Introduction
Esophageal cancer (EC) is the 8th most frequent and
the 6th most fatal cancer worldwide (1), with an occurrence of >1:1,000 in
high-risk northern Chinese populations, but 20-fold lower in
low-risk Western African populations (2). Esophageal squamous cell carcinoma (ESCC)
or esophageal adenocarcinoma (EAC) is found in almost 95% of EC
cases (1). ESCC is the main subtype
of EC in China, whereas EAC is the most common subtype of EC in
Western countries.
ESCC, which arises from the squamous epithelium of
the esophagus, is associated with the consumption of tobacco
(3). The intake of substantial
amounts of alcohol, particularly in conjunction with smoking,
dramatically increases the risk of squamous-cell carcinoma
(although not adenocarcinoma) (4). At
present, surgical resection is the main surgical treatment for EC.
For patients who have curative surgery and do not progress to lymph
node metastasis, the 5-year survival rate is 20–30%; whereas, for
patients with >1 lymph node metastasis, the 5-year survival rate
is only 13% (5). Therefore, it is
important to understand the molecular characteristics of EC for
clinical biomarkers and therapeutic modalities.
Biomarkers have been investigated for many years, in
terms of modifications in genomic DNA, specific mRNA/protein
molecules or metabolites (6).
Active or mature miRNAs are 18–22 nucleotides,
single-stranded RNA molecules with 50 phosphate and 30 hydroxyl
groups (7). A mature miRNA can target
hundreds of mRNAs and one mRNA can be targeted by various miRNAs
(8). miRNAs are potential biomarkers
with crucial cellular roles in healthy and diseased cells (8). The reasons for adopting miRNAs as
anti-cancer drugs are the dysregulation of miRNAs in cancer and the
alteration of cancer phenotypes via targeting miRNA (9). The in vivo stability of miRNAs
has been successfully used in preclinical models of miRNA-based
treatments (10). miR-34a acts as an
important tumor suppressor in various cancers (11). CDK4/6, cyclin D1, E2F3, MYCN, SIRT1
and Bcl2, which are correlated with cell cycle and apoptosis
control, are downregulated by miR-34a (11).
The present study aimed to explore the role of
miR-34a in ESCC and the possible molecular mechanisms.
Materials and methods
Tissue samples
A total of 30 cases of ESCC tissues and 30 cases of
adjacent non-tumor esophageal tissues were collected from 30
patients (17 male: 13 female) who aged at 47.21±8.33 between
February, 2014 and January, 2016, from Chibi Pu Spinning Hospital.
No patient had received radiotherapy or chemotherapy prior to
surgery. The use of human tissues was approved by the Ethics
Committee of Chibi Pu Spinning Hospital. Patients agreed to the use
of their samples in scientific research.
Cell culture
Human esophageal epithelial cells (HEEC), Het-1A,
and ESCC TE-8 and TE-1 cells were obtained from the Institute of
Biochemistry and Cell Biology of the Chinese Academy of Sciences
(Shanghai, China). Cell lines were cultured in RPMI-1640 medium
(Hyclone; GE Healthcare, Logan, UT, USA) containing 10%
heat-inactivated fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific Inc., MA, USA), 100 U/mL penicillin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), and 100 mg/mL streptomycin
(Sigma-Aldrich; Merck KGaA) in an incubator at 37°C and 5%
CO2.
Cell transfections
The miRNA-34a mimics and miRNA-negative control (NC)
mimics were obtained from Qiagen GmbH (Hilden, Germany). HEEC, TE-8
and TE-1 cells were transfected with miR-34a mimics or miRNA-NC
mimics using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), incubated at 37°C for 48 h and prepared
for the following experiments. The target sequences of miRNA-34a
mimics were as follows: 5′-UGGCAGUGUCUUAGCUGGUUGU-3′.
The miRNA-34a inhibitor and miRNA-NC inhibitor were
purchased from Applied Biological Materials (ABM, Vancouver,
Canada). HEEC cells were transfected with miR-34a inhibitor or
miRNA-NC inhibitor by Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific Inc.), incubated at 37°C for 48 h and
prepared for the following experiments.
RNA isolation and quantitative
real-time PCR
Total RNA from 30 samples of ESCC and non-tumor
tissues, and ESCC cell lines transfected with miR-34a mimics and
miRNA-NC mimics was isolated by TRIzol (Thermo Fisher Scientific,
Inc.). miRNA was isolated from 30 samples of ESCC and non-tumor
tissues, and ESCC cell lines transfected with miR-34a mimics and
miRNA-NC mimics, using a miRNA Extraction kit (Qiagen GmbH). RNA
integrity was assessed using a Nano Drop ND-1000 spectrophotometer.
Reverse transcription was conducted using a miRNA cDNA Synthesis
Kit (Qiagen GmbH). qPCR amplification was conducted using SYBR
green Premix Ex Taq II (Qiagen GmbH) with the Step One Plus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The experiment was performed using an ABI Prism 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR was performed in a total volume of 25.0 µl:
10 µl SYBR Premix Ex Taq (2X), 1 µl PCR Forward Primer, 1 µl PCR
Reverse Primer, 0.5 µl ROX Reference Dye II (50X)*3, 2 µl cDNA and
8 µl double-distilled water. The PCR reaction was performed at an
initial denaturation of 10 min at 95°C, 95°C (5 sec), 63°C (30
sec), and 72°C (30 sec) for a total of 40 cycles, with a final
extension step at 72°C for 5 min. The expression levels of genes
were calculated by 2−ΔΔCq (ΔΔCq=ΔCq ESCC-ΔCq
corresponding normal tissues) (12).
Primers used were: forkhead box M1 (FOXM1) forward:
5′-TCTCAGCACCACTCCCTTG-3′; FOXM1 reverse:
5′-GGATCTTGCTGAGGCTGTC-3′; GAPDH forward:
5′-TGCACCACCAACTGCTTAGC-3′; GAPDH reverse:
5′-GGCATGGACTGTGGTCATGAG-3′; miR-34a forward:
5′-CCCAGAACATAGACACGCTGGA-3′; miR-34a reverse:
5′-ATCAGCTGGGCACCTAGGACA-3′; U6 forward: 5′-CTCGCTTCGGCAGCACA-3′;
and U6 reverse: 5′-CTCGCTTCGGCAGCACA-3′. The expression level of
miRNA was normalized using U6 as an internal control. The
expression level of mRNA was normalized using GAPDH as an internal
control.
Western blot analysis
Cells were collected and lysed in RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). A total of 10 µl protein extract separated on 6–15%
SDS-PAGE and transferred onto a PVDF membrane (Immobilon 0.45 µm;
EMD Millipore, Billerica, MA, USA). The blots were immersed in the
primary antibodies of MMP-2 (cat. no: ab37150; 1:1,000; Abcam,
Cambridge, MA, USA), MMP-9 (cat. no: ab76003; 1:1,000; Abcam),
FOXM1 (cat. no: ab180710; 1:1,000; Abcam) and GAPDH (cat. no:
ab8245; 1:1,000; Abcam) which were diluted with 5% non-fat milk
overnight at 4°C; then treated with a secondary antibody (cat. no:
ab97080; 1:5,000; Abcam) for 2 h at room temperature. Finally, the
bands were exposed using electrochemiluminescence. Protein
expression was normalized to GAPDH.
Luciferase reporter assay
For the luciferase reporter assay, TE-8 and TE-1
cells were co-transfected with 20 mM miR-34a mimic or the negative
control and 200 ng of psiCHECK-2-FOXM1-3′-UTR-WT or
psiCHECK-2-FOXM1-MUT, and 10 ng Renilla luciferase vector using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 48 h transfection, cells were
collected and analyzed via the Dual-Luciferase Reporter Assay
System (Promega Corporation, Madison, WI, USA). Luciferase activity
was detected using the GloMax fluorescence reader (Promega
Corporation). The pRL-CMV Renilla luciferase reporter was the
control for contrast correction in transfection efficiency.
Wound healing assay
The migratory ability of TE-1 cells was evaluated by
wound-healing assay. TE-1 cells in 6-well plates were scratched
with a 20 µl pipette tip (Eppendorf, Hamburg, Germany) and grown in
RPMI-1640 medium with 1% FBS with 5% CO2 at 37°C. The
extent of migration was assessed by phase contrast microscopy at 0
and 30 h, respectively. Wound healing capacity was monitored under
a microscope after 30 h.
Cell proliferation assay
To explore the effect of miR-34a on the cell
viability of TE-1 cells, the cell viability was measured using a
CCK8 cell counting kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) based on the manufacture's protocol. Briefly,
1×104 TE-1 cells were seeded into 96-well plates and
allowed to grow overnight in complete DMEM (Hyclone; GE Healthcare)
at 37°C. The following day, 10 µl CCK8 solution was added into each
well and incubated for 2 h. The absorbance of TE-1 cells at 450 nm
was measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). At 24, 48 and 72 h after miR-34a mimics or
miR-NC mimics transfection, the cell numbers were analyzed using
the CCK8 kit.
Statistical analysis
SPSS software v13.0 (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Data are expressed as the mean ±
SD. A paired sample t-test was used to compare FOXM1 protein
expression. Spearman's correlation analysis was used to analyze the
correlation between FOXM1 and miR-34a. *P<0.05, **P<0.01 and
***P<0.001 were considered to indicate a statistically
significant difference.
Results
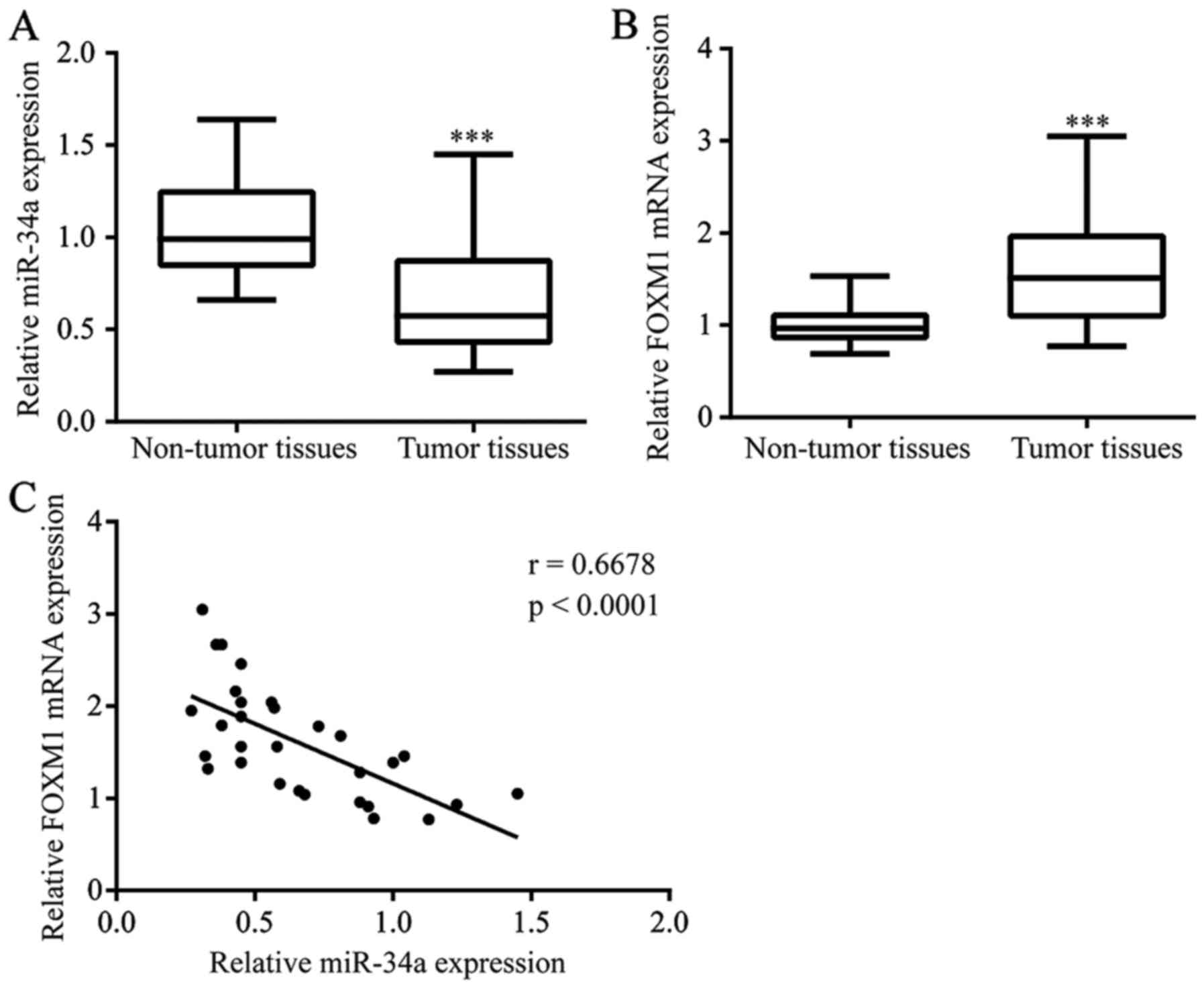
Decreased expression of miR-34a in
tumor tissues from ESCC
To evaluate the potential role of miR-34a in ESCC,
we detected miR-34a levels in 30 tumor tissues and matched normal
tissues from ESCC patients. A significant decrease in miR-34a was
observed in tumor tissues (Fig. 1A).
FOXM1 was reported to be a target gene of miR-34a in hepatocellular
carcinoma (13). We examined FOXM1
mRNA levels in samples from ESCC patients. Overexpression of FOXM1
mRNA was detected in tumor tissues compared with their
non-cancerous matched tissues (Fig.
1B). Importantly, there was a negative association between
miR-34a levels and FOXM1 mRNA levels in ESCC tumor tissues
(Fig. 1C).
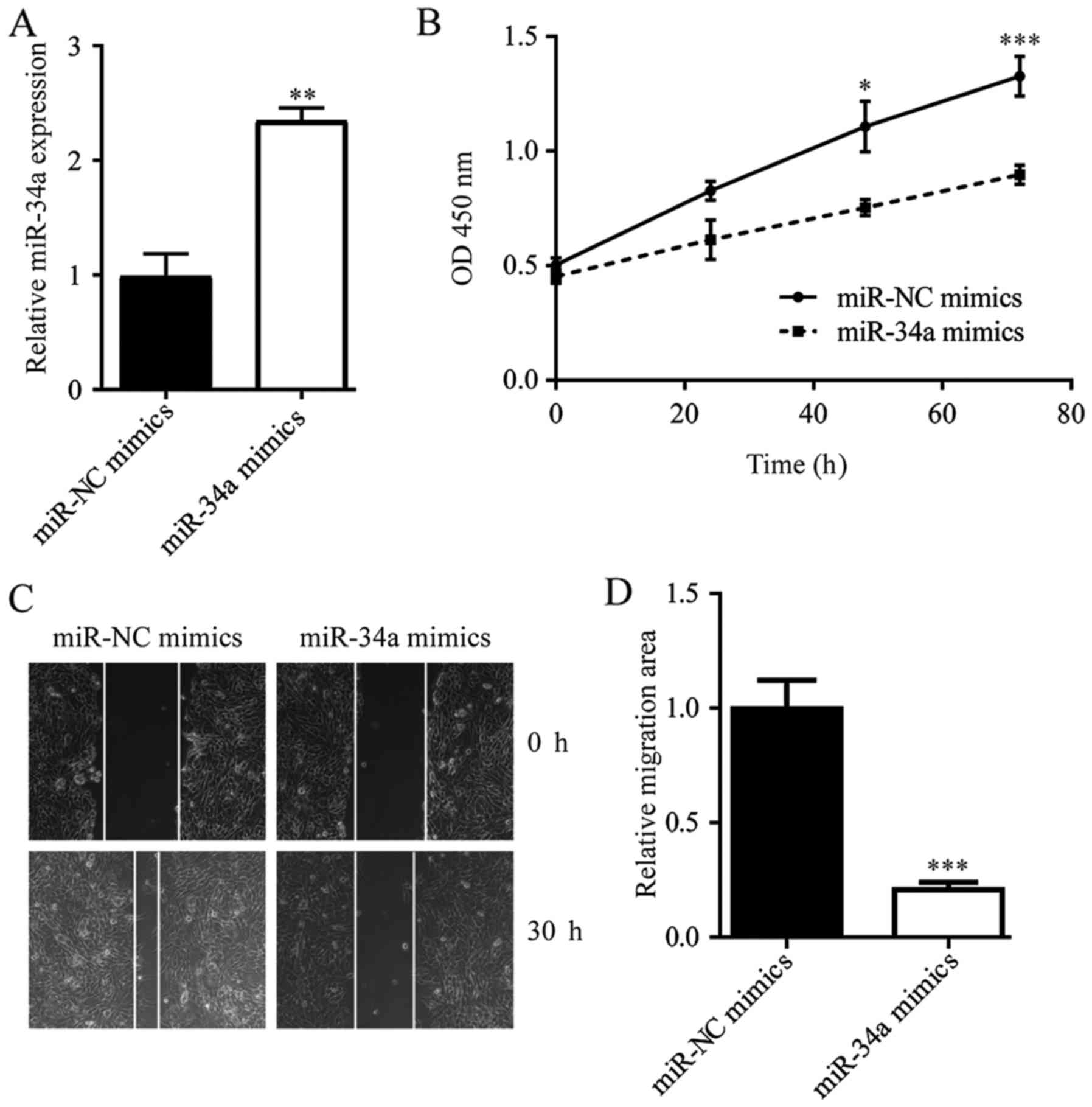
miR-34a inhibits cell growth and cell
migration in ESCC cells
To identify the function of miR-34a in ESCC, we
overexpressed miR-34a in ESCC TE-1 cells by transfection of miR-34a
mimics (Fig. 2A). Overexpression of
miR-34a induced a decrease in the cell proliferation rate (Fig. 2B). In addition, in a wound healing
assay, miR-34a overexpression reduced the wound closure areas of
TE-1 cells, indicating that the migration ability of TE-1 cells was
inhibited upon miR-34a elevation (Fig. 2C
and D). These data suggested that miR-34a could inhibit ESCC
progression via the inhibition of cell growth and the cell
migration ability of cancer cells.
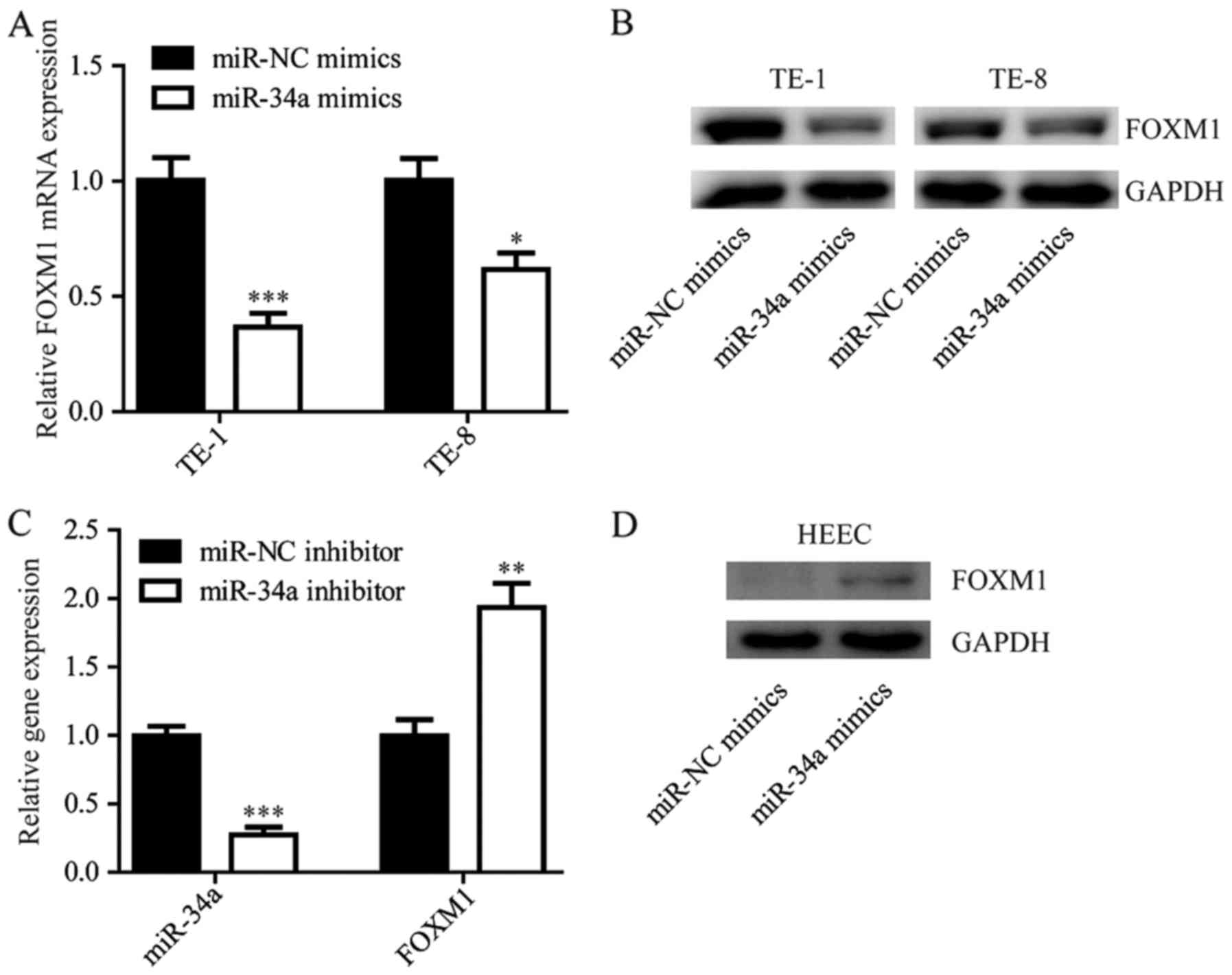
miR-34a negatively regulates FOXM1
expression in ESCC cells
The negative correlation between miR-34a and FOXM1
suggested that there might be a regulatory relationship between
miR-34a and FOXM1. Notably, we found that the overexpression of
miR-34a reduced FOXM1 expression at both the mRNA and protein level
in the cell lines tested (TE-1 and TE-8; Fig. 3A and B). In the immortal esophageal
epithelium cell line HEEC, inhibition of miR-34a by transfection of
the miR-34a inhibitor elevated FOXM1 expression (Fig. 3C and D).
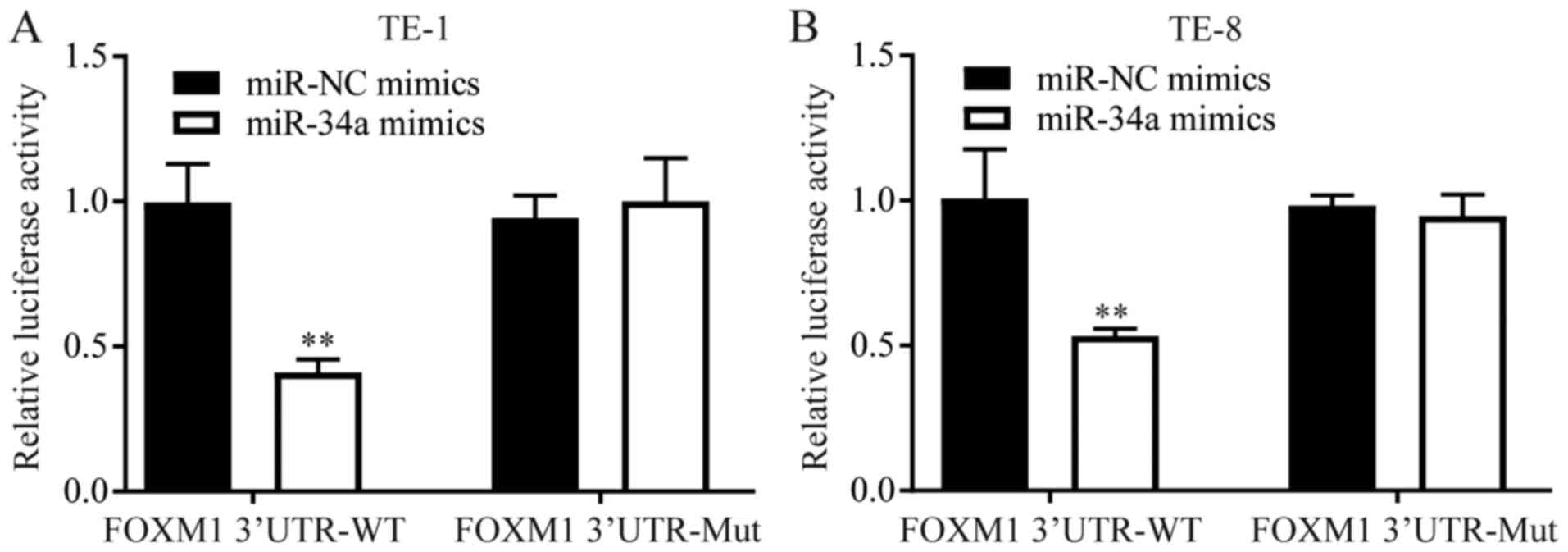
miR-34a directly represses FOXM1
expression in ESCC
FOXM1 was reported to be a target gene of miR-34a in
hepatocellular carcinoma (13). The
binding sites between miR-34a and FOXM1 were in consistent with
that of the hepatocellular carcinoma (13). A dual luciferase reporter assay was
used to further explore the regulatory relationship between miR-34a
and FOXM1 in ESCC cells. In both TE-1 and TE-8 cells,
overexpression of miR-34a decreased the luciferase activity of
cells transfected with FOXM1 3′UTR-WT, but not 3′UTR-Mut (Fig. 4).
miR-34a suppresses FOXM1 target gene
expression in ESCC cells
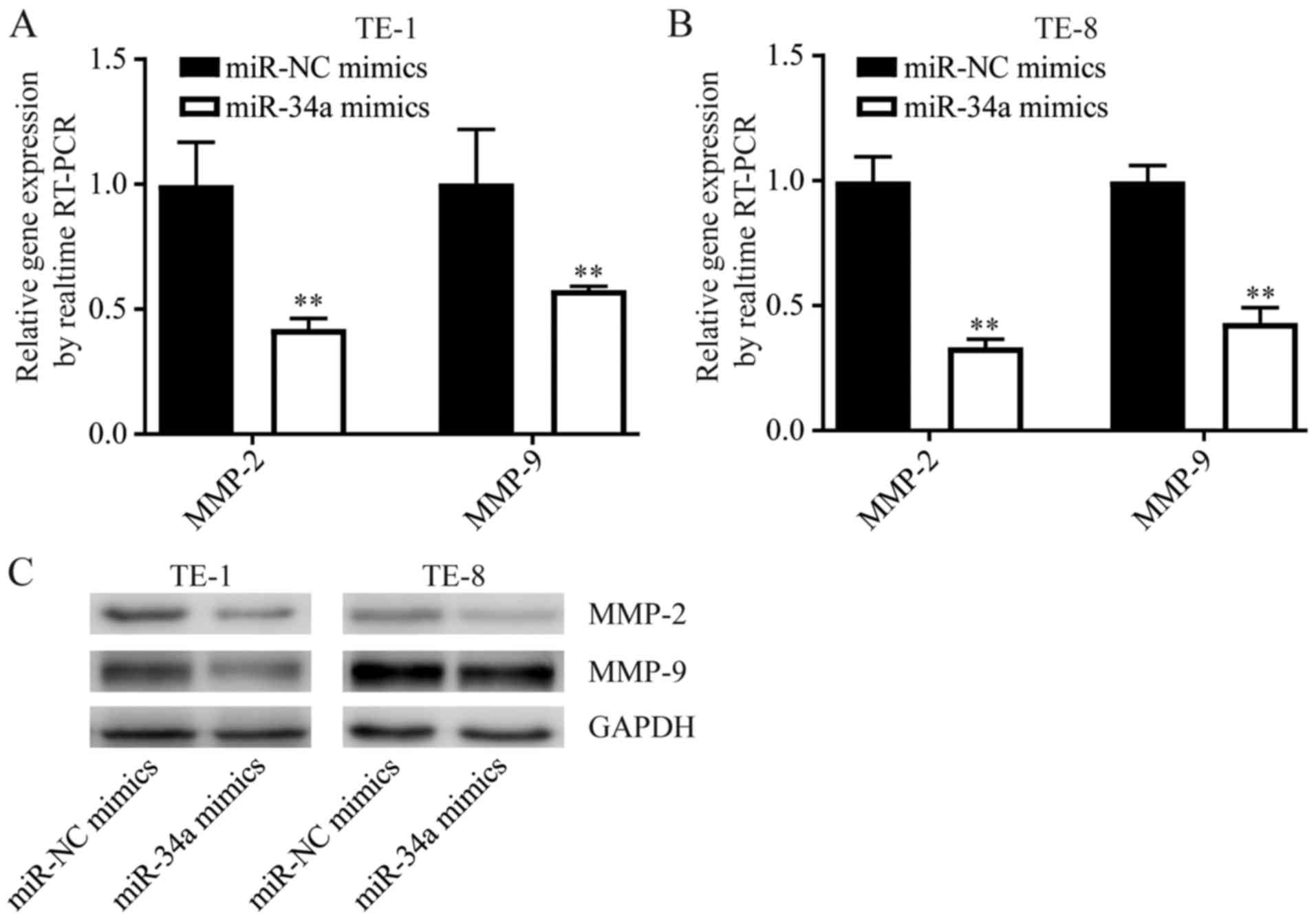
FOXM1 promoted cancer progression via elevating the
expression of MMP-2 and MMP-9 (14).
Consistently, overexpression of miR-34a downregulated MMP-2 and
MMP-9, two target genes of FOXM1 involved in cell migration
regulation, in both TE-1 and TE-8 cells (Fig. 5). These data suggested that miR-34a
functioned as a tumor suppressor through tight control of FOXM1 and
its target gene expression.
Discussion
EC incidence ranks fifth among cancers worldwide,
and EC is the fourth leading cause of cancer-related mortality in
China (15). About 70% of all EC
occurs in China and ESCC accounts for more than 90% of EC cases
(16). Despite the development in
surgical techniques, chemotherapy, radiation and perioperative
management, the five-year survival rate of ESCC is as low as only
10% (17). Thus, it is essential to
develop new therapeutic approaches to improve patient outcomes.
Increasing evidence has shown that alterations in
the expression of miRNAs are associated with the development,
prognosis and survival rate of EC (18). Studies have reported the lack of
expression of miR-34a in ESCC and colon cancer (19,20).
Consistent with those reports, we demonstrated the down-regulation
of miR-34a in ESCC patients compared with paired cancer-adjacent
normal tissues.
Our in vitro experiment showed that miR-34a
overexpression inhibited ESCC cell proliferation and migration,
indicating the tumor suppressor role of miR-34a in ESCC, which is
consistent with previous studies in ESCC (21) and breast cancer (22).
MMP family proteolytic enzymes are essential for the
remodeling of the extracellular matrix, whose degradation is a
prerequisite for the invasion and metastasis of cancer (23). In the present study, the protein
expression levels of MMP2 and MMP9 were decreased after miR-34a
mimics treatment compared with NC treatment.
miR-34a acts as an important tumor suppressor in
various cancers by targeting and downregulating CDK4/6, cyclin D1,
E2F3, MYCN, SIRT1 and Bcl2 (11).
FOXM1, a transcription factor of the forkhead box family, is
involved in the regulation of cell proliferation (24). Aberrant FOXM1 plays an important role
in angiogenesis, cell cycle acceleration and metastasis (25–27). FOXM1
promoted cancer progression via elevating the expression of MMP-2
and MMP-9 (14). Furthermore, FOXM1
is overexpressed in many human solid cancers, including breast
cancer and colon cancer (28,29).
Although the effects of miR-34a (30,31) as
well as FOXM1 (32,33) have already been investigated in ESCC,
and miR-34a could regulate the progression of hepatocellular
carcinoma via targeting FOXM1 (13),
it has not been investigated whether miR-34a could regulate the
progression of ESCC via targeting FOXM1. Consistent with these
studies, we demonstrated the upregulation of FOXM1 in ESCC patients
compared with paired cancer-adjacent normal tissues. Importantly,
the FOXM1 level was negatively correlated with the miR-34a level.
We also found that miR-34a interacted with a putative binding site
in the FOXM1 3′UTR and downregulated the expression of FOXM1.
There are further molecular mechanisms that could be
involved, for instance, we only interpret the down regulation of
MMP2 and MMP9 as a consequence of the miR-34a-mediated down
regulation of FOXM1, which will be further explored to show that
the observed effect is actually mediated by FOXM1.
These results suggest that miR-34a is possibly a
therapeutic target for ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported with Funding of
Hubei Province, China (grant no: WJ2015MB22).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX was involved in the study conception and design.
HZ, LY, XX, ML, RG, DL, QH and YL were involved in acquisition of
data. HZ, GD and YX were involved in the analysis and
interpretation of data. HZ and YX prepared and revised the
manuscript.
Ethics approval and consent to
participate
The present study was performed under the
supervision of the Ethics Committee of Chibi Pu Spinning Hospital.
Written consents were provided by all patients.
Patient consent for publication
All patients consented the publication of data in
this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hongo M, Nagasaki Y and Shoji T:
Epidemiology of esophageal cancer: Orient to occident. Effects of
chronology, geography and ethnicity. J Gastroenterol Hepatol.
24:729–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou SL and Wang LD: Circulating
microRNAs: Novel biomarkers for esophageal cancer. World J
Gastroenterol. 16:2348–2354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
David S and Meltzer SJ: MicroRNA
involvement in esophageal carcinogenesis. Curr Opin Pharmacol.
11:612–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown LM, Hoover R, Silverman D, Baris D,
Hayes R, Swanson GM, Schoenberg J, Greenberg R, Liff J, Schwartz A,
et al: Excess incidence of squamous cell esophageal cancer among US
Black men: Role of social class and other risk factors. Am J
Epidemiol. 153:114–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C, Wang C, Chen X, Yang C, Li K,
Wang J, Dai J, Hu Z, Zhou X, Chen L, et al: Expression profile of
microRNAs in serum: A fingerprint for esophageal squamous cell
carcinoma. Clin Chem. 56:1871–1879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jankowski JA and Odze RD: Biomarkers in
gastroenterology: Between hope and hype comes histopathology. Am J
Gastroenterol. 104:1093–1096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patnaik SK, Mallick R and Yendamuri S:
MicroRNAs and esophageal cancer. J Gastrointestinal Oncol. 1:55–63.
2010.
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang J, Zhang SY, Gao YM, Liu YF, Liu YB,
Zhao ZG and Yang K: MicroRNAs as oncogenes or tumour suppressors in
oesophageal cancer: Potential biomarkers and therapeutic targets.
Cell Prolif. 47:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei JS, Song YK, Durinck S, Chen QR, Cheuk
AT, Tsang P, Zhang Q, Thiele CJ, Slack A, Shohet J and Khan J: The
MYCN oncogene is a direct target of miR-34a. Oncogene.
27:5204–5213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Chen W, Miao R, Zhou Y, Wang Z,
Zhang L, Wan Y, Dong Y, Qu K and Liu C: miR-34a induces cellular
senescence via modulation of telomerase activity in human
hepatocellular carcinoma by targeting FoxM1/c-Myc pathway.
Oncotarget. 6:3988–4004. 2015.PubMed/NCBI
|
|
14
|
Chen H, Zou Y, Yang H, Wang J and Pan H:
Downregulation of FoxM1 inhibits proliferation, invasion and
angiogenesis of HeLa cells in vitro and in vivo. Int J Oncol.
45:2355–2364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rice TW, Rusch VW, Apperson-Hansen C,
Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE,
et al: Worldwide esophageal cancer collaboration. Dis Esophagus.
22:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogawa R, Ishiguro H, Kuwabara Y, Kimura M,
Mitsui A, Katada T, Harata K, Tanaka T and Fujii Y: Expression
profiling of micro-RNAs in human esophageal squamous cell carcinoma
using RTPCR. Med Mol Morphol. 42:102–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Xu XY, Chen QS and Huang C:
Clinical significance of microRNA-34a in esophageal squamous cell
carcinoma. Genet Mol Res. 14:17684–17691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi H, Zhou S, Liu J, Zhu J, Xue J, Gu L
and Chen Y: miR-34a inhibits the in vitro cell proliferation and
migration in human esophageal cancer. Pathol Res Pract.
212:444–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao X, Huang X, Ye F, Chen B, Song C, Wen
J, Zhang Z, Zheng G, Tang H and Xie X: The miR-34a-LDHA axis
regulates glucose metabolism and tumor growth in breast cancer. Sci
Rep. 6:217352016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cathcart J, Pulkoski-Gross A and Cao J:
Targeting matrix metalloproteinases in cancer: Bringing new life to
old ideas. Genes Dis. 2:26–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye H, Kelly TF, Samadani U, Lim L, Rubio
S, Overdier DG, Roebuck KA and Costa RH: Hepatocyte nuclear factor
3/fork head homolog 11 is expressed in proliferating epithelial and
mesenchymal cells of embryonic and adult tissues. Mol Cell Biol.
17:1626–1641. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D,
Huang S, Tan D and Xie K: Critical role and regulation of
transcription factor FoxM1 in human gastric cancer angiogenesis and
progression. Cancer Res. 69:3501–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monteiro LJ, Khongkow P, Kongsema M,
Morris JR, Man C, Weekes D, Koo CY, Gomes AR, Pinto PH, Varghese V,
et al: The Forkhead Box M1 protein regulates BRIP1 expression and
DNA damage repair in epirubicin treatment. Oncogene. 32:4634–4645.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schimmel J, Eifler K, Sigurðsson JO,
Cuijpers SA, Hendriks IA, Verlaan-de Vries M, Kelstrup CD,
Francavilla C, Medema RH, Olsen JV and Vertegaal AC: Uncovering
SUMOylation dynamics during cell-cycle progression reveals FoxM1 as
a key mitotic SUMO target protein. Mol Cell. 53:1053–1066. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Madureira PA, Varshochi R, Constantinidou
D, Francis RE, Coombes RC, Yao KM and Lam EW: The Forkhead box M1
protein regulates the transcription of the estrogen receptor alpha
in breast cancer cells. J Biol Chem. 281:25167–25176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li D, Peng Z, Tang H, Wei P, Kong X, Yan
D, Huang F, Li Q, Le X, Li Q and Xie K: KLF4-mediated negative
regulation of IFITM3 expression plays a critical role in colon
cancer pathogenesis. Clin Cancer Res. 17:3558–3568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuo J, Zhu K, Wang Y and Yu Z:
MicroRNA-34a suppresses invasion and metastatic in esophageal
squamous cell carcinoma by regulating CD44. Mol Cell Biochem.
443:139–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie J, Ge X, Geng Y, Cao H, Zhu W, Jiao Y,
Wu J, Zhou J and Cao J: miR-34a inhibits the migration and invasion
of esophageal squamous cell carcinoma by targeting Yin Yang-1.
Oncol Rep. 34:311–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song L, Wang X and Feng Z: Overexpression
of FOXM1 as a target for malignant progression of esophageal
squamous cell carcinoma. Oncol Lett. 15:5910–5914. 2018.PubMed/NCBI
|
|
33
|
Hui MK, Chan KW, Luk JM, Lee NP, Chung Y,
Cheung LC, Srivastava G, Tsao SW, Tang JC and Law S: Cytoplasmic
Forkhead box M1 (FoxM1) in esophageal squamous cell carcinoma
significantly correlates with pathological disease stage. World J
Surg. 36:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|