Introduction
The anticancer agent 5-fluorouracil (5-FU) is well
known as the key drug in regimens for the treatment of
gastrointestinal cancer (1,2). Large inter- and intraindividual
variations in the pharmacokinetics of 5-FU have been investigated,
and this high pharmacokinetic variation is one of the factors
contributing to treatment failure (1). Monitoring 5-FU concentration in plasma
(known as therapeutic drug monitoring (TDM)) is proposed to improve
clinical outcomes, including alleviation of 5-FU toxicity (2). However, identification of the factors
contributing to the plasma 5-FU concentration, target plasma
levels, and appropriate blood sampling time in individual
chemotherapeutic regimens remains a challenge.
In a previous study by our research group, Japanese
patients with esophageal squamous cell carcinoma (ESCC) were
followed for 5 years after treatment with definitive 5-FU/cisplatin
(CDDP)-based chemoradiotherapy and the relation between prognosis
and the plasma 5-FU concentration was evaluated (3,4). The
chemoradiotherapy consisted of the continuous infusion of 5-FU at
400 mg/(m2·day) for 5 days in weeks 1 and 2, and plasma
concentrations of 5-FU were determined at eight sampling points up
to the end of a second course. We found that the plasma
concentration of 5-FU is a possible key determinant of the clinical
response in patients with ESCC after the treatment with definitive
5-FU/CDDP-based chemoradiotherapy. However, circadian variations in
the plasma 5-FU concentration were observed during continuous
infusion of 5-FU, and the repeated treatment cycle also affected
the plasma 5-FU concentration, thereby complicating the analysis of
the degree to which these factors affect plasma 5-FU
concentrations. These effects make it difficult to analyze the data
(5) and to determine appropriate
blood sampling time points and plasma 5-FU levels. In our previous
study, to normalize the circadian rhythm and the treatment cycle
effects on plasma 5-FU concentration, an eight-point average value
was used to investigate the association with the clinical response
(3). To realize a personalized
5-FU/CDDP-based chemoradiotherapy regimen based on the plasma 5-FU
concentration to improve the clinical response, more detailed
analysis of the circadian rhythm and the treatment cycle effects on
the plasma 5-FU concentration has remained an important issue for
further research.
Recently, we successfully evaluated the circadian
variations of 5-FU after intravenous administration of 5-FU or oral
administration of capecitabine, which is a prodrug of 5-FU, in rats
using population model analysis (6,7). One of
the advantages of population analysis is the quantitative
evaluation of covariate effects on the drug plasma concentrations.
The results of these studies could point to a new chronomodulated
schedule for the administration of capecitabine (7). Therefore, population analysis is
considered effective at evaluating the factors contributing to
plasma concentrations of 5-FU.
In the current study, we performed population
analysis to evaluate the effects of the circadian rhythm and
treatment cycle on the plasma 5-FU concentration in patients with
ESCC after treatment with definitive 5-FU/CDDP-based
chemoradiotherapy.
Materials and methods
The data source
The 5-FU plasma concentration and clinical outcome
data from 49 patients with ESCC after treatment with definitive
5-FU/CDDP-based chemoradiotherapy in our previous study (3) were used as the source of data for
population analysis. A summary of the patients' characteristics is
shown in Table I. The treatment
protocol consisted of the prolonged infusion of 5-FU at 400
mg/(m2·day) for days 1–5 and 8–12, the infusion of CDDP
at 40 mg/(m2·day) on days 1 and 8, and radiation at 2
Gy/day on days 1 to 5, 8 to 12, and 15 to 19, with a second course
repeated after a 2-week interval. Blood samples were collected at
eight sampling points (on day 3 at 17:00 h, day 4 at 05:00 h, day
10 at 17:00 h, and day 11 at 05:00 h in the first cycle, and day 38
at 17:00 h, day 39 at 05:00 h, day 45 at 17:00 h, and day 46 at
05:00 h in the second course). In the current study, the plasma
sample on day 1 and day 2 could not be obtained from patients
because the sample collection time was limited during definitive
5-FU/CDDP-based chemoradiotherapy. The sampling in the early h of
the infusion could underestimate the plasma concentration-time
curve (AUC) value of 5-FU due to a wide variety of factors,
including fluctuating rates of 5-FU metabolism before steady-state
conditions are reached and collecting before the infusion pump was
fully primed with drug (8). Moreover,
it is necessary to minimize the effects of high plasma CDDP levels
on plasma concentrations of 5-FU. Based on the half-life of CDDP
(as unbound plasma concentration of platinum) in distribution and
elimination phase is approximately 31.2 min and 20.1 h (9,10),
respectively, day 3 and 4 in the regimen was considered to be
appropriate sampling time to analyze the circadian and treatment
cycles effects of plasma concentration of 5-FU. Further details of
these data including the patient characteristics, treatment
protocol, and clinical responses in this clinical study, which was
conducted with the authorization of the Ethical Committee for
Genetic Studies of the Kobe University Graduate School of Medicine
(Kobe, Japan) and followed the medical research council guidelines
of Kobe University, have been described in our previous report
(3).
 | Table I.Demographic and clinicopathologic
characteristics and clinical outcome. |
Table I.
Demographic and clinicopathologic
characteristics and clinical outcome.
| Characteristics | Values |
|---|
| Demographic and
clinicopathologic characteristics |
|
|
Male/female | 46/3 |
| Age,
yeara | 64.5±7.4
(48–78) |
| Height,
cma | 163.5±6.6
(150–180) |
| Weight,
kga | 56.1±9.6
(33–79) |
|
Performance status,
0/1/2/unknown | 24/20/4/1 |
|
Differentiation,
well/moderate/poor/unknown | 7/28/8/6 |
|
T1/T2/T3/T4 | 16/6/15/12 |
|
N0/N1 | 22/27 |
|
M0/M1ab | 41/8 |
| Stage
I/II/III/IVa | 12/10/19/8 |
| Clinical
outcomec |
|
|
Complete response rate | 23 (46.9%) |
| Grade
3/4 leucopenia | 21 (42.9%) |
| Grade
3/4 stomatitis | 7 (14.3%) |
| Grade
3/4 cheilitis | 8 (16.3%) |
Population analysis
This analysis was performed using a nonlinear
mixed-effects modeling program, Phoenix®
NLME™ software (v7.0; Certara USA, Inc., Princeton, NJ,
USA). The first-order conditional estimation with the extended
least-squares (FOCE-ELS) method was used to estimate the population
parameters and their variability. Population model choice was based
on Akaike's Information Criteria (AIC), goodness-of-fit plots,
including observed (OBS) vs. population prediction (PRED) and vs.
individual predicted concentrations (IPRED), and the coefficient of
variation (CV) in parameter estimates. As a cutoff criterion for
model improvement, a drop in AIC of 2 or more was applied, which is
a threshold for choosing one model over another (11).
A pharmacokinetic compartment model with a cosinor
method was employed to evaluate circadian variations in the plasma
drug concentrations (12). However,
this method is unsuitable for the evaluation of the circadian
variation of the plasma 5-FU concentration in the current study
because these data were obtained at only two dosing time points
(05:00 and 17:00 h), which is too small a sample size to describe
time-course alteration via a pharmacokinetic compartment model with
a cosine curve. Therefore, population analysis using simple model
equations is necessary in this case. Our research group previously
found that the circadian rhythm and treatment cycle are significant
factors contributing to inter- and intrapatient variations of the
plasma 5-fluorouracil concentrations in patients with ESCC
(3,4).
Thus, in the current study, both the circadian rhythm and treatment
cycle served as covariates for the model equation. To determine the
final model equation, different model equations (additive,
multiplicative, and power) were initially tested based on Akaike's
Information Criteria (AIC), goodness-of-fit plots, and the CV of
parameter estimates. In the final model, the plasma 5-fluorouracil
concentration was defined using the following equation:
C5-FU=Basis×Circkcirc×Cyckcyc
Where C5-FU is the plasma 5-FU
concentration, and Basis is the plasma 5-FU concentration
excluding the effects of the treatment cycle and circadian rhythm.
Circ and Cyc are circadian rhythm and repeated
treatment cycle effects on the plasma 5-FU concentration,
respectively; kcirc and kcyc
are the constants governing Circ and Cyc. In the
source data, the plasma 5-FU concentration at 17:00 h was higher
than that at 05:00 h in the same treatment cycle; thus, to describe
the effects of the circadian rhythm on the plasma 5-FU level,
kcirc was fixed at 0 and 1.0 when the time point
was 05:00 h and 17:00 h, respectively. Similarly, the plasma 5-FU
concentration in the second cycle was higher than that in the first
cycle at the same sampling time; therefore, to describe the effects
of the treatment cycle on the plasma 5-FU level,
kcyc was fixed at 0 in the first cycle and at 1.0
in the second cycle. The Basis value means the plasma
concentration of 5-FU on day 3 at 17:00 h.
The interindividual variability was described by an
exponential function. The individual parameter estimate
(Ai) is the product of the population parameter
estimate (θA) and the random effect for parameter
Ai (ηi), which followed a
normal distribution with mean 0 and variance ω2 and was
determined using the following equation:
Ai=θA×eηi
To describe the residual variability between the
observed and predicted plasma concentrations
(Cobs and Cpred), different
error models (additive, multiplicative, and power models) were
tested and chosen based on AIC and CV of the parameter estimates.
The residual variability was characterized using the random effect
(ε), which followed a normal distribution with mean 0 and
variance σ2, as per the following power error model:
Cobs=Cpred+Cpred0.5×ε
To perform statistical analysis on patients'
characteristics and clinical outcomes, post hoc population
parameter estimates for individual patients were obtained by this
population analysis.
To confirm the stability of the final population
model, the model was assessed by nonparametric bootstrap sampling
(n=1,000). This bootstrap procedure was performed for comparison
with the population model parameters estimated from the original
dataset and to obtain the confidence intervals for the model
parameters.
Statistical analysis
Two-group comparisons of population parameters were
made by the Mann-Whitney U test. Comparisons across multiple
groups were performed using the Kruskal-Wallis test with post-hoc
comparisons by the Scheffe test. Correlation between population
parameters and patients' characteristics, including clinical
efficacy and toxicity, were assessed by the Pearson or Spearman
rank correlation test. The patients' characteristics in the
correlation test were age, height, weight, body surface area (BSA),
sex, TNM staging, the clinical response, leucopenia, stomatitis,
and cheilitis according to our previous report (3). P<0.05 was considered to indicate a
statistically significant difference.
Results
Population analysis
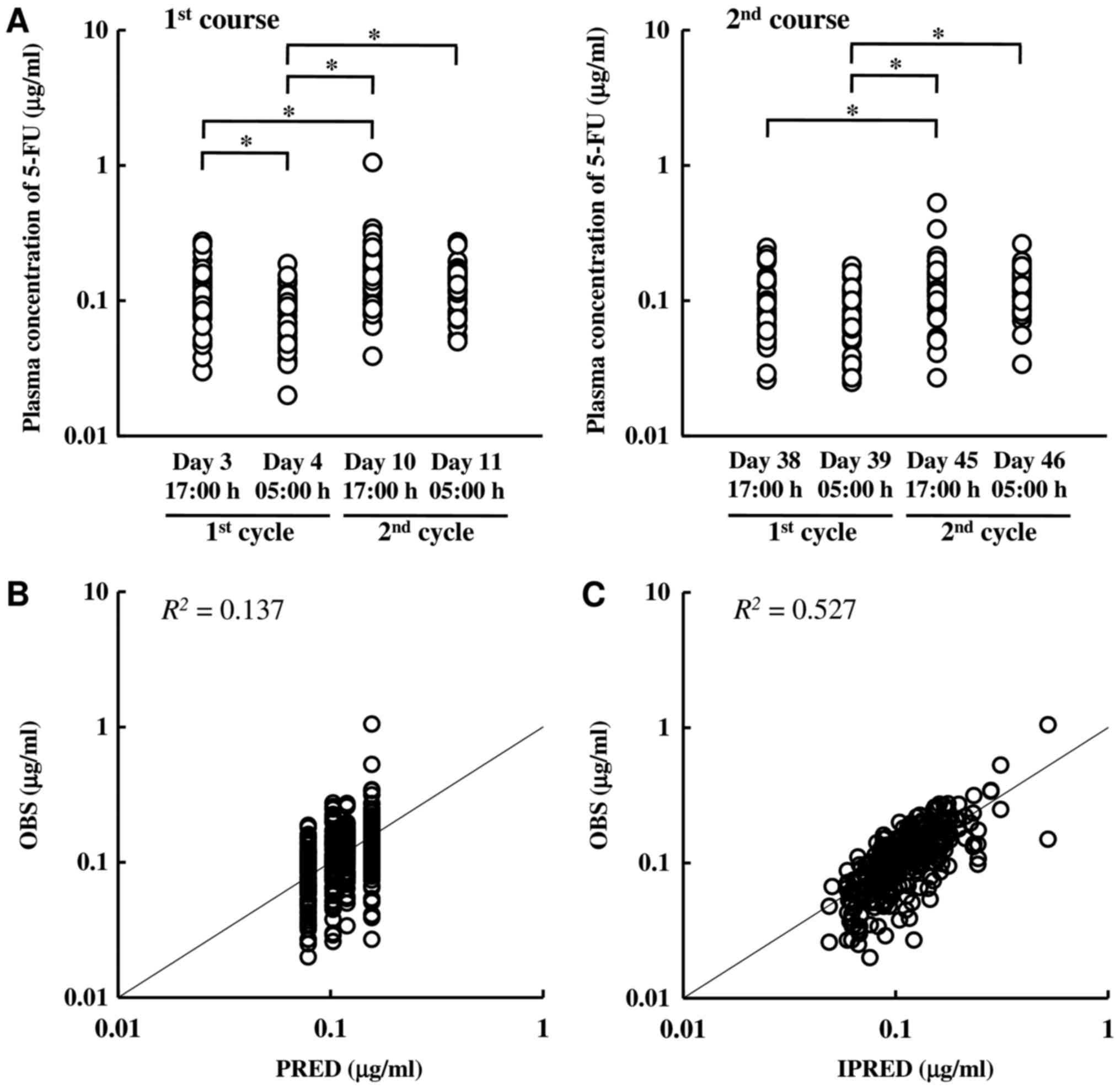
Fig. 1 shows the
plasma 5-FU concentration and goodness-of-fit plots of the final
population model. Significant circadian variations and treatment
cycle effects were observed; the plasma 5-FU concentration at 17:00
h was higher than that at 05:00 h, and that level in the second
cycle was higher than that in the first cycle. However, no
significant circadian variations in the second cycle were observed,
possibly due to large interindividual variability in the second
cycle. The goodness-of-fit plots indicated that the population
model with a random effect adequately described the individual
predictions of the plasma 5-FU concentration. The population
parameter estimates and the results of the bootstrap validation are
described in Table II. These
parameter and random effects were estimated with relatively high
precision because all were <39.8% and were very similar to the
mean of the bootstrap procedure. Population analysis with the
covariates of circadian variations and repeated treatment cycle
effects revealed that the basis plasma 5-FU concentration without
the covariates (Basis) was 0.078 µg/ml at the steady state
during the prolonged infusion of 5-FU at 400
mg/(m2·day), with relatively low inter- and
intraindividual variability (13.5 and 24.2%, respectively). The
population parameters indicated that the plasma 5-FU concentration
was 1.3-fold higher in the evening than in the morning, and that
level in the second cycle was 1.5-fold greater than that in the
first cycle.
 | Table II.Population parameters of 5-FU
regarding circadian and repeated treatment cycle effects in
Japanese patients with ESCC. |
Table II.
Population parameters of 5-FU
regarding circadian and repeated treatment cycle effects in
Japanese patients with ESCC.
|
|
| Final model | Bootstrap
(n=1,000) |
|---|
|
|
|
|
|
|---|
| Parameters | Unit | Estimate | CV, % | Mean | Median | 2.5th-97.5th
percentiles |
|---|
| Fixed effect
parameters |
|
Basis | µg/ml | 0.078 |
5.4 | 0.079 | 0.079 | 0.070–0.088 |
|
Circ |
| 1.308 |
5.4 | 1.31 | 1.30 | 1.183–1.460 |
|
Cyc |
| 1.522 |
4.9 | 1.52 | 1.51 | 1.369–1.699 |
| Inter-individual
variability |
|
ω (Basis) | % | 24.2 | 26.2 | 23.7 | 23.7 | 18.595–28.288 |
|
ω (Circ) | % | 23.3 | 39.8 | 22.6 | 22.3 | 0.237–33.038 |
|
ω (Cyc) | % | 16.8 | 36.0 | 16.1 | 16.2 | 0.087–25.068 |
| Residual
variability |
| σ | % | 13.5 |
4.5 | 13.4 | 13.3 | 11.086–16.434 |
Population parameters and clinical
responses
The population parameters of the patients with a
survival period of 5 years or more and with less than 5 years are
shown in Table III, and for the
patients with a complete response (CR), partial response (PR),
stable disease (SD), and progressive disease (PD), the parameters
are given in Table IV. No
significant differences between survival time groups were observed
in any population parameters. However, significant differences (in
the population parameters) were observed in therapeutic efficacy,
with higher Basis values in patients with CR than in those
with PR (P=0.034). Cyc values in patients with CR were
slightly higher than those in patients with PR, although the
different was not significant (P=0.062).
 | Table III.Association between prognosis after
treatment with a definitive 5-FU/cisplatin-based chemoradiotherapy
and population parameters of 5-FU regarding treatment cycle and
circadian variation in 49 Japanese patients with ESCC. |
Table III.
Association between prognosis after
treatment with a definitive 5-FU/cisplatin-based chemoradiotherapy
and population parameters of 5-FU regarding treatment cycle and
circadian variation in 49 Japanese patients with ESCC.
| Parameters | Total (n=49) | Survival of ≥5
years (n=21) | Survival of <5
years (n=28) |
P-valuea |
|---|
| Basis,
µg/ml | 0.079±0.015 | 0.081±0.016 | 0.077±0.014 | 0.480 |
| Circ | 1.305±0.205 | 1.346±0.277 | 1.274±0.123 | 0.657 |
| Cyc | 1.525±0.138 | 1.534±0.169 | 1.519±0.113 | 0.952 |
 | Table IV.Association between clinical outcome
after treatment with a definitive 5-FU/cisplatin-based
chemoradiotherapy and population parameters of 5-FU regarding
treatment cycle and circadian variation in 49 Japanese patients
with ESCC. |
Table IV.
Association between clinical outcome
after treatment with a definitive 5-FU/cisplatin-based
chemoradiotherapy and population parameters of 5-FU regarding
treatment cycle and circadian variation in 49 Japanese patients
with ESCC.
| Parameters | CR (n=23) | PR (n=21) | SD (n=2) | PD (n=2) |
P-valuea |
|---|
| Basis,
µg/ml | 0.084±0.016 | 0.075±0.011 | 0.062, 0.067 | 0.061, 0.095 | 0.034 |
| Circ | 1.312±0.257 | 1.305±0.163 | 1.324, 1.339 | 1.263, 1.310 | 0.751 |
| Cyc | 1.564±0.163 | 1.489±0.104 | 1.529, 1.682 | 1.454, 1.476 | 0.062 |
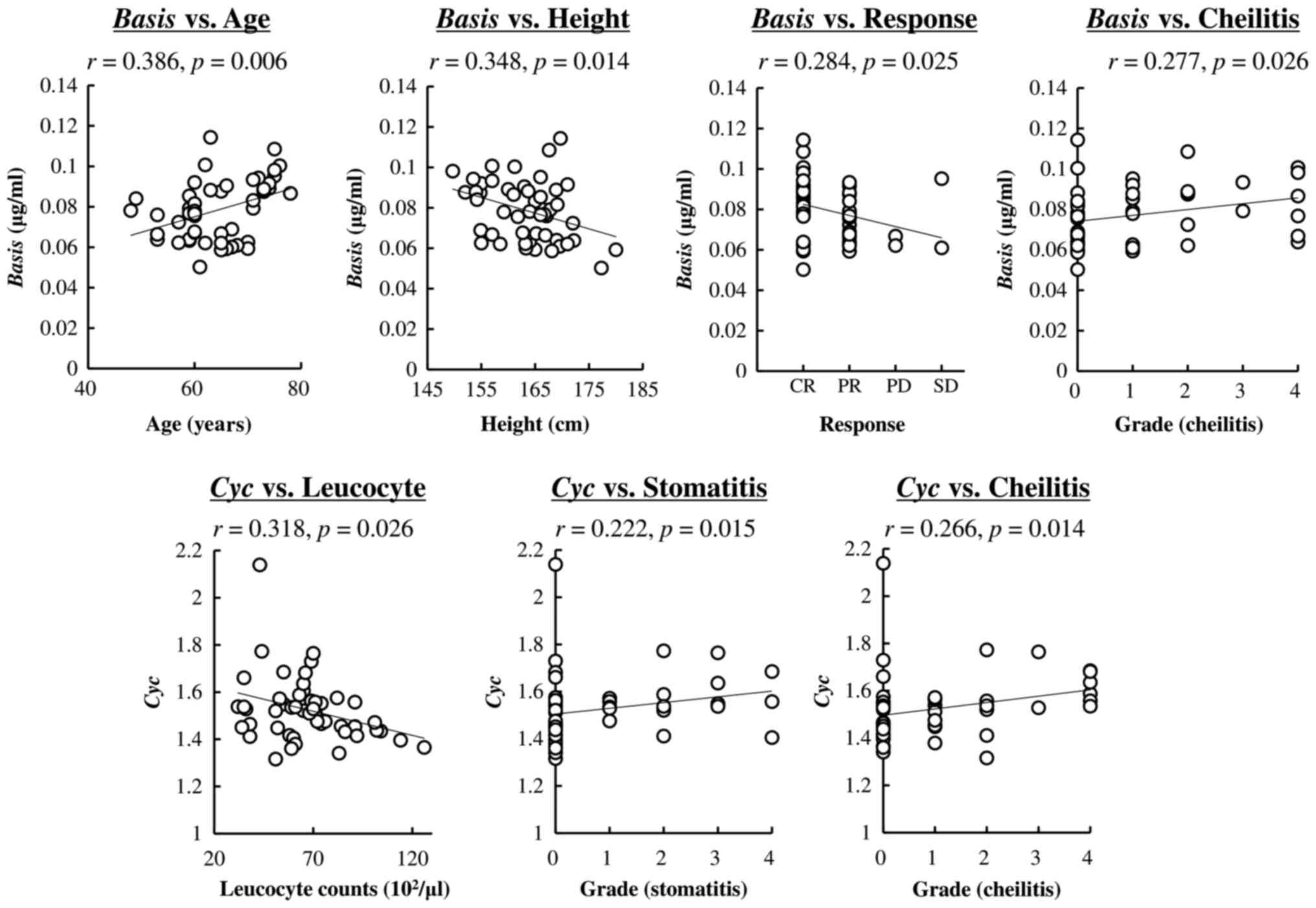
Fig. 2 presents a
significant linear correlation between population parameters and
patients' characteristics. There were significant correlations in
Basis vs. age and height, whereas no correlations were
observed with BSA, sex, or TNM staging. Clinical responses (CR, PR,
SD, and PD) significantly correlated with Basis values
(P=0.025). The grade of cheilitis after the second course also
varied according to Basis values. A significant negative
correlation was observed between Cyc and leucocyte counts
obtained before the start of chemoradiotherapy. Stomatitis and
cheilitis after the second course also correlated with Cyc
values. There were no significant correlations between Circ
values and patients' characteristics.
Discussion
In the field of oncology, researchers are focusing
on the TDM of 5-FU, which improves the clinical outcome. However,
circadian variation and repeated treatment cycle effects on the
plasma concentration of 5-FU exist as confounding factors in the
determination of the appropriate blood sampling time and plasma
5-FU level. Unfortunately, the correlations between the size of
these effects on plasma 5-FU concentration and the clinical
response, including long-term survival and toxicities, are still
unknown. In the current study, to evaluate the circadian rhythm and
treatment cycle effects on the plasma 5-FU concentration,
population analysis was performed using previously reported data on
patients with ESCC after definitive 5-FU/CDDP-based
chemoradiotherapy, and correlations with clinical responses were
investigated.
The population analysis revealed that the estimated
Basis parameter, which shows the plasma concentration of
5-FU on day 3 at 17:00 h, significantly correlated with the
clinical response (CR, PR, PD, and SD), and higher values were
observed in patients with CR, whereas there was no correlation with
survival time after chemoradiotherapy. Although the 5-FU dose was
determined based on BSA, there was no correlation between the
plasma 5-FU level and BSA; older and shorter patients had higher
estimated Basis. Clinical studies have shown that in the
FOLFIRI and FOLFOX regimens for the treatment of colorectal cancer,
pharmacokinetic-guided dose adjustment of 5-FU can enhance and
improve the clinical efficacy as compared to BSA-based dosage
(1,2).
Our results suggest that, in agreement with the regimens for
colorectal cancer, in the definitive 5-FU/CDDP-based
chemoradiotherapy for the treatment of ESCC, dose adjustment based
on the plasma 5-FU level could be a valuable approach to improving
clinical responses. The results of the current study also suggest
that the appropriate blood sampling time for estimating the
clinical response is the evening of day 3. Dose management based on
the patient's age and height with monitoring of the plasma 5-FU
level on the evening of day 3 may be an effective approach to
improving clinical efficacy in patients with ESCC after the
definitive 5-FU/CDDP-based chemoradiotherapy. However, the number
of sampling time points within the day was small in the current
study; further studies are necessary to determine the optimal blood
sampling time.
A subsequent cycle of treatment was estimated to
lead to a 1.5-fold greater plasma 5-FU level. Of note, Cyc
significantly correlated with pretherapeutic leucocyte counts:
Lower leucocyte counts had greater effects on the magnitude of
plasma 5-FU level elevation in the second cycle. Higher 5-FU
concentrations in the plasma after repeated treatment with 5-FU in
animals and patients have been reported (4,5,13,14). This
5-FU elevation is related to a decrease in clearance and hepatic
dihydropyrimidine dehydrogenase (DPD) activity levels (13,14).
Repeated 5-FU administration also leads to the loss of the
circadian rhythm in hepatic DPD activity (4,13). The
plasma ratio of dihydrouracil/uracil (UH2/Ura) is known
to be an indirect response marker of DPD activity in the liver and
peripheral blood mononuclear cells (15), and our previous study using a rat
model of colorectal cancer found that this ratio can assess the
higher AUC of 5-FU and lower DPD activity during repeated 5-FU
administration (14). Nevertheless,
the measurement of dihydrouracil and uracil concentration in plasma
needs highly sensitive analysis using high-performance liquid
chromatography or liquid chromatography with tandem mass
spectrometry, which require much more experimental time and is
expensive. Therefore, according to the present results, leucocyte
counts obtained by the routine diagnostic test before the
definitive 5-FU/CDDP-based chemoradiotherapy could be more valuable
for estimating the elevation in plasma 5-FU concentration during a
subsequent cycle of treatment. However, the mechanism of their
correlation is still unknown and further studies are needed.
The analysis in the current study detected a
correlation between the plasma concentration of 5-FU and its
toxicity. The occurrence of cheilitis and stomatitis after the
second course of the definitive 5-FU/CDDP-based chemoradiotherapy
was affected by the plasma concentration of 5-FU on day 3 at 17:00
h (parameter Basis) and the elevation in plasma 5-FU
concentration during a subsequent treatment cycle (parameter
Cyc). Some clinical studies have revealed that
pharmacokinetics-guided dose adjustment of 5-FU can reduce the
toxicity (2,16,17). Thus,
the plasma 5-FU concentration on the evening of day 3 and
pretherapeutic leucocyte counts could be important markers for
evaluating both clinical efficacy and toxicity.
Our data showed that circadian variations in the
plasma concentration of 5-FU during continuous infusion were
relatively small and did not contribute to the clinical response or
toxicity, in line with the previous report (5). Fleming et al (5), raised the possibility that the benefit
from chronomodulated chemotherapy with 5-FU observed in clinical
studies is related to proliferation and/or drug metabolic rhythms
in tumor tissue, not circadian variations in the plasma 5-FU level.
It has been shown that there is significant circadian variation in
cell proliferation (18–21). Results of population PK-PD model
analysis in rats show that not only fluctuations in plasma 5-FU
concentration but also the cell sensitivity to 5-FU affect the
onset and severity of its toxicity (22). The modulation of the infusion rate
throughout the day according to cell proliferation may improve the
clinical response to 5-FU/CDDP-based chemoradiotherapy.
The results of the current study show a relation
between the factors affecting plasma 5-FU concentration and the
clinical response in patients with ESCC after treatment with the
definitive 5-FU/CDDP-based chemoradiotherapy. To obtain a better
clinical response to this chemoradiotherapy, the measurement of
plasma 5-FU concentration on the evening of day 3 and leucocyte
counts before chemotherapy may represent a good therapeutic
strategy for the definitive 5-FU/CDDP-based chemoradiotherapy
because it would help to predict the clinical response. This
measurement of the plasma component may aid the decision on whether
to continue chemotherapy to sequential cycles. However, this study
has some limitations. First, we evaluated only two blood sampling
time points (5:00 h or 17:00 h) throughout the day and furthermore,
the sample size was small. Second, it is difficult to exclude
radiation effects from our original data to analyze the circadian
rhythm chemotherapy. The radiation may be one of covariates for
plasma concentrations of 5-FU (23).
However, in the current study, all patients have been treated with
2 Gy/day on days 1–5 and then covariate analysis of radiation could
not be performed in the current original data set. Therefore, the
results of this study could apply to only the definitive
5-FU/CDDP-based chemoradiotherapeutic regimen, not the other
5-FU-bsed regimen. Finally, the current analysis was retrospective,
i.e., using previously reported data. To demonstrate the usefulness
of measuring the plasma concentration of 5-FU and leucocyte counts
before chemotherapy, we suggest a prospective study with a larger
sample size.
In conclusion, the findings of the present study
provide evidence that plasma concentration of 5-FU, excluding the
circadian and repeated treatment cycle effects, depends on age,
height, and the clinical response in patients with ESCC after
treatment with the definitive 5-FU/CDDP-based chemoradiotherapy.
The circadian variations in plasma 5-FU concentration were
relatively small, and the magnitude of the repeated cycle effect
correlated with leucocyte counts before chemotherapy. These results
suggest that the initial dosage of 5-FU based on age and height may
offer a higher plasma concentration of 5-FU and clinical response.
The measurement of leucocyte counts before the start of
chemoradiotherapy may estimate the increase in the plasma 5-FU
level after a sequential cycle and help to avoid severe toxicity.
To clarify the advantages of these proposed chemoradiotherapy
regimens, additional prospective studies with a larger sample size
are needed.
Acknowledgements
The authors would like to thank Professor Toshiyuki
Sakaeda of the Department of Pharmacokinetics, Kyoto Pharmaceutical
University (Kyoto, Japan) for his guidance and help.
Funding
The present study was supported in part by a
Grant-in-Aid for Young Scientists (B) from JSPS KAKENHI (grant no.
15K18937).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK and SK analyzed the clinical data and wrote the
manuscript. TT designed the study and was a major contributor to
writing of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The source of data was obtained from the previous
reported study that was conducted with the authorization of the
Ethical Committee for Genetic Studies of the Kobe University
Graduate School of Medicine (Kobe, Japan) and followed the medical
research council guidelines of Kobe University, Japan. Informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saif MW, Choma A, Salamone SJ and Chu E:
Pharmacokinetically guided dose adjustment of 5-fluorouracil: A
rational approach to improving therapeutic outcomes. J Natl Cancer
Inst. 101:1543–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JJ, Beumer JH and Chu E: Therapeutic
drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol.
78:447–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuwahara A, Yamamori M, Kadoyama K,
Nishiguchi K, Nakamura T, Miki I, Tamura T, Okuno T, Omatsu H and
Sakaeda T: Effects of plasma concentrations of 5-fluorouracil on
long-term survival after treatment with a definitive
5-fluorouracil/cisplatin-based chemoradiotherapy in Japanese
patients with esophageal squamous cell carcinoma. J Exp Clin Cancer
Res. 30:942011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuwahara A, Yamamori M, Nishiguchi K,
Okuno T, Chayahara N, Miki I, Tamura T, Kadoyama K, Inokuma T,
Takemoto Y, et al: Effect of dose-escalation of 5-fluorouracil on
circadian variability of its pharmacokinetics in Japanese patients
with Stage III/IVa esophageal squamous cell carcinoma. Int J Med
Sci. 7:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fleming GF, Schumm P, Friberg G, Ratain
MJ, Njiaju UO and Schilsky RL: Circadian variation in plasma
5-fluorouracil concentrations during a 24 h constant-rate infusion.
BMC Cancer. 15:692015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobuchi S, Ito Y, Nakano Y and Sakaeda T:
Population pharmacokinetic modelling and simulation of
5-fluorouracil incorporating a circadian rhythm in rats.
Xenobiotica. 46:597–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobuchi S, Yazaki Y, Ito Y and Sakaeda T:
Circadian variations in the pharmacokinetics of capecitabine and
its metabolites in rats. Eur J Pharm Sci. 112:152–158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaldate RR, Haregewoin A, Grier CE,
Hamilton SA and McLeod HL: Modeling the 5-fluorouracil area under
the curve versus dose relationship to develop a pharmacokinetic
dosing algorithm for colorectal cancer patients receiving FOLFOX6.
Oncologist. 17:296–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cisplatin (BRIPLATIN®) [Drug
information]. Bristol-Myers Squibb. 2017.
|
|
10
|
Kitajima K, Fukuoka M, Kobayashi S,
Kusunoki Y, Takada M, Negoro S, Matsui K, Sakai N, Ryu S and
Takifuji N: Studies on the appropriate administration of cisplatin
based on pharmacokinetics and toxicity. Gan To Kagaku Ryoho.
14:2517–2523. 1987.(In Japanese). PubMed/NCBI
|
|
11
|
Mould DR and Upton RN: Basic concepts in
population modeling, simulation and model-based drug
development-part 2: Introduction to pharmacokinetic modeling
methods. CPT Pharm Syst Pharmacol. 17:e382013. View Article : Google Scholar
|
|
12
|
Yang QJ, Bukuroshi P, Quach HP, Chow ECY
and Pang KS: Highlighting vitamin D receptor-targeted activities of
1α, 25-dihydroxyvitamin D (3) in mice via physiologically based
pharmacokinetic-pharmacodynamic modeling. Drug Metab Dispos.
46:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobuchi S, Ito Y, Okada K, Imoto K, Kuwano
S and Takada K: Pre-therapeutic assessment of plasma
dihydrouracil/uracil ratio for predicting the pharmacokinetic
parameters of 5-fluorouracil and tumor growth in a rat model of
colorectal cancer. Biol Pharm Bull. 36:907–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobuchi S, Kuwano S, Imoto K, Okada K,
Nishimura A, Ito Y, Shibata N and Takada K: A predictive biomarker
for altered 5-fluorouracil pharmacokinetics following repeated
administration in a rat model of colorectal cancer. Biopharm Drug
Dispos. 34:365–376. 2013.PubMed/NCBI
|
|
15
|
Jiang H, Lu J and Ji J: Circadian rhythm
of dihydrouracil/uracil ratios in biological fluids: A potential
biomarker for dihydropyrimidine dehydrogenase levels. Br J
Pharmacol. 141:616–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gamelin E, Delva R, Jacob J, Merrouche Y,
Raoul JL, Pezet D, Dorval E, Piot G, Morel A and Boisdron-Celle M:
Individual fluorouracil dose adjustment based on pharmacokinetic
followup compared with conventional dosage: Results of a
multicenter randomized trial of patients with metastatic colorectal
cancer. J Clin Oncol. 26:2099–2105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Capitain O, Asevoaia A, Boisdron-Celle M,
Poirier AL, Morel A and Gamelin E: Individual fluorouracil dose
adjustment in FOLFOX based on pharmacokinetic follow-up compared
with conventional body-area-surface dosing: A phase II,
proof-of-concept study. Clin Colorectal Cancer. 11:263–267. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchi KN, Moore JG, Hrushesky WJ, Sothern
RB and Rubin NH: Circadian rhythm of cellular proliferation in the
human rectal mucosa. Gastroenterology. 101:410–415. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang R, Lu Z, Liu T, Soong SJ and Diasio
RB: Relationship between circadian-dependent toxicity of
5-fluorodeoxyuridine and circadian rhythms of pyrimidine enzymes:
Possible relevance to fluoropyrimidine chemotherapy. Cancer Res.
53:2816–2822. 1993.PubMed/NCBI
|
|
20
|
Jilma B, Hergovich N, Stohlawetz P,
Eichler HG, Bauer P and Wagner OF: Circadian variation of
granulocyte colony stimulating factor levels in man. Br J Haematol.
106:368–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abolmaali K, Balakrishnan A, Stearns AT,
Rounds J, Rhoads DB, Ashley SW and Tavakkolizadeh A: Circadian
variation in intestinal dihydropyrimidine dehydrogenase (DPD)
expression: A potential mechanism for benefits of 5FU
chrono-chemotherapy. Surgery. 146:269–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobuchi S, Ito Y and Sakaeda T: Population
pharmacokinetic-pharmacodynamic modeling of 5-fluorouracil for
toxicities in rats. Eur J Drug Metab Pharmacokinet. 42:707–718.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh CH, Hou ML, Chiang MH, Tai HC, Tien
HJ, Wang LY, Tsai TH and Chen YJ: Head and neck irradiation
modulates pharmacokinetics of 5-fluorouracil and cisplatin. J
Transl. 11:2312013. View Article : Google Scholar
|