Introduction
Gastric cancer is prevalent worldwide, with ~800,000
gastric cancer-associated mortalities occurring each year, making
it the second most common cause of cancer-associated mortality
(1). With the recent advances in
medical technologies, gastric cancer can often be removed with
minimally invasive surgical techniques if identified early
(2). Endoscopic submucosal dissection
is the least invasive and, therefore, the most widely used of these
surgical procedures (2). It is
important for all gastric cancer tissue to be removed by the
endoscopic submucosal dissection, since residual cancerous tissue
can lead to recurrence (3). Resected
cancerous tissues are pathologically evaluated to determine whether
all cancerous areas have been removed; however, pathological
assessment is rarely straightforward. Interpretations of stained
cancerous tissues are, however, not consistent between
pathologists, meaning that different conclusions may be drawn by
different pathologists (4).
Cancer markers could inform pathological evaluations
of cancer. An ideal marker would be one that is identifiable in
formalin-fixed paraffin-embedded (FFPE) tissue samples, which a
high proportion of medical institutions prefer as these tissues
store well and are easy to handle (5).
The first objective of the present study was to
quantitatively determine the levels of angiopoietin-like protein 2
(ANGPTL2) in cancerous and noncancerous areas of FFPE tissues to
evaluate whether ANGPTL2 is a marker relevant to pathological
diagnoses of gastric cancer.
ANGPTL2, a member of the ANGPTL family, contributes
to the onset and progression of chronic inflammation and to the
associated diseases caused by this (6–10). ANGPTL2
also regulates angiogenesis in the body (11). Endo et al (9) considered ANGPTL2 to be a potential
biomarker for diagnosing lung and breast cancer in humans.
Our previous studies showed ANGPTL2 to be widely
expressed in gastric cancer cell lines and patients with gastric or
colon cancer (12,13). These findings highlight the potential
of ANGPTL2 as a biomarker for identifying gastric and colon cancer
in clinical settings.
In the present study, mRNA levels of ANGPTL2
were determined in FFPE tumor tissues collected from patients with
mucosal (M), submucosal (SM), tunica muscularis propria (MP),
serosa-exposed (SE) and subserosal (SS) gastric cancer. A second
objective was to evaluate whether ANGPTL2 mRNA is useful as
a marker of the extent of vascular invasion of gastric cancer,
which portends hematogenous metastasis (14).
Materials and methods
Patients and tissue samples
Serum samples were obtained from 15 patients who
attended the clinic between May 2013 and November 2014 at the
Nanpuh Hospital (Kagoshima, Japan). Patient characteristics,
including sex, age, body mass index (BMI), serum carcinoembryonic
antigen (CEA) levels and serum carbohydrate antigen 19-9 (CA19-9)
levels, are summarized in Table I.
Serum concentrations of CEA and CA19-9 were determined using an
electro-chemiluminescence immunoassay using the LUMIPULSE
G1200® (Fujirebio Diagnostics, Inc., Tokyo, Japan)
according to the manufacturer's protocol.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Variable | Mucosal (n=3) | Submucosal (n=3) | MP (n=4) | SE/SS (n=5) | Total (n=15) |
|---|
| Sex |
|
|
|
|
|
| Male | 2 | 3 | 3 | 4 | 12 |
|
Female | 1 | 0 | 1 | 1 | 3 |
| Age, years |
|
|
|
|
|
| Mean ±
SD | 66.0±7.9 | 80.0±9.8 | 56.8±15.4 | 65.0±17.6 | 66.0±15.1 |
|
Range | 60–75 |
69–88 | 43–75 | 36–78 | 36–88 |
| BMI,
kg/m2 |
|
|
|
|
|
| Mean ±
SD | 19.8±2.6 | 20.2±0.7 | 22.8±2.1 | 21.4±1.4 | 21.2±2.0 |
|
Range |
17.8–22.7 | 19.4–20.7 |
19.9–24.5 |
19.3–23.3 |
17.8–24.5 |
| CEA level,
ng/ml |
|
|
|
|
|
| Mean ±
SD | 1.4±0.1 | 4.6±4.2 | 6.4±6.2 | 28.7±58.0 | 12.5±33.4 |
|
Range | 1.3–1.4 | 2.0–9.4 |
2.0–15.5 |
0.6–132.4 |
0.6–132.4 |
| CA19-9 level,
U/ml |
|
|
|
|
|
| Mean ±
SD | 14.3±4.1 | 13.0±8.3 | 18.0±10.7 | 99.4±209.2 | 43.4±119.3 |
|
Range |
9.6–16.9 |
6.5–22.4 |
6.9–30.3 |
0.1–473.6 |
0.1–473.6 |
Tissues removed from the patients were immersed in
10%-formaldehyde neutral buffer solution. FFPE tissues were
prepared using the Tissue-Tek VIP6® (Sakura Finetek
Japan Co., Ltd., Nagano, Japan).
Table II shows the
sex, diagnosis, levels of ANGPTL2 mRNA, degree of
differentiation, tumor invasion depth, lymph node metastasis,
distant metastasis, tumor stage and degrees of lympho-vascular and
vascular invasion for each patient. Lympho-vascular invasion was
classified into four grades according to Japanese Classification of
Gastric Carcinoma (15): ly0, no
lymphatic invasion; ly1, minimal lymphatic invasion; ly2, moderate
lymphatic invasion; and ly3, extensive lymphatic invasion. Vascular
invasion was also classified into four grades according to Japanese
Classification of Gastric Carcinoma (15): v0, no venous invasion; v1, minimal
venous invasion; v2, moderate venous invasion; and v3, extensive
venous invasion. Of the 15 patients with gastric cancer, 9 were
diagnosed with adenocarcinoma, 5 with tubular adenocarcinoma and 1
with signet-ring cell carcinoma. The patient group included 3
patients diagnosed with M, 3 patients with SM, 4 patients with MP
and 5 patients with SE or SS cancer.
 | Table II.Level of ANGPTL2 mRNA, degree
of differentiation, tumor invasion depth, lymph node metastasis,
distant metastasis, tumor stage, and degrees of lympho-vascular
invasion and of vascular invasion. |
Table II.
Level of ANGPTL2 mRNA, degree
of differentiation, tumor invasion depth, lymph node metastasis,
distant metastasis, tumor stage, and degrees of lympho-vascular
invasion and of vascular invasion.
| Patient no. | Sex | Diagnosis | ANGPTL2
mRNAa | Degree of
differentiation | Tumor invasion
depth | Lymph node
metastasis | Distant
metastasis | Stage | Lympho-vascular
invasion | Vascular
invasion |
|---|
| 1 | Male | Tubular
adenocarcinoma | 1.11 | High | Mucosal | N0 | M0 | IA | 0 | 0 |
| 2 | Male | Adenocarcinoma | 0.99 | Poor | Mucosal | N0 | M0 | IA | 0 | 0 |
| 3 | Female | Signet-ring cell
carcinoma | 1.81 | Poor | Mucosal | N0 | M0 | IA | 0 | 0 |
| 4 | Male | Tubular
adenocarcinoma | 2.52 | High | Submucosal | N0 | M0 | IA | 0 | 1 |
| 5 | Male | Tubular
adenocarcinoma | 0.42 | Moderate | Submucosal | N0 | M0 | IA | 3+ | 0 |
| 6 | Male | Adenocarcinoma | 1.27 | Poor | Submucosal | N2 | M0 | IIA | 1 | 0 |
| 7 | Male | Adenocarcinoma | 1.46 | High | MP | N1 | M1 | IV | 1 | 0 |
| 8 | Male | Tubular
adenocarcinoma | 2.13 | Moderate | MP | N1 | M0 | IIA | 2 | 2 |
| 9 | Male | Adenocarcinoma | 0.69 | Poor | MP | N1 | M0 | IIA | 1 | 0 |
| 10 | Female | Adenocarcinoma | 1.02 | Poor | MP | N0 | M0 | IB | 0 | 0 |
| 11 | Female | Adenocarcinoma | 1.22 | Poor | Subserosal | N0 | M0 | IIA | 0 | 0 |
| 12 | Male | Tubular
adenocarcinoma | 1.64 | High | SE | N2 | M0 | IIIB | 2+ | 2+ |
| 13 | Male | Adenocarcinoma | 1.97 | Poor | SE | N1 | M0 | IIIA | 1 | 0 |
| 14 | Male | Adenocarcinoma | 2.81 | Poor | SE | N1 | M0 | IIIA | 1 | 2 |
| 15 | Male | Adenocarcinoma | 1.53 | Poor | SE | N3a | M0 | IIIC | 3 | 2 |
Among the patient group, 5 patients were diagnosed
with clinical stage IA, 1 with stage IB, 4 with stage IIA, 2 with
stage IIIA, 1 with stage IIIB, 1 with stage IIIC and 1 with stage
IV. Cancer staging was based on routine histopathological analysis
and clinical assessment, according to the Tumor Node Metastasis
(TNM) classification (16). Tumors
were classified according to the recommendations of the
International Union Against Cancer/TNM system (17). The characteristics of the subjects are
summarized in Table III.
 | Table III.Level of ANGPTL2 mRNA, degree of
differentiation, lymph node metastasis, distant metastasis, tumor
stage, and degrees of lympho-vascular invasion and of vascular
invasion by degree of tumor invasion depth. |
Table III.
Level of ANGPTL2 mRNA, degree of
differentiation, lymph node metastasis, distant metastasis, tumor
stage, and degrees of lympho-vascular invasion and of vascular
invasion by degree of tumor invasion depth.
| Parameters | Mucosal (n=3) | Submucosal
(n=3) | MP (n=4) | SE/SS (n=5) | Total (n=15) |
|---|
| ANGPTL2 mRNA
levela | 1.31±0.44 | 1.41±1.06 | 1.33±0.62 | 1.83±0.61 | 1.51±0.66 |
| Degree of
differentiation, n |
|
|
|
|
|
|
Poor | 2 | 1 | 2 | 4 | 9 |
|
Moderate | 0 | 1 | 1 | 0 | 2 |
|
High | 1 | 1 | 1 | 1 | 4 |
| Lymph node
metastasis, n |
|
|
|
|
|
| N0 | 3 | 2 | 1 | 1 | 7 |
| N1 | 0 | 0 | 3 | 2 | 5 |
| N2 | 0 | 1 | 0 | 1 | 2 |
|
N3a | 0 | 0 | 0 | 1 | 1 |
| Distant metastasis,
n |
|
|
|
|
|
| M0 | 3 | 3 | 3 | 5 | 14 |
| M1 | 0 | 0 | 1 | 0 | 1 |
| Tumor stage, n |
|
|
|
|
|
| IA | 3 | 2 | 0 | 0 | 5 |
| IB | 0 | 0 | 1 | 0 | 1 |
|
IIA | 0 | 1 | 2 | 1 | 4 |
|
IIIA | 0 | 0 | 0 | 2 | 2 |
|
IIIB | 0 | 0 | 0 | 1 | 1 |
|
IIIC | 0 | 0 | 0 | 1 | 1 |
| IV | 0 | 0 | 1 | 0 | 1 |
| Lympho-vascular
invasion, n |
|
|
|
|
|
| 0 | 3 | 1 | 1 | 1 | 6 |
| 1 | 0 | 1 | 2 | 2 | 5 |
|
2/2+ | 0 | 0 | 1 | 1 | 2 |
|
3/3+ | 0 | 1 | 0 | 1 | 2 |
| Vascular invasion,
n |
|
|
|
|
|
| 0 | 3 | 2 | 3 | 2 | 10 |
| 1 | 0 | 1 | 0 | 0 | 1 |
|
2/2+ | 0 | 0 | 1 | 3 | 4 |
Six consecutive slices, each 3-µm thick, were
prepared from the FFPE tissues. Hematoxylin and eosin (H&E)
staining was performed on one slice to identify the cancerous
areas. Tissue slices were stained with hematoxylin (for 10 min) and
eosin (1 min) at room temperature. A pathologist observing the
H&E-stained slices classified the cancerous and noncancerous
areas. For patients with M cancer, the cancerous and noncancerous
areas in the M layer of the remaining five slices were obtained
using microdissection. For patients with SM, MP or SE/SS cancer,
the cancerous and noncancerous areas in the SM layer were obtained
using microdissection. Tissues were also stained with Victoria blue
(Muto Pure Chemicals Co., Ltd., Tokyo, Japan) for 12 h at room
temperature to determine venous invasion.
Written informed consent was obtained from all
patients. The study design was approved by the Ethics Committee of
Nanpuh Hospital (Kagoshima Kyosaikai, Public Interest Inc.
Association, Japan). Clinical examinations were performed according
to the principles of the Declaration of Helsinki.
RNA extraction from FFPE tissue
Total RNA was extracted from FFPE tissue slices
using the PureLink FFPE Total RNA Isolation kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed with equipment of the Division
of Gene Research, Kagoshima University. The RT reaction was
performed using random primers (Toyobo Co., Ltd., Osaka, Japan) and
ReverTra Ace® (Toyobo Co., Ltd.), according to the
manufacturer's protocol, using 100 ng RNA. Cycle conditions were
95°C for 1 min, followed by 45 cycles of denaturation for 15 sec at
95°C, annealing and extension steps for 30 sec at 60°C each.
The amplification was performed using the
StepOnePlus™ Real-Time PCR System (Applied Biosciences; Thermo
Fisher Scientific, Inc.) using a SYBR Green Realtime PCR Master Mix
kit (Toyobo Co., Ltd.) according to the manufacturer's protocol.
The specific primers for human ANGPTL2 (purchased from
Thermo Fisher Scientific, Inc.) were 5′-GCCACCAAGTGTCAGCCTCA-3′
(forward) and 5′-TGGACAGTACCAAACATCCAACATC-3′ (reverse). Human
β-actin, used as a control, was amplified using the following
specific primers: Forward, 5′-AAGCCACCCCACTTCTCTCTAA-3′; and
reverse, 5′-AATGCTATCACCTCCCCTGTGT-3′ (Thermo Fisher Scientific,
Inc.). With the ANGPTL2 mRNA level in the noncancerous areas
taken to be 1.0, ANGPTL2 mRNA levels in the cancerous areas
were calculated with the 2−ΔΔCq method (18).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using SPSS version 23 (IBM SPSS, Armonk, NY,
USA). The correlations of the ANGPTL2 mRNA concentration
with the patient age, BMI and serum CEA and CA19-9 levels were
analyzed using Pearson's correlation analysis. The correlations of
the ANGPTL2 mRNA concentration with the degree of
differentiation, tumor invasion depth, lymph node metastasis,
distant metastasis, tumor stage, degree of lympho-vascular invasion
and degree of vascular invasion were analyzed using Spearman's rank
correlation analysis. A receiver operating characteristic (ROC)
curve was plotted to evaluate the ability of ANGPTL2 mRNA
level to predict vascular invasion. Youden's index method (19) was used to determine the optimal cutoff
for the ANGPTL2 mRNA level for assessing the presence of
vascular invasion in gastric cancer. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
To the best of our knowledge, this is the first
study to investigate whether ANGPTL2 in FFPE tissues is a useful
biomarker for diagnosing gastric cancer. To prepare FFPE tissues, a
tissue specimen removed from a patient was fixed in a formalin
solution to cross-link biological molecules, and then embedded in
paraffin. This procedure may denature mRNA and other biological
components. von Ahlfen et al (20), however, successfully extracted the
mRNA for telomere-binding protein from FFPE tissues (21), which indicated that ANGPTL2
mRNA may be extractable from FFPE tissues. In the present study,
the cancerous and noncancerous areas were distinguished from one
another by pathological diagnosis using H&E-stained
cross-sections.
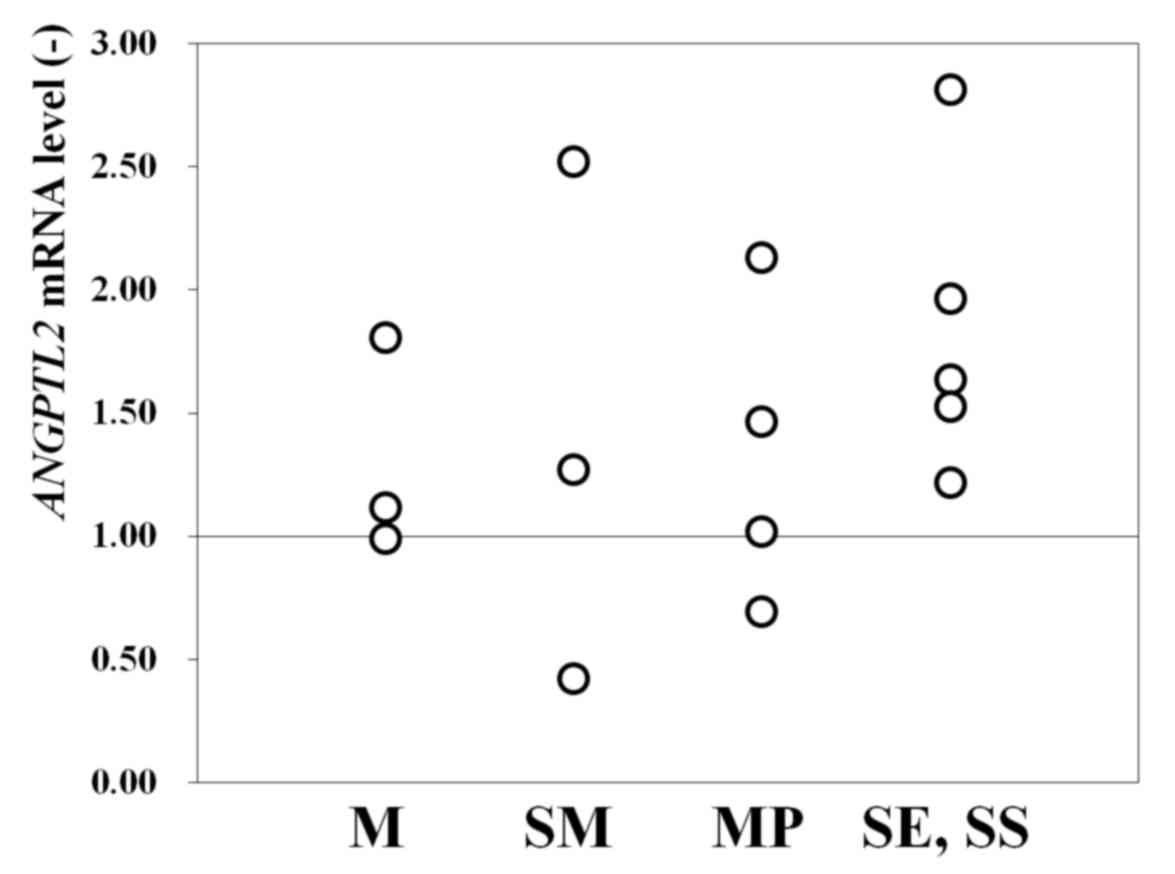
Of the 15 patients studied, 12 (80%) had a higher
ANGPTL2 mRNA level in cancerous areas compared with the
reference level (set as 1.0) in noncancerous areas (Fig. 1). In total, 2 of the 3 patients with M
cancer, 2 of the 3 patients with SM cancer, 3 of the 4 patients
with MP cancer and all 5 patients with SE/SS cancer had an
ANGPTL2 mRNA level >1.0 (Fig.
1). This finding indicated that ANGPTL2 mRNA is useful
as a biomarker for identifying cancerous areas in FFPE tissues, at
least for male patients (owing to the small female sample size).
Furthermore, the results indicated that the ANGPTL2 mRNA
level may have higher diagnostic precision in advanced cancer.
The association between ANGPTL2 mRNA
expression and BMI, as well as other factors, was also evaluated.
As shown in Table IV, ANGPTL2
mRNA levels in FFPE tissues were not correlated with age
(correlation coefficient, r=−0.31; P=0.26), BMI (r=0.09; P=0.75),
CEA level (r=0.04; P=0.90), CA19-9 level (r=0.03; P=0.91), degree
of tumor differentiation (r=0.21; P=0.46), depth of tumor invasion
(r=0.35; P=0.21), degree of lymph node metastasis (r=0.31; P=0.26),
degree of distant metastasis (r=0.00; P=1.00), tumor stage (r=0.33;
P=0.23) or degree of lympho-vascular invasion (r=0.07; P=0.80).
ANGPTL2 mRNA levels were, however, correlated with the
degree of vascular invasion (r=0.66; P=0.01).
 | Table IV.Correlations between
angiopoietin-like protein 2 mRNA concentration and various
parameters. |
Table IV.
Correlations between
angiopoietin-like protein 2 mRNA concentration and various
parameters.
| Parameter | Correlation
coefficient | P-value |
|---|
| Age, years | −0.31 | 0.26a |
| Body mass index,
kg/m2 | 0.09 | 0.75a |
| CEA level | 0.04 | 0.90a |
| CA19-9 level | 0.03 | 0.91a |
| Degree of
differentiation | 0.21 | 0.46b |
| Tumor invasion
depth | 0.35 | 0.21b |
| Lymph node
metastasis | 0.31 | 0.26b |
| Distant
metastasis | 0.00 | 1.00b |
| Stage | 0.33 | 0.23b |
| Lympho-vascular
invasion | 0.07 | 0.80b |
| Vascular
invasion | 0.66 | 0.01b |
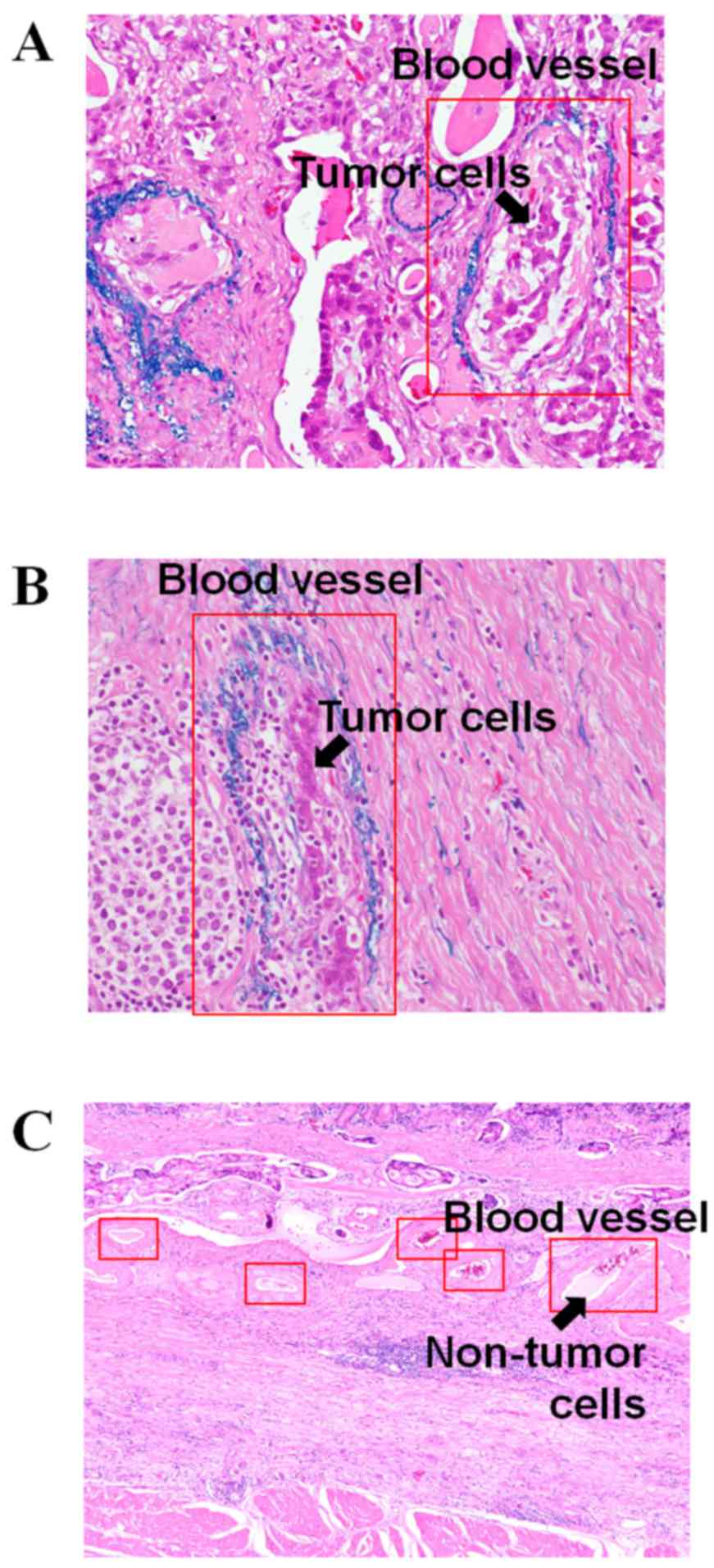
Micrographs of cancerous areas stained with Victoria
blue and H&E in patients with a high ANGPTL2 mRNA level
and a high degree of vascular invasion [patient no. 4 (mRNA level
2.52) and patient no. 14 (mRNA level 2.81)] are shown in Fig. 2. The arrows in Fig. 2A and B show the tumor cells in the
blood vessels. Fig. 2C shows the
micrograph of cancerous areas, stained with H&E, of patients
with a low ANGPTL2 mRNA level [patient no. 5 (mRNA level
0.42)]. The arrow in Fig. 2C
indicates no tumor cells in the blood vessels.
Since primary cancer with a high degree of vascular
invasion has often already metastasized (14,22,23),
accurate pathological diagnoses of vascular invasion could inform
assessments of metastatic status. Pathological diagnoses of
vascular invasion, however, are elusive. A biomarker of vascular
invasion would aid the detection of metastatic cancer. As shown in
Table IV, the ANGPTL2 mRNA
level was correlated with vascular invasion.
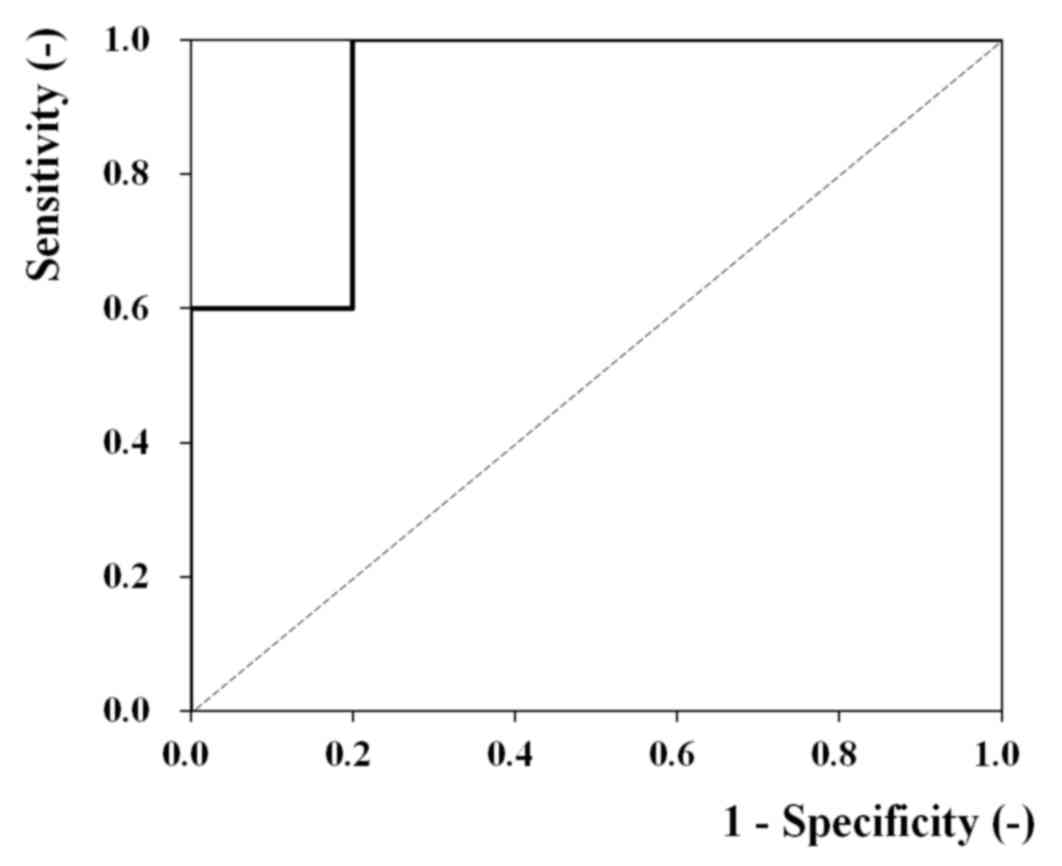
An ROC analysis was conducted to explore this
possibility. Fig. 3 contains the ROC
curve (n=15) for the analysis of ANGPTL2 mRNA levels and the
degree of vascular invasion. An area under the curve of 0.92 (95%
confidence interval, 0.78–1.00; P=0.01) indicated a high diagnostic
potential. The results of ROC analysis also indicated that
ANGPTL2 mRNA may be useful for assessing gastric cancer
metastasis.
Next, the optimal cutoff using by Youden's index
method for the ANGPTL2 mRNA level for assessing the presence
of vascular invasion in gastric cancer. The optimal cutoff was
determined to be a relative expression level of 1.50. All patients
with vascular invasion had a level ≥1.50, while only 20% lacking
vascular invasion had a level at or above this cutoff. Thus, the
cutoff of 1.50 produces a high true-positive rate and low
false-positive rate.
The present study demonstrated that ANGPTL2
mRNA in FFPE tissues is a potential biomarker for informing the
pathological diagnosis of gastric cancer. Since numerous medical
institutions retain FFPE tissues, this discovery may lead to a
widely usable diagnostic procedure for more accurately assessing
gastric cancer compared with the conventional pathological
diagnosis. The present study also showed that ANGPTL2 mRNA
may be predictive of vascular invasion, which is an indicator of
metastasis in gastric cancer. An increase in the number of samples
of cancer metastasis is necessary to clarify whether the
ANGPTL2 mRNA level is actually correlated with
metastasis.
The present study focused on ANGPTL2, and
comparisons with other tumor markers were not performed. Previous
studies have reported microRNAs, human epidermal growth factor
receptor 2 and proteomic profiling in FFPE as gastric cancer
biomarkers (24–26). Our future studies will measure and
compare other biomarkers.
Acknowledgements
The present study was supported in part by the
Division of Gastrointestinal Surgery, the Division of Diagnostic
Pathology, and the Division of Clinical Laboratory of Nanpuh
Hospital.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY, TT, HN and TY conceived and designed the
experiments. Data collection and experiments were performed by ST,
EH, AT and ET. TY analyzed the data and all authors contributed to
the writing of the manuscript.
Ethics approval and consent to
participate
The study design was approved by the Ethics
Committee of Nanpuh Hospital (Kagoshima Kyosaikai, Public Interest
Inc. Association, Japan). Written informed consent was obtained
from all patients.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loei H, Tan HT, Lim TK, Lim KH, So JB,
Yeoh KG and Chung MC: Mining the gastric cancer secretome:
Identification of GRN as a potential diagnostic marker for early
gastric cancer. J Proteome Res. 11:1759–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ono H, Kondo H, Gotoda T, Shirao K,
Yamaguchi H, Saito D, Hosokawa K, Shimoda T and Yoshida S:
Endoscopic mucosal resection for treatment of early gastric cancer.
Gut. 48:225–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanabe S, Koizumi W, Mitomi H, Nakai H,
Murakami S, Nagaba S, Kida M, Oida M and Saigenji K: Clinical
outcome of endoscopic aspiration mucosectomy for early stage
gastric cancer. Gastrointest Endosc. 56:708–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukai K and Shimoda T: Proceedings of the
Xth international congress on histochemistry and cytochemistry.
Acta Histochemica Cytochemica. 29:92–93. 1996.
|
|
5
|
Kokkat TJ, Patel MS, McGarvey D, LiVolsi
VA and Baloch ZW: Archived formalin-fixed paraffin-embedded (FFPE)
blocks: A valuable underexploited resource for extraction of DNA,
RNA, and protein. Biopreserv Biobank. 11:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabata M, Kadomatsu T, Fukuhara S, Miyata
K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadomatsu T, Tabata M and Oike Y:
Angiopoietin-like proteins: Emerging targets for treatment of
obesity and related metabolic diseases. FEBS J. 278:559–564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aoi J, Endo M, Kadomatsu T, Miyata K,
Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K, et al:
Angiopoietin-like protein 2 is an important facilitator of
inflammatory carcinogenesis and metastasis. Cancer Res.
71:7502–7512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endo M, Nakano M, Kadomatsu T, Fukuhara S,
Kuroda H, Mikami S, Hato T, Aoi J, Horiguchi H, Miyata K, et al:
Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical
driver of metastasis. Cancer Res. 72:1784–1794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada T, Tsukano H, Endo M, Tabata M,
Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M,
et al: Synoviocyte-derived angiopoietin-like protein 2 contributes
to synovial chronic inflammation in rheumatoid arthritis. Am J
Pathol. 176:2309–2319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshinaga T, Shigemitsu T, Nishimata H,
Takei T and Yoshida M: Angiopoietin-like protein 2 is a potential
biomarker for gastric cancer. Mol Med Rep. 11:2653–2658. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshinaga T, Shigemitsu T, Nishimata H,
Kitazono M, Hori E, Tomiyoshi A, Takei T and Yoshida M:
Angiopoietin-like protein 2 as a potential biomarker for colorectal
cancer. Mol Clin Oncol. 3:1080–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanigawa N, Amaya H, Matsumura M,
Shimomatsuya T, Horiuchi T, Muraoka R and Iki M: Extent of tumor
vascularization correlates with prognosis and hematogenous
metastasis in gastric carcinomas. Cancer Res. 56:2671–2676.
1996.PubMed/NCBI
|
|
15
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma - 2nd English Edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rindi G, Klöppel G, Couvelard A, Komminoth
P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A,
et al: TNM staging of midgut and hindgut (neuro) endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
451:757–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jun KH, Lee JS, Kim JH, Kim JJ, Chin HM
and Park SM: The rationality of N3 classification in the 7th
edition of the international union against cancer TNM staging
system for gastric adenocarcinomas: A case-control study. Int J
Surg. 12:893–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
von Ahlfen S, Missel A, Bendrat K and
Schlumpberger M: Determinants of RNA quality from FFPE samples.
PLoS One. 2:e12612007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis F, Maughan NJ, Smith V, Hillan K and
Quirke P: Unlocking the archive-gene expression in
paraffin-embedded tissue. J Pathol. 195:66–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang YC, Nagasue N, Kohno H, Taniura H,
Uchida M, Yamanoi A, Kimoto T and Nakamura T: Clinicopathologic
features and long-term results of alpha-fetoprotein-producing
gastric cancer. Am J Gastroenterol. 85:1480–1485. 1990.PubMed/NCBI
|
|
23
|
Kono K, Amemiya H, Sekikawa T, Iizuka H,
Takahashi A, Fujii H and Matsumoto Y: Clinicopathologic features of
gastric cancers producing alpha-fetoprotein. Dig Surg. 19:359–365.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinugasa H, Nouso K, Tanaka T, Miyahara K,
Morimoto Y, Dohi C, Matsubara T, Okada H and Yamamoto K: Droplet
digital PCR measurement of HER2 in patients with gastric cancer. Br
J Cancer. 112:1652–1655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sousa JF, Ham AJ, Whitwell C, Nam KT, Lee
HJ, Yang HK, Kim WH, Zhang B, Li M, LaFleur B, et al: Proteomic
profiling of paraffin-embedded samples identifies
metaplasia-specific and early-stage gastric cancer biomarkers. Am J
Pathol. 181:1560–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|