3 cells in a time- and dose-dependent
manner. CBDCA exerts its effects through numerous different
molecular mechanisms, including apoptosis, necrosis and autophagy
may be triggered by different apoptotic signaling pathways.
Accordingly, the aim of the present study was also to investigate
whether the mechanism of the antitumor effects of CBDCA were
similar to those of 9-HPbD. Therefore, the expression level of ROS
and the apoptosis/necrosis rate of HN-3 cells were measured
following treatment with different concentrations. The results
indicated that the ROS concentration in HN-3 cells increased as the
concentration of CBDCA increased, but additional increases in CBDCA
concentration resulted in the reduced expression of ROS. This trend
was also observed for the degree of apoptosis of HN-3 cells.
Notably, the optimum concentration for cell apoptosis was identical
to that obtained from the detection of ROS concentration. These
results indicated that apoptosis induced by CBDCA in HN-3 cells is
associated with ROS production.
In a previous study, the induced generation of ROS
by treatment with CBDCA was reported and demonstrated to some
extent (26). However, the direct
association between CBDCA treatment and generation of ROS has not
been established. Therefore, in the present study, CBDCA with GSH,
a reductant that inhibits the expression of ROS, or
H2O2, an oxidant that promotes the release of
ROS (39,40), were combined to treat HN-3 cells and
then their viability, migration ability and apoptosis state was
assessed. As expected, the results demonstrated that the combined
treatment of CBDCA with H2O2 synergistically
suppressed the migration ability and viability, and enhanced the
apoptosis of HN-3 cells, compared with CBDCA treatment alone,
whereas the combined treatment of CBDCA and GSH produced opposite
results. These results indicated that CBDCA promotes apoptosis by
the excessive production of ROS in human LSCC cells. Notably, one
of the limitations of the present study was that all studies were
performed based on HN-3 cells. In future studies, the research
scope would be extended by additionally utilizing LSCC cell lines,
including TU212 and TU686, and including a number of normal control
cell lines.
In conclusion, the results of the present study
demonstrated that CBDCA exerts promising antitumor effects in LSCC
cells, inhibits cell proliferation and migration, and promotes
apoptosis through the excessive expression of ROS in LSCC cells.
These results may provide a novel therapeutic strategy for the
treatment of LSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172557) and the
Project of Shanghai Municipal Health and Family Planning Commission
(grant no. 201640104), and partially supported by Leading Foreign
Research Institute Recruitment Program through the National
Research Foundation of Korea funded by the Ministry of Education,
Science and Technology (grant no. 2012K1A4A3053142), and Beckman
Laser Institute Korea, Dankook University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PJH and HTW made substantial contributions to the
concept and design of the present study, and the examination of the
manuscript. PJH, RFG and WJM conducted experiments and produced the
manuscript. PSC and JCA conducted experiments and analyzed the
experimental data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang W, Yan Y, Gu M, Wang X, Zhu H, Zhang
S and Wang W: High expression levels of Wnt5a and Ror2 in laryngeal
squamous cell carcinoma are associated with poor prognosis. Oncol
Lett. 14:2232–2238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riga M, Chelis L, Danielides V, Vogiatzaki
T, Pantazis TL and Pantazis D: Systematic review on T3 laryngeal
squamous cell carcinoma; still far from a consensus on the optimal
organ preserving treatment. Eur J Surg Oncol. 43:20–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen Z, Cao B, Lin L, Zhou C, Ye D, Qiu S,
Li Q and Cui X: The clinical signification of claudin-11 promoter
hypermethylation for laryngeal squamous cell carcinoma. Med Sci
Monit. 23:3635–3640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CACancerJ Clin. 59:225–249. 2009.
View Article : Google Scholar
|
|
6
|
Dirix P, Lambrecht M and Nuyts S:
Radiotherapy for laryngeal squamous cell carcinoma: Current
standards. Expert Rev Anticancer Ther. 10:1461–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia CX, Zhu Q, Zhao HX, Yan F, Li SL and
Zhang SM: Usefulness of ultrasonography in assessment of laryngeal
carcinoma. Brit J Radiol. 86:201303432013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Lv K, Chen W, Jing Z, Jie L, Wu J,
Li Z, Hao Q, Wong TS, Yang W, et al: miR-375 and miR-205 regulate
the invasion and migration of laryngeal squamous cell carcinoma
synergistically via AKT-mediated EMT. Biomed Res Int.
2016:96527892016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phang CW, Karsani SA and Abd Malek SN:
Induction of apoptosis and cell cycle arrest by flavokawain C on
HT-29 human colon adenocarcinoma via enhancement of reactive oxygen
species generation, upregulation of p21, p27 and GADD153, and
inactivation of inhibitor of apoptosis proteins. Pharmacogn Mag. 13
Suppl 2:S321–S328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakayama K, Murata S, Ito H, Iwasaki K,
Villareal MO, Zheng YW, Matsui H, Isoda H and Ohkohchi N:
Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen
species. Oncol Lett. 14:2015–2024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allison SE, Chen Y, Petrovic N, Zhang J,
Bourget K, Mackenzie PI and Murray M: Activation of ALDH1A1 in
MDA-MB-468 breast cancer cells that over-express CYP2J2 protects
against paclitaxel-dependent cell death mediated by reactive oxygen
species. Biochem Pharmacol. 143:79–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YH: Diallyl trisulfide induces
apoptosis and mitotic arrest in AGS human gastric carcinoma cells
through reactive oxygen species-mediated activation of
AMP-activated protein kinase. Biomed Pharmacother. 94:63–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang YT, Huang CY, Tang JY, Liaw CC, Li
RN, Liu JR, Sheu JH and Chang HW: Reactive oxygen species mediate
soft corals-derived sinuleptolide-induced antiproliferation and DNA
damage in oral cancer cells. Onco Targets Ther. 10:3289–3297. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:10882017. View Article : Google Scholar
|
|
15
|
Shagieva G, Domnina L, Makarevich O,
Chernyak B, Skulachev V and Dugina V: Depletion of mitochondrial
reactive oxygen species downregulates epithelial-to-mesenchymal
transition in cervical cancer cells. Oncotarget. 8:4901–4913. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He P, Ahn JC, Shin JI and Chung PS:
Photoactivation of 9-hydroxypheophorbide alpha triggers apoptosis
through the reactive oxygen species-mediated mitochondrial pathway
and endoplasmic reticulum stress in AMC-HN-3 laryngeal cancer
cells. Int J Oncol. 36:801–808. 2010.PubMed/NCBI
|
|
17
|
He P, Ahn JC, Shin JI, Hwang HJ, Kang JW,
Lee SJ and Chung PS: Enhanced apoptotic effect of combined modality
of 9-hydroxypheophorbide alpha-mediated photodynamic therapy and
carboplatin on AMC-HN-3 human head and neck cancer cells. Oncol
Rep. 21:329–334. 2009.PubMed/NCBI
|
|
18
|
Baek MW, Cho HS, Kim SH, Kim WJ and Jung
JY: Ascorbic acid induces necrosis in human laryngeal squamous cell
carcinoma via ROS, PKC, and calcium signaling. J Cell Physiol.
232:417–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castrellon AB, Pidhorecky I, Valero V and
Raez LE: The role of carboplatin in the neoadjuvant chemotherapy
treatment of triple negative breast cancer. Oncol Rev. 11:3242017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Castria TB, da Silva EM, Gois AF and
Riera R: Cisplatin versus carboplatin in combination with
third-generation drugs for advanced non-small cell lung cancer.
Cochrane Database Syst Rev. 16:CD0092562013.
|
|
21
|
Collins IM, Roberts-Thomson R, Faulkner D,
Rischin D, Friedlander M and Mileshkin L: Carboplatin dosing in
ovarian cancer: Problems and pitfalls. Int J Gynecol Cancer.
21:1213–1218. 2011.PubMed/NCBI
|
|
22
|
Chikazawa K, Netsu S and Konno R: Outcomes
of concurrent radiotherapy and weekly paclitaxel/carboplatin
therapy in cervical cancer: A retrospective study. Eur J Gynaecol
Oncol. 37:511–516. 2016.PubMed/NCBI
|
|
23
|
Pisters KM, Vallières E, Crowley JJ,
Franklin WA, Bunn PA Jr, Ginsberg RJ, Putnam JB Jr, Chansky K and
Gandara D: Surgery with or without preoperative paclitaxel and
carboplatin in early-stage non-small-cell lung cancer: Southwest
Oncology Group Trial S9900, an intergroup, randomized, phase III
trial. J Clin Oncol. 28:1843–1849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: Comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng CF, Juan SH, Chen JJ, Chao YC, Chen
HH, Lian WS, Lu CY, Chang CI, Chiu TH and Lin H: Pravastatin
attenuates carboplatin-induced cardiotoxicity via inhibition of
oxidative stress associated apoptosis. Apoptosis. 13:883–894. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao WJ, Zhang HK, Shen B and Pei-Jie HE:
Role of reactive oxygen series generation in the apoptosis of HN-3
human laryngeal carcinoma cells induced by carboplatin. Chin J
Ophthalmol Otorhinolaryngol. 15:384–387. 2015.
|
|
27
|
Kim SY, Chu KC, Lee HR, Lee KS and Carey
TE: Establishment and characterization of nine new head and neck
cancer cell lines. Acta Otolaryngol. 117:775–784. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morin C and Fortin S: Docosahexaenoic acid
monoglyceride increases carboplatin activity in lung cancer models
by targeting EGFR. Anticancer Res. 37:6015–6023. 2017.PubMed/NCBI
|
|
29
|
Ryu H, Song IC, Choi YS, Yun HJ, Jo DY,
Kim JM, Ko YB and Lee HJ: ERCC1 expression status predicts the
response and survival of patients with metastatic or recurrent
cervical cancer treated via platinum-based chemotherapy. Medicine
(Baltimore). 96:e94022017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bayés M, Rabasseda X and Prous JR:
Gateways to clinical trials. Methods Find Exp Clin Pharmacol.
29:697–735. 2007.PubMed/NCBI
|
|
31
|
Yazawa H, Hiraiwa T, Ito F and Fujimori K:
Long-term recurrence-free survival of a patient with advanced pure
primary ovarian squamous cell carcinoma treated with dose-dense
paclitaxel combined with carboplatin. Obstet Gynecol Sci.
60:587–592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han S, Hong Y, Liu T, Wu N and Ye Z: The
efficacy and safety of paclitaxel and carboplatin with versus
without bevacizumab in patients with non-small-cell lung cancer: A
systematic review and meta-analysis. Oncotarget. 9:14619–14629.
2017.PubMed/NCBI
|
|
33
|
Liu JJ, Kim Y, Yan F, Ding Q, Ip V, Jong
NN, Mercer JF and Mckeage MJ: Contributions of rat Ctr1 to the
uptake and toxicity of copper and platinum anticancer drugs in
dorsal root ganglion neurons. Biochem Pharmacol. 85:207–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knox RJ, Friedlos F, Lydall DA and Roberts
JJ: Mechanism of cytotoxicity of anticancer platinum drugs:
Evidence that cis-diamminedichloroplatinum(II) and
cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only
in the kinetics of their interaction with DNA. Cancer Res.
46:1972–1979. 1986.PubMed/NCBI
|
|
35
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ardizzoni A, Boni L, Tiseo M, Fossella FV,
Schiller JH, Paesmans M, Radosavljevic D, Paccagnella A, Zatloukal
P, Mazzanti P, et al: Cisplatin-versus carboplatin-based
chemotherapy in first-line treatment of advanced non-small-cell
lung cancer: An individual patient data meta-analysis. J Natl
Cancer Inst. 99:847–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He P, Shen B, Chung PS, Ahn JC and Zhou L:
Photosensitizer effect of 9-hydroxypheophorbide α on diode
laser-irradiated laryngeal cancer cells: Oxidative stress-directed
cell death and migration suppression. Oncol Lett. 12:1889–1895.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishimura G, Tsukuda M, Mikami Y, Matsuda
H, Horiuchi C, Taguchi T, Takahashi M, Kawakami M, Watanabe M, Niho
T, et al: Efficacy of concurrent chemoradiotherapy for T1 and T2
laryngeal squamous cell carcinoma regarding organ preservation.
Anticancer Res. 29:661–666. 2009.PubMed/NCBI
|
|
39
|
Park WH and You BR: Antimycin A induces
death of the human pulmonary fibroblast cells via ROS increase and
GSH depletion. Int J Oncol. 48:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singsai K, Akaravichien T, Kukongviriyapan
V and Sattayasai J: Protective effects of streblus asper leaf
extract on H2O2-induced ROS in SK-N-SH Cells and MPTP-induced
parkinson's disease-like symptoms in C57BL/6 mouse. Evid Based
Complement Alternat Med. 2015:9703542015. View Article : Google Scholar : PubMed/NCBI
|