Introduction
Glioblastoma is one of the most common primary brain
tumors in adults, and glioblastoma alone accounts for ~70% of
high-grade gliomas (1). Despite the
advances in chemotherapy, radiotherapy, surgical resection and most
recently immunotherapy, the prognosis of patients with glioblastoma
remains poor, with a median survival of 12–15 months (2–6).
Temozolomide (TMZ) is the most common drug in glioblastoma
chemotherapy and is used throughout the whole treatment of
glioblastoma (7). However, the
efficacy of TMZ is limited partly due to the high activity of DNA
repair in tumor cells, which reduces the effect of this alkylating
agent and leads to a resistant phenotype. Multiple theories have
been developed to explain the TMZ resistance in glioblastoma
(8,9),
including DNA repair mechanism (10),
overexpression of epidermal growth factor receptor and galectin-1
(11,12), malfunction of p53 (13), Murine double minute 2 (14), and phosphatase and tensin homolog
(15), as well as involvement of
miRNAs (16,17). The P53 pathway is inactivated in
almost 50% of human tumors (18).
Malfunction of P53 generally leads to a poorer prognosis for cancer
patients, and accumulating studies have demonstrated that
chemoresistance is associated with P53 inactivation (18–20). Since
TMZ functions by inserting alkyl groups into DNA to cause DNA
damage and inhibit cell division, mutation of P53 inhibits its
regulative role in DNA replication and repair, thus enhances the
resistance of glioblastoma cells to TMZ (21,22).
Recently, BACH1 has been found to promote TMZ resistance in
glioblastoma through antagonizing the function of p53 (23). Thus, it is critical to inspect P53
activity in glioblastoma cells with varying genetic background and
to adjust the therapeutic plan according to specific
conditions.
Ubiquitination is a critical regulatory event in
cancer, particularly ubiquitination and deubiquitination of the
proteins that affects p53 pathway activity. Deubiquitinating
enzymes mediate the removal of ubiquitin and are divided into four
subclasses based on their Ub-protease domains, including
ubiquitin-specific proteases (USPs), ubiquitin C-terminal
hydrolases, Otubain proteases and Machado-Joseph disease proteases
(24). USPs have been reported to
serve key roles in the progression of glioblastoma. For instance,
USP15 amplification confers poor prognosis in individuals with
glioblastoma through transforming growth factor-β (TGF-β)
signaling, while USP13 maintains glioblastoma stem cells by
antagonizing Myc ubiquitination (25). Furthermore, USP7 was identified as a
stabilizer of P53 by directly binding to P53 (26), and USP2 was found to facilitate the
p53-mediated intrinsic apoptotic pathway in glioblastoma (27).
USP4 is a negative regulator of P53 by stabilizing
ARF-BP1 and HDAC2, and has been found to be overexpressed in
several types of human cancer (28,29). In
breast cancer, USP4 crosslinks protein kinase B and TGF-β pathway
to promote cancer cell migration (30). USP4 has also been demonstrated to
control the potential of brain metastasis in patients with lung
adenocarcinoma (31). Recently,
increased USP4 levels were reported following intracerebral
hemorrhage in adult rats, and this enzyme participated in neuronal
apoptosis (32), indicating the
critical role of USP4 in neuronal cells and apoptosis. However, the
role of USP4 in glioblastoma is not currently clear, particularly
when TMZ treatment is involved.
In the present study, the aim was to elucidate the
role of USP4 in glioblastoma. The expression level of USP4 in
glioblastoma tissues and cell lines was examined, while the
chemoresistance of TMZ upon USP4 knockdown was tested. The study
also inspected the role of P53 in USP4-mediated TMZ resistance, and
the use of a specific inhibitor of P53 revealed the cancer
promoting role of USP4 via the regulation of P53 activity.
Materials and methods
Cell culture
Human glioblastoma U251 and U87 cells were obtained
from the American Type Culture Collection (Manassas, VA, USA).
Primary human astrocytes were purchased from Procell Life Science
& Technology Co., Ltd (Wuhan, China). Cells were maintained in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2. All TMZ treatments were performed with 100 µM TMZ
(T257; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 24 h, at
37°C. The groups were divided as follow: Scramble (cells
transfected with scramble siRNA), siRNA-1 (cells transfected with
siRNA-1 targeting USP4) and siRNA-2 (cells transfected with siRNA-2
targeting USP4). TMZ+ indicates cells treated with 100 µM TMZ while
PFT+ indicates cells treated with 10 µM PFT. PFT treatment were
performed as previously described (33), with 10 µM PFT (Tocris Bioscience,
Bristol, UK) for 24 h at 37°C. The cell lines were transfected with
50 nM scramble small interfering RNA (siRNA) or each of the two
siRNAs designed for USP4 [siRNA-1: 5′-GGCUCUGGAACAAAUACAU-3′ and
siRNA-2: 5′-GGUCGCAGAUGUGUAUAAU-3′]. All transfections were
performed using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 24 h of siRNA transfection, cells were treated with TMZ with
or without 10 µM PFT for 24 h at 37°C prior to further
analysis.
Human glioblastoma samples
Human glioblastoma tissue samples and their adjacent
controls (n=4) were obtained from patients who underwent surgical
resection at the Department of Neurology at Xijing Hospital of the
Fourth Military Medical University (Xi'an, China). These samples
were confirmed as glioblastoma by pathological diagnosis (34). The present study was approved by the
Ethics Committee of the Fourth Military Medical University. Written
informed consent was obtained from all the patients.
USP4 small interfering RNA (siRNA)
transfection in U251 and U87 cells
USP4 siRNA was designed and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China), according to the sequence
information of the USP4 gene (GenBank accession no. NM_001251877).
The two siRNA sequences used in the study were as follows:
(siRNA-1), 5′-GGCUCUGGAACAAAUACAU-3′; and siRNA-2,
5′-GGUCGCAGAUGUGUAUAAU-3′. A siRNA with a scrambled sequence
(5′-UUCUCCGAACGUGUCACGU-3′) was used as the negative control. U251
and U87 cells were seeded in 60-mm dishes at a density of
5×105 cells/dish and incubated overnight at 37°C in a
humidified atmosphere containing 5% CO2. Subsequently,
cells were transfected with siRNAs using Lipofectamine®
3000 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 24 h, cell lysates were collected
for use in subsequent experiments.
Western blotting. U251 and U87 cells were washed
with PBS twice and lysed in radioimmunoprecipitation assay lysis
buffer (Thermo Fisher Scientific, Inc.). Proteins were quantified
with a Pierce BCA Protein Assay kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 10 µg protein for each sample was
separated by 10% SDS-polyacrylamide gel electrophoresis and then
transferred onto a nitrocellulose membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked at room temperature
with 5% milk in Tris-buffered saline/Tween 20 (TBST; containing 20
mM Tris-HCl, 150 mM NaCl and 0.1% Tween 20, pH 7.5) for at least 1
h and then incubated with each primary antibody overnight at 4°C.
Primary antibodies against USP4 (ab38510; 1:500), p53 (ab131442;
1:1,000), poly(ADP-ribose) polymerase (PARP; ab32064; 1:1,000) were
used, which were purchased from Abcam (Cambridge, MA, USA). Equal
loading of samples was verified by immunoblotting for GAPDH
(ab128915; 1:10,000; Abcam). Subsequently, the membrane was washed
with TBST buffer for at least five times for 5 min each and then
incubated with goat anti-rabbit secondary antibody (sc-2004,
1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at room temperature. The protein bands were visualized using a
Pierce™ ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from U251 and U87 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to manufacturer's protocol. For each
sample, 500 ng total RNA was reverse-transcribed using a Promega
Reverse Transcription System (Promega Corporation, Madison, WI,
USA), and the resulting complementary DNA was diluted 40 times.
qPCR was then performed using a QuantiTect SYBR® Green
PCR kit (204141; Qiagen China Co., Ltd., Shanghai, China) in
96-well optical reaction plates and the ABI StepOne Plus Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The PCR reaction cycle was 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and an annealing/elongation step at
60°C for 30 sec. The following primer sequences were used: Human
GAPDH, 5′-GAAGATGGTGATGGGATTTC-3′ (forward) and
5′-GAAGGTGAAGGTCGGAGTC-3′ (reverse); human USP4,
5′-CCTGGGCTCTGTGGACTTG-3′ (forward) and
5′-TGTTGATTTCGGCTTCATACTC-3′ (reverse). USP4 transcription level
was normalized to GAPDH as reference gene. Relative expressions
values for are presented as fold-increase in relation to control.
The actual values were calculated using the 2−ΔΔCq
method (35).
MTT assay
U251/U87 cells transfected with control siRNA or
USP4 siRNA were treated with 100 µM TMZ for 24 h at 37°C.
TMZ-treated and untreated cells were plated at a density of
1×104 cells/well in a 48-well plate. Viable cells were
then stained with 0.25 mg/ml MTT [also known as
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] for 1
h. The media were then removed, and the formazan crystals produced
were dissolved by the addition of dimethyl sulfoxide. Cell
viability was determined according to the absorbance at 540 nm.
Flow cytometry
Wild-type U251/U87 cells and U251/U87 cells
transfected with control or USP4 siRNA were treated with 100 µM TMZ
or 10 µM PFT for 24 h. The TMZ-treated and untreated cells were
seeded into 6-well plates at a density of 1×106
cells/well for 24 h. Subsequently, the cells were collected and
stained with FITC Annexin V Apoptosis Detection Kit I (cat. no.
556547; BD Biosciences, San Jose, CA, USA) according to the
manufacturer's protocol, and then analyzed by flow cytometry
(FACSCalibur; BD Biosciences).
Immunohistochemical analysis
Tissues were fixed with 10% formalin for 2 h at room
temperature, embedded in paraffin and then sectioned into 5-mm
slides for USP4 expression analysis. The specimens were blocked for
1 h at room temperature with 10% sheep serum (Beyotime Institute of
Biotechnology, Haimen, China). The primary antibody was anti-USP4
rabbit polyclonal antibody (ab3850; Abcam; 1:100 dilution) at 4°C
overnight, and the secondary antibody was goat anti-rabbit
horseradish peroxidase-conjugated antibody (ab6721; Abcam; 1:1,000)
at room temperature for 1 h. The detailed IHC procedure was
described previously (36).
Statistical analysis
Data are presented as the mean ± standard error of
at least three separate experiments. To assess significant
differences between two groups, Student's t-test was performed.
Statistical calculations were performed using IBM SPSS software
(version 20.0; IBM Corp., Armonk, NY, USA). P-values of <0.05
were considered to denote differences that were statistically
significant.
Results
Increased USP4 expression in
glioblastoma tissues and cell lines
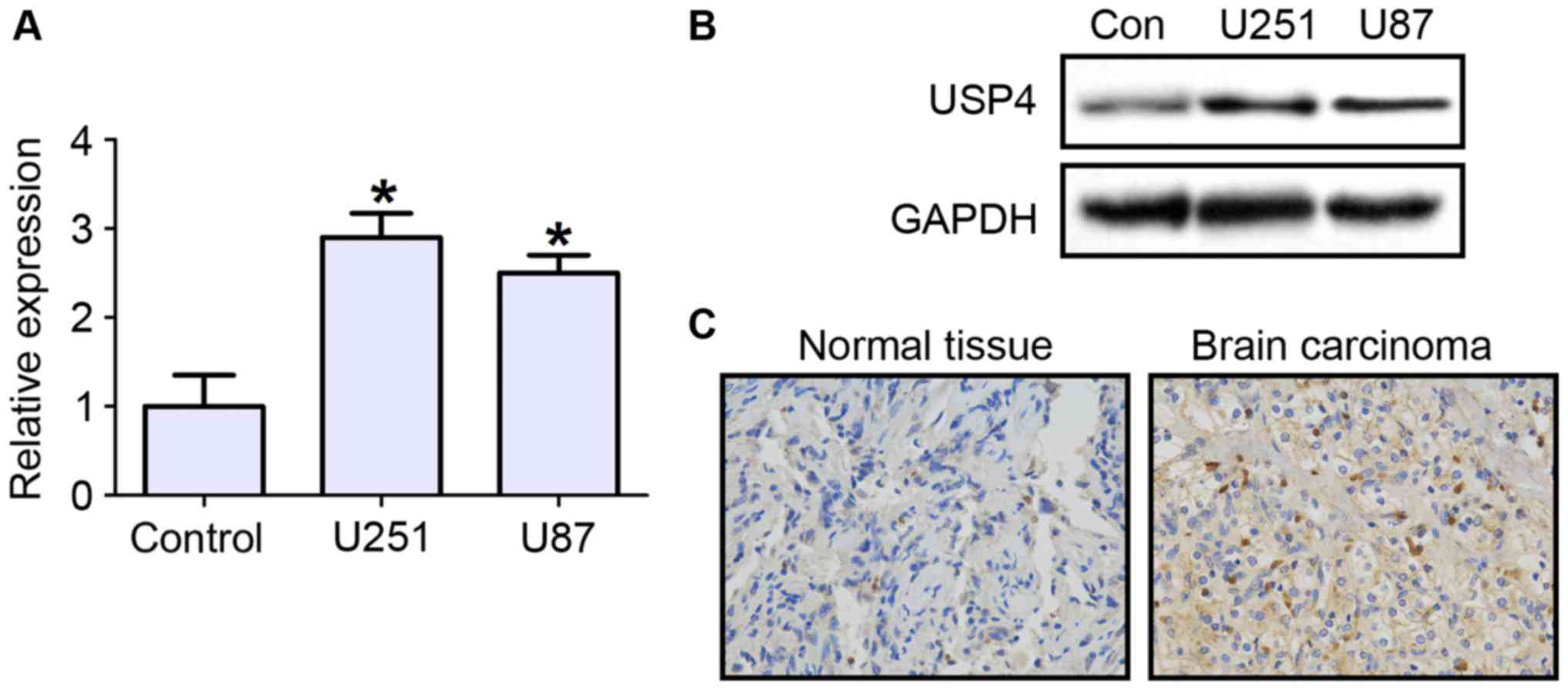
To investigate the role that USP4 plays in
glioblastoma, the mRNA and protein expression levels of USP4 were
first examined in the glioblastoma cell lines U251 and U87. The
RT-qPCR results demonstrated that UPS4 mRNA expression was
significantly increased in U251 and U87 cell lines by ~3 folds, as
compared with primary human astrocytes as negative control cells
(Fig. 1A). The protein level of USP4
was also evidently increased in the two cell lines (Fig. 1B). Next, the expression of USP4 in
glioblastoma tissues was tested by immunohistochemical staining.
The results revealed that USP4 was highly expressed in cancer
tissues compared with control tissues (Fig. 1C).
Downregulation of USP4 ameliorates
chemoresistance of U251 and U87 cells to TMZ
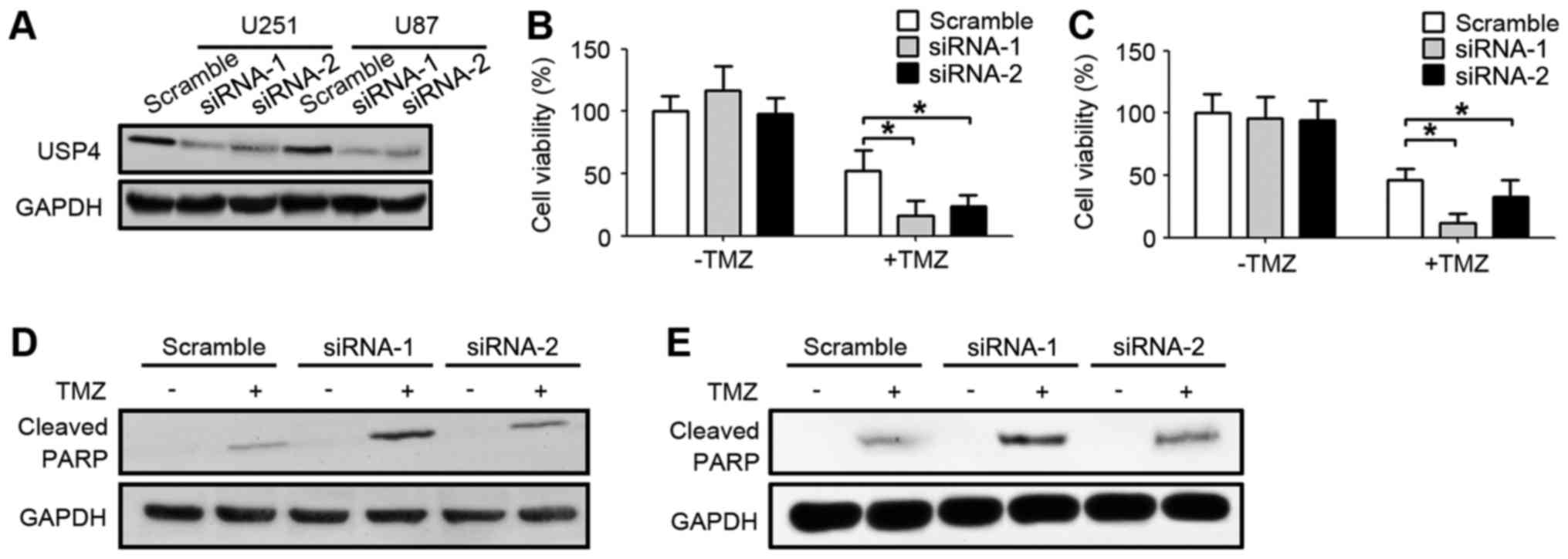
In order to further explore the function of the
elevated USP4 in glioblastoma, USP4 was knocked down by two
different siRNA sequences in U251 and U87 glioblastoma cell lines.
As shown in Fig. 2A, USP4 were
efficiently knocked down by the two siRNAs in U251 and U87 cell
lines. Subsequent to the UPS4 knockdown with these two siRNA
sequences, glioblastoma cells were treated with TMZ. Notably, USP4
knockdown alone did not affect the cell viability, which was
assessed by an MTT assay. However, when USP4 knockdown cells were
treated with TMZ, the cell viability was significantly decreased
compared with the scramble-transfected cells treated with TMZ,
suggesting that USP4 attenuated the anti-cancer function of TMZ in
the two glioblastoma cell lines (Fig. 2B
and C). Furthermore, the level of cleaved PARP was also
examined by western blotting in order to analyze the apoptotic
status. The results demonstrated that cleaved PARP levels were
elevated when cells were treated with TMZ. In addition, a marked
increase in cleaved PARP was observed in cells transfected with
USP4 siRNA, suggesting that USP4 inhibited the apoptosis induced by
TMZ (Fig. 2D and E).
USP4 negatively regulates P53
expression
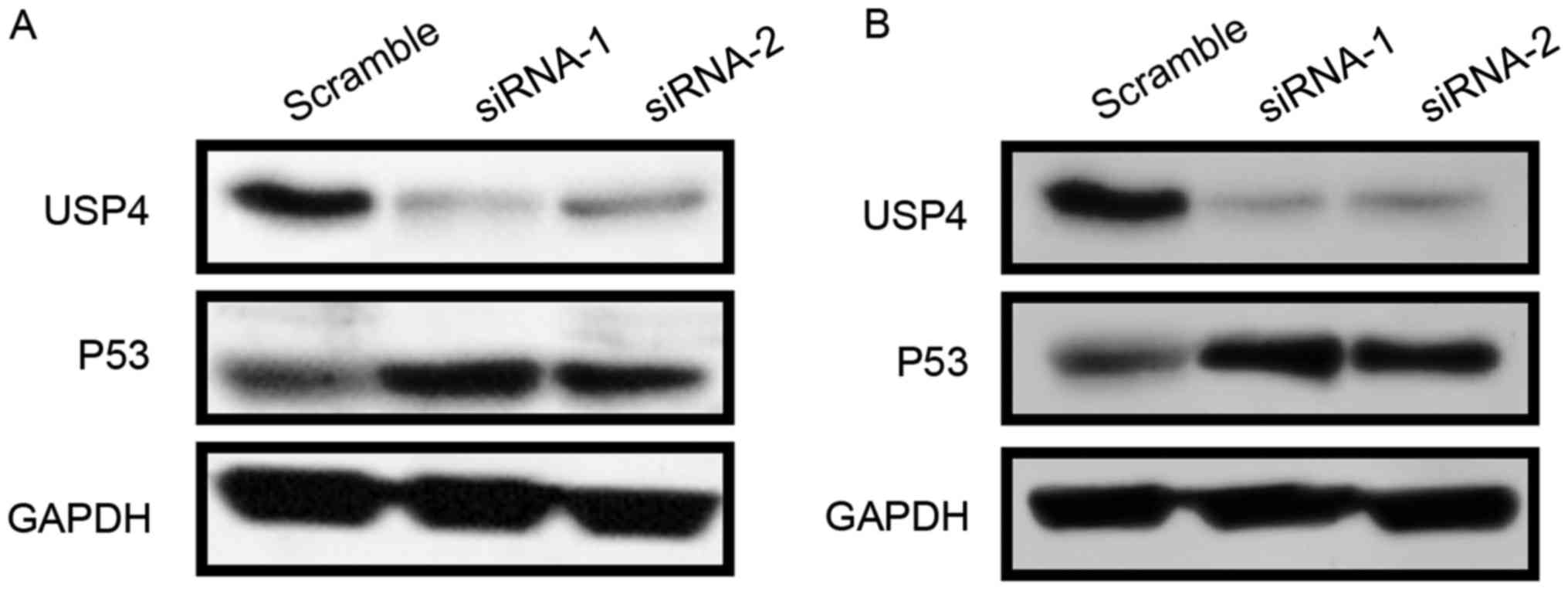
Since knockdown of USP4 promotes apoptosis, the
expression of the apoptosis-associated protein P53 was then
examined. The results revealed that USP4 knockdown by the two siRNA
sequences led to a strong induction of P53 expression in U251 and
U87 cells (Fig. 3A and B).
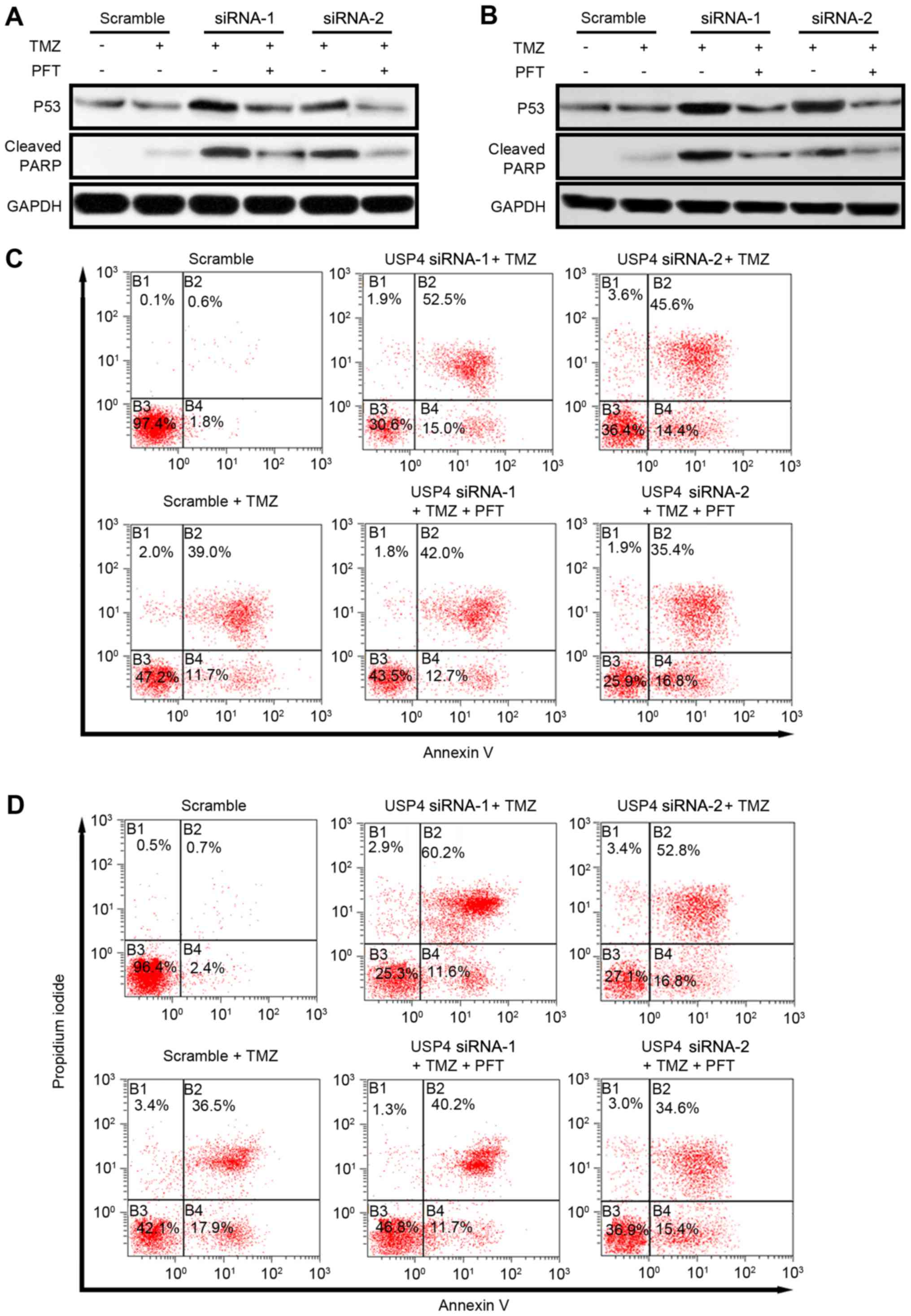
USP4 mediates chemoresistance via inhibiting P53 in
glioblastoma. To further investigate the role of P53 in
USP4-mediated chemoresistance, P53 activity was inhibited by a
P53-specific inhibitor, pifithrin-α hydrobromide (PFT). As reported
earlier, USP4 knockdown resulted in an increase in P53 expression,
as well as apoptosis indicated by cleaved PARP. Following the
administration of PFT, P53 protein level is slightly affected but
the level of cleaved PARP reduced dramatically revealed by western
blotting suggesting that USP4 facilitates chemoresistance by
inhibiting P53 in both cell lines (Fig.
4A and B). Furthermore, the apoptosis mediated by USP4
knockdown was also analyzed by flow cytometry. In U251 cells, the
results indicated that TMZ treatment increased the Annexin V and
propidium iodide (PI) double-positive cells from 0.6 to 39%. When
USP4 knockdown cells were treated with TMZ, the Annexin V and PI
double-positive cells increased to 52.5% (siRNA-1) and 45.6%
(siRNA-2), suggesting that an improved chemotherapy effect may be
acquired when USP4 is absent in glioblastoma. Finally, when PFT was
added in TMZ-treated USP4 knockdown cells, the percentage of
apoptotic cells was decreased to 42.0% (siRNA-1) and 35.4%
(siRNA-2), suggesting that USP4-mediated chemoresistance is
dependent on P53 activity (Fig. 4C).
Similarly, in U87 cells, USP4 knockdown facilitated the drug effect
of TMZ, while P53 inhibitor reversed this effect (Fig. 4D).
Discussion
Glioblastoma is the most common primary malignancy
of the brain. In spite of decades of research on this disease,
little progress has been made to improve the survival rate of
patients. Resistance to anticancer drugs is a problem in numerous
types of cancer, particularly in glioblastoma (6). Combining radiation therapy with TMZ is
currently the first-line therapy for glioblastoma. However, the
efficiency of TMZ remains limited owing to inherent and acquired
resistance of glial tumor cells. It is, thus, urgent to determine
the underlying mechanism that genetic alterations of patients
facilitate drug resistances.
The present study findings revealed that USP4 is
significantly upregulated in glioblastoma tissues, as well as in
glioblastoma cell lines. It was observed that, when USP4 was
knocked down, glioblastoma cells became more sensitive to TMZ
treatment, suggesting the pro-cancer role of USP4. Furthermore, the
results demonstrated that P53 was increased in USP4 knockdown U251
and U87 cells. Taken together, the results of the present study
suggested that USP4 mediated chemoresistance through inhibiting
apoptosis in a P53-dependent manner.
Due to the critical role of p53 in a variety of
tumors, targeting p53 and its altered signaling pathways is of
great importance in order to gain a better prognosis, particularly
in malignancies such as glioblastoma. Thus, it is critical to
understand the genetic background that affects the normal function
of P53 signaling. The findings of the current study suggested a
de novo role of USP4 in chemoresistance via P53 signaling,
providing new evidence for future drug discovery and clinical
treatment of glioblastoma. However, despite extensive studies, the
data regarding a link between altered p53 and efficacy of
chemotherapy in patients with glioblastoma remain controversial
(37). Certain studies have argued
that the p53 status correlates with a favorable response of
patients to therapy (38,39), while other studies revealed no such
correlation (40,41). Thus, it is of great use to further
explore the role of P53 in chemoresistance and to develop therapies
that simultaneously target P53 and USP4 in order to improve the
outcome of chemotherapy.
In conclusion, the results reported in the current
study suggested a pro-cancer role of USP4 in glioblastoma by
facilitating chemoresistance. Thus, to acquire better benefits for
glioblastoma patients with elevated USP4 expression, it is useful
to develop drugs that antagonize USP4. In addition, USP4 function
in various types of cancer requires further investigations.
Experiments in breast cancer indicated that USP4 was downregulated
in breast cancer tissues, while USP4 overexpression led to
inhibition of breast cancer cell proliferation (42). Furthermore, how USP4 affects P53
signaling and whether USP4 mediates TMZ resistance through other
types of signaling remain unclear. However, USP4 appears to be a
potential target for future drug discovery for glioblastoma.
Acknowledgements
The authors thank Dr Gang Wu (Department of
Neurology, Xijing Hospital of the Fourth Military Medical
University) for providing glioblastoma tissue samples obtained from
patients.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81402544 and
31370834).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
Cell culture and associated treatments were
performed by NQ and FH. NQ and YG performed qPCR assays, FH and LL
performed western blotting. NQ and YG performed flow cytometry. LW
performed cell viability analysis. GZ performed the histological
examination of the brain tissue. NQ, FH and LL wrote the
manuscript. YD and JZ designed this study and contributed to
manuscript revision. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fourth Military Medical University (Xi'an, China).
Written informed consent was obtained from all patients.
Patient consent for publication
Written informed consent was obtained from all the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott CB, Scarantino C, Urtasun R, Movsas
B, Jones CU, Simpson JR, Fischbach AJ and Curran WJ Jr: Validation
and predictive power of Radiation Therapy Oncology Group (RTOG)
recursive partitioning analysis classes for malignant glioma
patients: A report using RTOG 90-06. Int J Radiat Oncol Biol Phys.
40:51–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson CM, Lim M and Drake CG:
Immunotherapy for brain cancer: Recent progress and future promise.
Clin Cancer Res. 20:3651–3659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson TA, Karajannis MA and Harter DH:
Glioblastoma multiforme: State of the art and future therapeutics.
Surg Neurol Int. 5:642014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oike T, Suzuki Y, Sugawara K, Shirai K,
Noda SE, Tamaki T, Nagaishi M, Yokoo H, Nakazato Y and Nakano T:
Radiotherapy plus concomitant adjuvant temozolomide for
glioblastoma: Japanese mono-institutional results. PLoS One.
8:e789432013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part I:
Resistance mechanisms and strategies to overcome resistance of
glioblastoma to temozolomide. Drug Discov Today. 20:899–905.
2015.
|
|
9
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part II:
RNA interference as a promising way to sensitize glioblastomas to
temozolomide. Drug Discov Today. 20:772–779. 2015.
|
|
10
|
Esteller M, Garcia-Foncillas J, Andion E,
Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB and Herman JG:
Inactivation of the DNA-repair gene MGMT and the clinical response
of gliomas to alkylating agents. N Engl J Med. 343:1350–1354. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang PH, Xu AM and White FM: Oncogenic
EGFR signaling networks in glioma. Sci Signal. 2:re62009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz A, Haklai R, Elad-Sfadia G, Ballan E
and Kloog Y: Galectin-1 binds oncogenic H-Ras to mediate Ras
membrane anchorage and cell transformation. Oncogene. 20:7486–7493.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burton EC, Lamborn KR, Forsyth P, Scott J,
O'Campo J, Uyehara-Lock J, Prados M, Berger M, Passe S, Uhm J, et
al: Aberrant p53, mdm2, and proliferation differ in glioblastomas
from long-term compared with typical survivors. Clin Cancer Res.
8:180–187. 2002.PubMed/NCBI
|
|
14
|
Wang AL, Liu ZX, Li G and Zhang LW:
Expression and significance of P53 protein and MDM-2 protein in
human gliomas. Chin Med J (Engl). 124:2530–2533. 2011.PubMed/NCBI
|
|
15
|
Kato H, Kato S, Kumabe T, Sonoda Y,
Yoshimoto T, Kato S, Han SY, Suzuki T, Shibata H, Kanamaru R and
Ishioka C: Functional evaluation of p53 and PTEN gene mutations in
gliomas. Clin Cancer Res. 6:3937–3943. 2000.PubMed/NCBI
|
|
16
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizoguchi M, Guan Y, Yoshimoto K, Hata N,
Amano T, Nakamizo A and Sasaki T: Clinical implications of
microRNAs in human glioblastoma. Front Oncol. 3:192013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toledo F and Wahl GM: Regulating the p53
pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer.
6:909–923. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robles AI and Harris CC: Clinical outcomes
and correlates of TP53 mutations and cancer. Cold Spring Harb
Perspect Biol. 2:a0010162010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hermisson M, Klumpp A, Wick W, Wischhusen
J, Nagel G, Roos W, Kaina B and Weller M: O6-methylguanine DNA
methyltransferase and p53 status predict temozolomide sensitivity
in human malignant glioma cells. J Neurochem. 96:766–776. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Chen JX, Liu YH, You C and Mao Q:
Mutant TP53 enhances the resistance of glioblastoma cells to
temozolomide by up-regulating O(6)-methylguanine
DNA-methyltransferase. Neurol Sci. 34:1421–1428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nie E, Jin X, Wu W, Yu T, Zhou X, Zhi T,
Shi Z, Zhang J, Liu N and You Y: BACH1 promotes temozolomide
resistance in glioblastoma through antagonizing the function of
p53. Sci Rep. 6:397432016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang X, Zhou W, Wu Q, Huang Z, Shi Y, Yang
K, Chen C, Xie Q, Mack SC, Wang X, et al: Deubiquitinase USP13
maintains glioblastoma stem cells by antagonizing FBXL14-mediated
Myc ubiquitination. J Exp Med. 214:245–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li M, Chen D, Shiloh A, Luo J, Nikolaev
AY, Qin J and Gu W: Deubiquitination of p53 by HAUSP is an
important pathway for p53 stabilization. Nature. 416:648–653. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang CL, Wang JY, Liu ZY, Ma XM, Wang XW,
Jin H, Zhang XP, Fu D, Hou LJ and Lu YC: Ubiquitin-specific
protease 2a stabilizes MDM4 and facilitates the p53-mediated
intrinsic apoptotic pathway in glioblastoma. Carcinogenesis.
35:1500–1509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Berger FG, Yang J and Lu X: USP4
inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO
J. 30:2177–2189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Hao Q, Luo J, Xiong J, Zhang S, Wang
T, Bai L, Wang W, Chen M, Wang W, et al: USP4 inhibits p53 and
NF-κB through deubiquitinating and stabilizing HDAC2. Oncogene.
35:2902–2912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Zhou F, Drabsch Y, Gao R,
Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu
CX and ten Dijke P: USP4 is regulated by AKT phosphorylation and
directly deubiquitylates TGF-β type I receptor. Nat Cell Biol.
14:717–726. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang SJ, Lee HW, Kim HR, Lee H, Shin CH,
Yun SI, Lee DH, Kim DH, Kim KK, Joo KM and Kim HH:
Ubiquitin-specific protease 4 controls metastatic potential through
β-catenin stabilization in brain metastatic lung adenocarcinoma.
Sci Rep. 6:215962016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, Liu C, Liu H, Gong L, Tao T, Shen
Y, Zhu S and Shen A: Increased expression of ubiquitin-specific
protease 4 participates in neuronal apoptosis after intracerebral
hemorrhage in adult rats. Cell Mol Neurobiol. 37:427–435. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fraser M, Leung BM, Yan X, Dan HC, Cheng
JQ and Tsang BK: p53 is a determinant of X-linked inhibitor of
apoptosis protein/Akt-mediated chemoresistance in human ovarian
cancer cells. Cancer Res. 63:7081–7088. 2003.PubMed/NCBI
|
|
34
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Romanowska M, Evans A, Kellock D, Bray SE,
McLean K, Donandt S and Foerster J: Wnt5a exhibits layer-specific
expression in adult skin, is upregulated in psoriasis, and
synergizes with type 1 interferon. PLoS One. 4:e53542009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masui K, Cloughesy TF and Mischel PS:
Review: Molecular pathology in adult high-grade gliomas: From
molecular diagnostics to target therapies. Neuropathol Appl
Neurobiol. 38:271–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schiebe M, Ohneseit P, Hoffmann W,
Meyermann R, Rodemann HP and Bamberg M: Analysis of mdm2 and p53
gene alterations in glioblastomas and its correlation with clinical
factors. J Neurooncol. 49:197–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Birner P, Piribauer M, Fischer I,
Gatterbauer B, Marosi C, Ungersböck K, Rössler K, Budka H and
Hainfellner JA: Prognostic relevance of p53 protein expression in
glioblastoma. Oncol Rep. 9:703–707. 2002.PubMed/NCBI
|
|
40
|
Kraus JA, Wenghoefer M, Glesmann N, Mohr
S, Beck M, Schmidt MC, Schröder R, Berweiler U, Roggendorf W, Diete
S, et al: TP53 gene mutations, nuclear p53 accumulation, expression
of Waf/p21, Bcl-2, and CD95 (APO-1/Fas) proteins are not prognostic
factors in de novo glioblastoma multiforme. J Neurooncol.
52:263–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rich JN, Hans C, Jones B, Iversen ES,
McLendon RE, Rasheed BK, Dobra A, Dressman HK, Bigner DD, Nevins JR
and West M: Gene expression profiling and genetic markers in
glioblastoma survival. Cancer Res. 65:4051–4058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Jiang D, Zhang Q, Liu X and Cai Z:
Ubiquitin-specific protease 4 inhibits breast cancer cell growth
through the upregulation of PDCD4. Int J Mol Med. 38:803–811. 2016.
View Article : Google Scholar : PubMed/NCBI
|