Introduction
In 2012, gastric cancer was the most common
malignancy globally, particularly in Eastern Asia (1). Additionally, in 2010, it was the second
most common cancer and third most common cause of cancer-associated
mortalities in China (2). The
conventional therapies of gastric cancer, including chemotherapy
and radiotherapy, have notable difficulties directly associated
with hypoxia (3). Hypoxia occurs in
solid tumor types, including gastric, liver, breast, pancreatic
cancer, as a result of an inadequate supply of oxygen, due to
exponential cellular proliferation and inefficient vascular supply;
therefore, it is an adverse prognostic indicator in cancer as it is
associated with resistance to radiotherapy and chemotherapy
(4). The mechanisms of
hypoxia-induced chemotherapy resistance are complex (5–8) due to
hypoxia inducible factor-1 (HIF-1) acting as a transcription factor
to upregulate numerous genes with varying functions, including the
regulation of drug efflux, proliferation, angiogenesis and
metabolic changes (9,10). Improving the hypoxia condition can
significantly enhance the effect of gastric cancer chemotherapy
(11).
With profound progress in biomedical science,
numerous targeted therapeutic methods have been investigated in a
number of cancer types, including gastric cancer (12). In this aspect, mesenchymal stem cells
(MSCs) have a notable potential as a tool for targeted therapy, due
to these cells being easily transduced in vitro and can be
used as gene-delivery vehicles for gene therapy (13). To date, a large number of genes with
tumor-suppressive functions have been successfully engineered into
MSCs and have been tested in a number of cancer models, including
interferon-α (IFN-α) in melanoma (14), IFN-γ in leukemia (15), interleukin-12 in cervical cancer
(16,17), and tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) in breast and hepatocellular
carcinoma (18,19). In the present study, the aim was to
produce hemoglobin protein-expressing MSCs, and then to use these
MSCs as a vehicle to supply oxygen to the hypoxic gastric cancer
cells. Additionally, whether this method could enhance the effect
of gastric cancer chemotherapy was investigated.
Materials and methods
Cell culture
Gastric cancer cell lines MKN-45 and SGC-7901 were
preserved by the Key Laboratory of Digestive System Tumors, Lanzhou
University Second Hospital (Lanzhou, China). The gastric cancer
cell lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere containing 5% CO2 at 37°C. MSCs
from bone marrow were preserved by the Key Laboratory of Digestive
System Tumors, Lanzhou University Second Hospital and cultured in
low-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS in a humidified
atmosphere containing 5% CO2 at 37°C. Subsequently, the
morphology of the cultured cells was observed with a light inverted
phase-contrast microscope (Nikon TS-100F; ×20).
Chemicals
Cisplatin (CDDP) and 5-fluorouracil (5-fu) were
obtained from Qilu Pharmaceuticals (Jinan, China) and Shanghai
Xudong Haipu Pharmaceuticals Co., Ltd., (Shanghai, China),
respectively. Prior to their use, CDDP and 5-fu were immediately
dissolved in 0.9% sodium chloride injection/PBS/DMEM to a working
concentration of 0.4 and 2 mg/ml, respectively.
Plasmid construct
The hemoglobin subunit α 2 (HBA2) and hemoglobin
subunit β (HBB) genes were chemically synthetized by GenePharma
Gene Technology, Co., (Shanghai, China). The sequences of HBA2 and
HBB are located on GeneBank (https://cipotato.org/genebankcip/). Subsequently, the
products were ligated with linearized pEX-2 Vector (GenePharma Gene
Technology, Co., Shanghai, China; concentration, 500 ng/ml) using
T4 DNA ligase (Takara Bio, Inc., Otsu, Japan) at 22°C for 60 min.
The recombinant plasmids were transformed into Top 10 competent
cells with the CaCl2 method (20). To
identify the positive clones, plasmids were extracted with a
mini-plasmid extract kit (Takara Bio, Inc.), according to the
manufacturer's protocols. The clone was confirmed by DNA sequencing
(forward primer, 5′-TCAAGCCTCAGACAGTGGTTC-3′; and reverse primer,
5′-CCTCACATTGCCAAAAGACG-3′). This was conductedon the next day
after transfection. Construction plasmid helper-SL3 and envelope
plasmid helper-SL4 were also purchased in GenePharma Gene
Technology, Co. GFP report gene was added to mark the positive
cells.
Lentivirus packaging
293T cells (Genomeditech, Shanghai, China) were
preserved by Key Laboratory of Digestive System Tumors, Lanzhou
University Second Hospital, and maintained with high-glucose DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS. The
293T cells at 90% confluence were used for lentivirus packaging.
The plasmid of pEX-2 containing the hemoglobin gene coding
sequence, the construction plasmid helper-SL3 and the envelope
plasmid helper-SL4 were co-transfected (5 µl/1×105
cells) into 293T cells mediated by Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 72 h, the
culture medium containing packaged lentivirus particles were
collected, filtered and stored at −80°C for further processing. The
lentiviral titer was detected by cell counting using a fluorescent
microscope (magnification, ×40).
Infection of MSCs with obtained
lentiviral particles
MSCs were directly infected with obtained
(containing hemoglobin genes and the GFP report gene) or empty
lentiviral particles (only containing the GFP report gene) by
adding DMEM containing 5 µg/mg polybrene (EMD Millipore, Billerica,
MA, USA). The concentration of lentiviral particles was 40
µl/1×105 cells. After 72 h, the expression of GFP was
observed by fluorescent microscopy (magnification, ×40) to observe
transfection efficiency. Blank control was also set, which
contained MSCs without any modification. The MSC-hemo-GFP, which
contained hemoglobin genes and the GFP report gene, and MSC-GFP,
which only contained the GFP report gene, groups were collected and
washed twice with 0.5% PBS solution, and then suspended with 0.5%
PBS solution for the next application.
Inducible expression of
hemoglobin
Induction of hemoglobin expression was achieved by
the addition of isopropyl-b-D-thiogalactopyranoside (IPTG) and
hemin. The IPTG and hemin addition occurred at an optical density
at 600 nm of ~0.6, with an IPTG final concentration of 0.2 mmol/L
and a hemin final concentration of 25 µg/ml. Following culturing in
a humidified atmosphere containing 5% CO2 at 37°C for 2
h, hemin was added again to the MSC-hemo group to a final
concentration of 50 µg/ml. After 6 h at 37°C, the cells were
collected for the next examination.
Observing the expression level of
hemoglobin with western blotting
The cells were lysed with radio immunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
to prepare the protein samples for western blotting. The
quantification of the extracted proteins was conducted with a BCA
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Subsequently, 40 µg protein samples were
loaded onto a 12% SDS-PAGE gel, and transferred to a polyvinylidene
difluoride (EMD Millipore) membrane. Following being blocked with
1% bovine serum albumin solution for 1 h at room temperature, the
membrane was incubated with mouse anti-hemoglobin polyclonal
antibody (1:1,000; cat. no. ab17925; Abcam, Shanghai, China)
overnight at 4°C, and then alkaline phosphatase-conjugated goat
anti-mouse IgG secondary antibodies (1:10,000; cat. no. zk9600;
OriGene Technologies, Inc., Beijing, China) were applied to the
membrane for 2 h at room temperature. Protein expression was
detected with an electro-chemiluminescence (ECL) assay
(Hypersensitive ECL chemiluminescence substrate kit; Yanxi
Biotechnology, Co., Shanghai, China), according to the
manufacturer's protocols (Pierce; Thermo Fisher Scientific, Inc.).
The detecting system was Quantity One 4.62 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Additionally, blank control (MSCs),
positive control (recombinant hemoglobin positive control;
purchased from Shanghai Yu Bo Biotechnology, Co., Ltd., Shanghai,
China) and β-actin (cat. no. ab8227; Abcam) were set to ensure the
quality of western-blotting detection. The 3 groups used were:
Non-transformed MSCs, which represented the blank control group;
0.45 µg hemoglobin as the positive control; and the MSC-hemo group,
which were transfected with 40 µl/1×105 cells lentiviral
particles.
Effect of the function of oxygen-laden
MSC-hemo cells on gastric cancer cell chemotherapy
A Transwell assay was conducted, and because GFP
report gene was involved in the lentivirus particles, the results
were observed with afluorescent microscope (×40). In the present
study, two chemotherapeutics, 5-fu and CDDP, were tested. For these
chemotherapeutics, three interventions were set: Only
chemotherapeutic intervention, as a control group; chemotherapeutic
and non-transformed MSC interventions; and chemotherapeutic and
oxygen-laden MSC-hemo interventions. These chemotherapeutics were
investigated in two different gastric cancer cell lines, MKN45 and
SGC-7901. The interventions were conducted as follows: Gastric
cancer cells (MKN45 or SGC-7901) were seeded (1×105
cells/well) in the lower well of Transwell plates (Corning Costar,
Cambridge, MA, USA), which were 6.5 mm in diameter with 8 µm pore
filters and contained 600 µl DMEM (Gibco; Thermo Fisher Scientific,
Inc.). Chemotherapeutics (2 mg/ml 5-fu or 0.4 mg/ml CDDP) were
added into every plate of all tested groups. For the oxygen-laden
MSC-hemo group, the cells were suspended in serum-free DMEM (Gibco;
Thermo Fisher Scientific, Inc.) and seeded (1×105
cells/well) in the upper well of Transwell plates, and then they
were placed in an atmosphere containing 100% oxygen. For the
control group, 0.9% NaCl was added in the upper well of the
Transwell plates. Furthermore, for the group containing the
non-transformed MSCs, MSCs were suspended in serum-free DMEM and
seeded (1×105 cells/well) in the upper well of the
Transwell plates. Subsequently, the upper wells were placed on the
lower wells. Following culturing for 24 h in a humidified
atmosphere containing 5% CO2 at 37°C, the cells in the
lower wells were collected to conduct an MTT assay, in order to
assess the effect of the three different interventions.
MTT assay
An MTT assay was conducted as described
subsequently. MTT powder (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved (final concentration, 5 mg/ml) in PBS and
filtered. MTT solution was added to the cells on plates and
incubation continued for 4 h at 37°C. Supernatants were removed and
200 µl 0.04 M HCl in isopropanol was added to each well. Optical
densities were measured at 450 nm using Varioskan Flash (Thermo
Fisher Scientific, Inc.) as the detection system. MTT assays were
conducted in triplicate.
Statistical analysis
SPSS v17.0 was used to analyze data (SPSS, Inc.,
Chicago, IL, USA). Data were expressed as the mean ± standard
deviation. Results were analyzed using one-way analysis of variance
(ANOVA) to assess the statistical significance of overall
differences between all treatment groups and to evaluate the MTT
assay data. When the ANOVA test determined a value of P<0.05,
data were further analyzed with the Student's Newman-Keuls-q test,
in order to assess the statistical differences between every two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphology of cultured cells
As depicted in Fig.
1A, after culturing for 48 h, the MSCs grew in a
fibroblast-like shape, >80% of the cells fused together.
Additionally, the gastric cell lines MKN45 and SGC-7901 also
exhibited normal characteristics of gastric cancer cells, as
depicted in Fig. 1B.
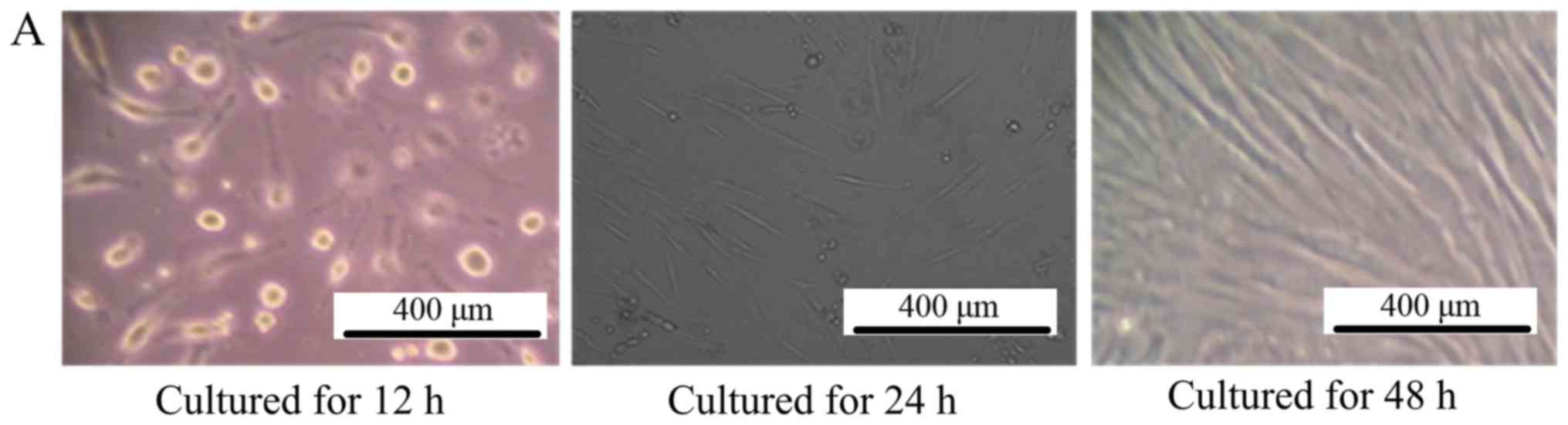 | Figure 1.Morphology of cultured cells. (A) The
morphology of mesenchymal stem cells observed by inverted
phase-contrast microscope (magnification, ×100). The left picture
is the MSCs cultured for 12 h, where all the cells adhered to the
bottom of the culture dish and cells were observed to be round. For
the middle picture, which depicts MSCs cultured for 24 h, the cells
began to grow in a fibroblast-like manner, and the right picture is
the MSCs cultured for 48 h, depicting that ~80% of the cells had
fused together. (B) The morphology of MKN45 and SGC-7901 cells
observed by inverted phase-contrast microscope (magnification,
×100). The left picture is the MKN-45 cells cultured for 48 h,
where all the cells grew in a triangle or quadrangle manner. The
right picture is the SGC-7901 cells cultured for 48 h, where the
cells grew in classical short shuttle-like manner. MSCs,
mesenchymal stem cells. |
Identification of the recombinant
vector
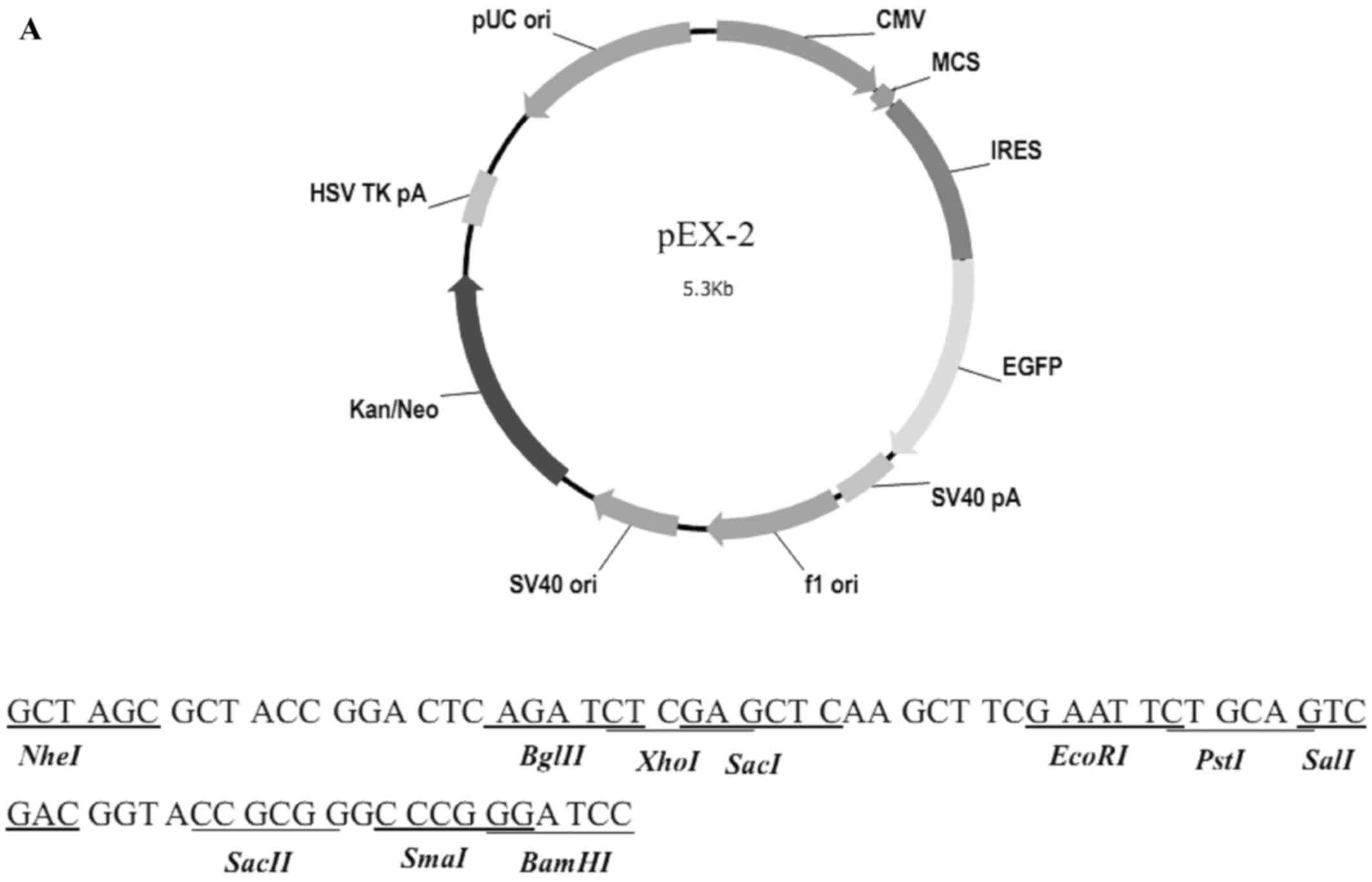
The structure of pEX-2 is depicted in Fig. 2A. The expression of hemoglobin genes
in the recombinant vector was identified by sequencing, and the
result demonstrated that the sequence was consistent with NM_000517
(HBA2) and NM_000518 (HBB) in Genebank (Fig. 2B).
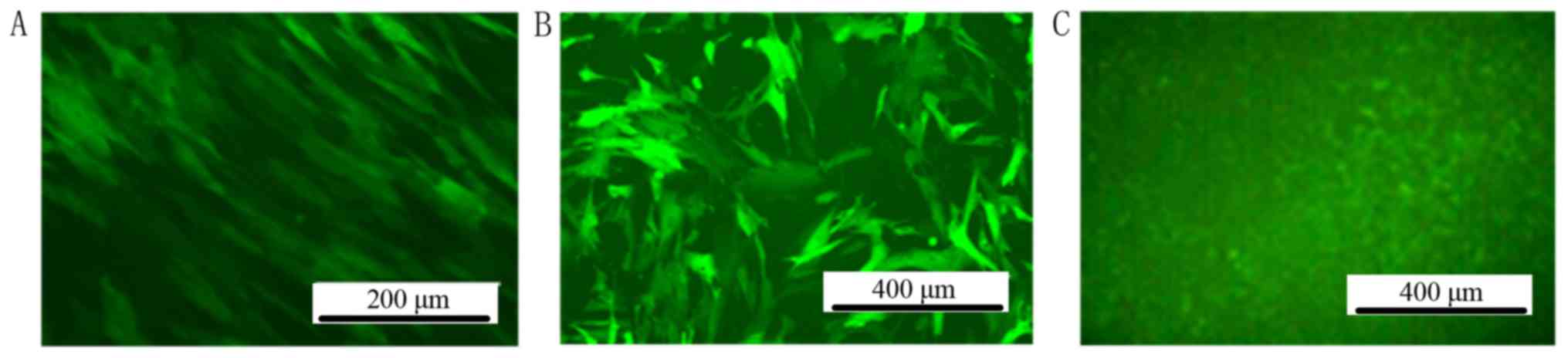
Screening of stable positive-infected
MSC clones by fluorescent microscopy
The titer of the lentiviral particles was determined
to be 1×109 TU/ml. MSCs were infected by lentiviral particles in
DMEM with 5 µg/ml polybrene. After 72 h infection by lentiviral
particles, GFP could be observed by fluorescent microscopy in the
MSC-hemo-GFP and MSC-GFP groups. No GFP expression was observed in
the blank control group (Fig. 3).
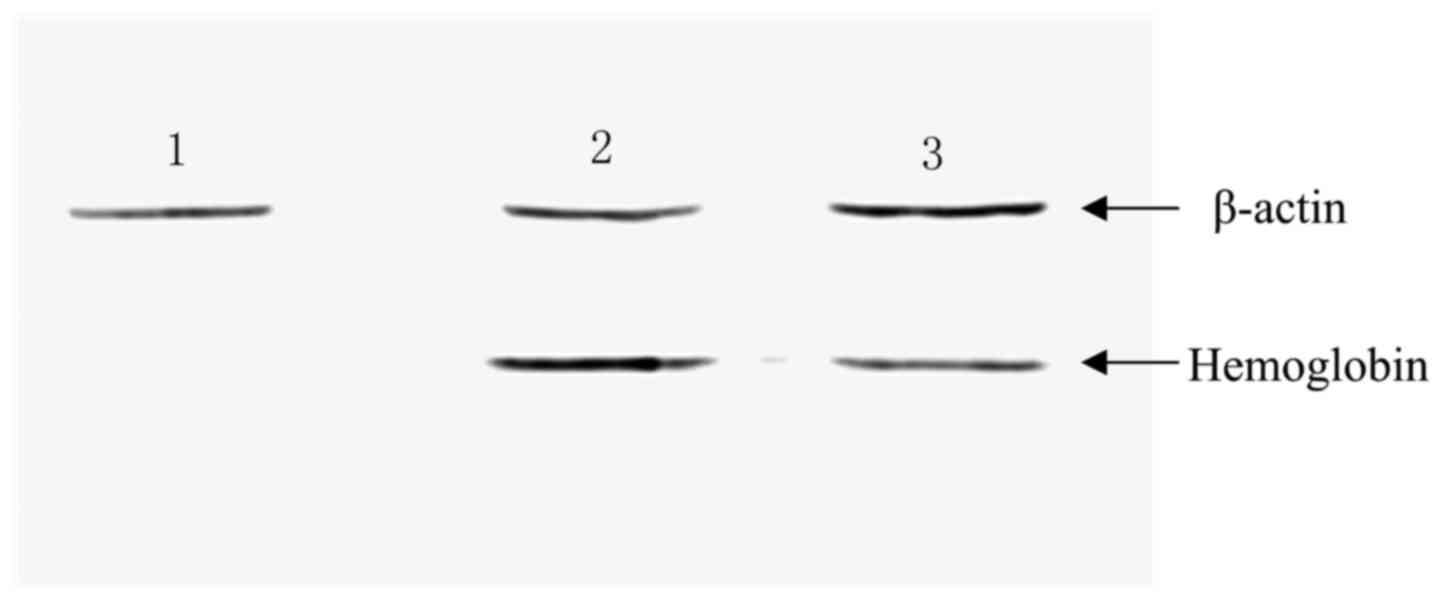
Observing the expression of hemoglobin
with western blotting
Subsequently, MSCs were infected with the
constructed lentiviral vectors, and the expression of hemoglobin
was detected with western blotting. As depicted in Fig. 4, hemoglobin was detected in the
MSC-hemo (Lane 3) and positive control groups (Lane 2); however,
hemoglobin protein was not expressed in the empty MSC control group
(Lane 1).
Effect of oxygen-laden MSC-hemo
function on the gastric cancer cell chemotherapy
The effect of oxygen-laden MSC-hemo function on the
gastric cancer cell chemotherapy was assessed with an MTT assay. As
depicted in Fig. 5, compared with the
5-fu treatment group (Fig. 5A), the
oxygen-laden MSC-hemo group significantly enhanced the effect of
5-fu treatment on MKN45 and SGC-7901 cells (P<0.05), while the
non-transformed MSC and 5-fu group had no significant difference
with the control group. In the CDDP treatment group (Fig. 5B), the identical results were
produced. This data indicated that the oxygen-laden MSC-hemo group
may contribute to the effect of the function of chemotherapeutics
on gastric cancer cell chemotherapy.
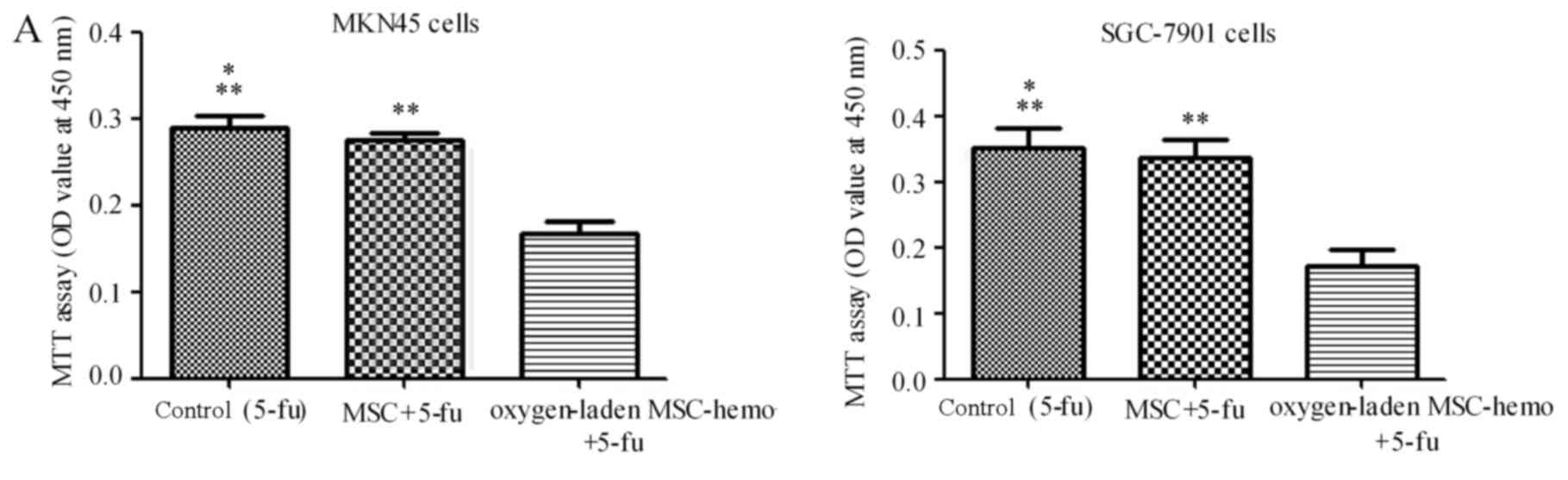 | Figure 5.The Oxygen-laden MSC-hemo group
significantly enhances the effect of chemotherapeutic treatments on
gastric cancer cells. (A) 5-fu treatment group for the MKN45 and
SGC-7901 cells. It demonstrated that the oxygen-laden MSC-hemo
group significantly enhanced the effect of 5-fu treatment on MKN45
and SGC-7901 cells, while the MSC group had no significant
difference, compared with the control group. (B) CDDP treatment
group for the MKN45 and SGC-7901 cells. It demonstrated that the
oxygen-laden MSC-hemo group significantly enhanced the effect of
CDDP treatment on MKN45 and SGC-7901 cells, while the MSC group had
no significant difference, compared with the control group.
*P>0.05, compared with MSC+5-fu or MSC+CDDP; **P<0,05,
compared with oxygen-laden MSC-hemo+5-fu or oxygen-laden
MSC-hemo+CDDP. MSCs, mesenchymal stem cells; CDDP, cisplatin; 5-fu,
5-fluroouracil. |
Discussion
Hypoxia is an integral characteristic of the tumor
microenvironment and a well-documented source of therapeutic
failure in clinical oncology (21).
It is a direct result of a lack of oxygen, which is caused by
microvascular defects that accompany the accelerated neoplastic
growth and indirectly caused by alterations in the proteome/genome,
angiogenesis and pH changes (4). The
majority of solid tumor cases >1 mm3 in volume
contain regions of hypoxia, particularly gastric cancer (22). The stomach is a hollow organ located
deep within the enterocoelia, and hypoxia is more severe for
gastric cancer (23).
Solid cancer types with hypoxia-induced phenotypes
are frequently resistant to chemotherapy and have a poor prognosis
(24). In particular, cancer cells
with reduced levels of oxygenation are more resistant to a number
of chemotherapeutic agents, including 5-fu and CDDP (25). This cellular response to hypoxia,
primarily mediated by HIF-1, may increase the aggressiveness of
cancer cells and contribute to poor responses to treatment
(26). With regards to the resistance
of gastric cancer cells to 5-fu, Nakamura et al (27) reported that the expression of HIF-1α
in gastric cancer tissue was an independent prognostic factor in
patients who were administered 5-fu adjuvant treatment following
resection. These authors also demonstrated that transfection of the
HIF-1α gene into gastric cancer cells increased their resistance to
5-fu in vitro and in vivo. Xuan et al
(3) also confirmed that in the MKN45
and AGS cell lines, HIF-1α expression is dependent on hypoxic
conditions and that the genetic enhancement of HIF-1α under
normoxic or hypoxic conditions can eliminate the sensitivity to
5-fu. Improvementof hypoxia in cancer is therefore a prime target
for the development of novel gastric cancer therapeutics. Reduced
treatment dosages and increased benefits for the patient are
envisaged as a consequence of these investigations. In conclusion,
the ability to increase oxygenation of tumors will revolutionize
contemporary cancer treatment. Successful treatment of hypoxic
cells has the potential to not only improve local control but also
impact overall patient survival.
Generally, oxygen is transported in the blood by
hemoglobin (28). Recombinant human
erythropoietin (rHuEPO) is currently administered to patients with
cancer, in order to protect against chemotherapy or
tumor-associated anemia, and clinical trial results indicate
numerous beneficial effects of rHuEPO treatment on the therapeutic
outcome of patients (29). In the
present study, a novel method was developed to increase the oxygen
supply for the gastric cancer cells to overcome the therapeutic
resistance of MKN45 and SGC-7901 cell lines to 5-fu and CDDP.
MSCs are a type of marrow stroma cells, which exist
in numerous tissues and are easy to obtain and amplify. Examples
include the bone marrow, umbilical cord blood and umbilical cord
(30). Due to their notable
differentiation potential, they are used in regenerative medicine
(31). In the last decade, increasing
numbers of researchers have determined another beneficial
characteristic of MSCs, that MSCs are able to home in on tumor
sites (32–34). This means that MSCs are ideal vehicles
for targeted gene therapies. At the same time, MSCs can avoid
immune rejection (35,36), which provides further convenience for
the use of MSCs as a tool for targeted therapy. In the present
study, MSCs were used as a vehicle for hemoglobin, with MSCs
infected by lentivirus vectors delivering the HBA2 and HBB genes.
Additionally, the present results demonstrated that these MSC-hemo
cells could continuously release hemoglobin protein following
induction with IPTG and hemin. Following placing in an atmosphere
containing 100% oxygen, the effect of MSC-hemo on gastric cancer
chemotherapy was evaluated. A total of two groups were set
according to the therapeutic drugs used (5-fu or CDDP).
Subsequently, three different intervention measures were conducted
on MKN45 and SGC-7901 cells in every group. The first intervention
measure was 0.9% NaCl and the therapeutic drug as a control, the
second measure was non-transformed MSCs and the therapeutic drug
and the final measure was the oxygen-laden MSC-hemo group and the
therapeutic drug. The results depicted in Fig. 5 indicated that the oxygen-laden
MSC-hemo group could significantly improve the effect of
chemotherapy, compared with the control group and the
non-transformed MSC group (P<0.05). Furthermore, the
non-transformed MSC+5-fu or MSC+CDDP groups demonstrated no
significant difference with the control group (P>0.05). These
results indicated that this method can successfully supply oxygen
to the gastric cancer cells and enhance the effect of
chemotherapeutics. This provides a novel method of thinking to
reduce the resistance to chemotherapy of gastric cancer and improve
the overall patient survival in clinical work. Furthermore, this is
the initial step and this requires further study to test this
method in vivo and in preclinical experiments.
To conclude, improving the tumor hypoxia environment
could provide notable benefit for cancer treatments, particularly
for gastric cancer. Using MSCs as a vehicle carrying oxygen to the
tumor microenvironment is a direct and effective method. With the
profound progress of biomedical science, targeted therapy had been
improved in the technology and security aspects (37). The present study demonstrated the
potential of MSCs as an effective delivery system that targets
tumors and reduces the resistance of anticancer drugs. This may
represent a prospective method for the treatment of gastric cancer
types.
Acknowledgements
The authors would like to thank Professor Liang Qiao
(University of Sydney, Sydney, Australia) for his suggestions.
Funding
This study was partly supported by the National
Natural Science Foundation of China (grant no. 31270532).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL conceived and designed the study. YZ was a major
contributor in acquiring data, data analysis and writing the
manuscript. WH helped with the acquisition of data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
IPTG
|
isopropyl-b-D-thiogalactopyranoside
|
|
HIF-1
|
hypoxia inducible factor-1
|
|
IFN-α
|
interferon-α
|
|
IFN-γ
|
interferon-γ
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
CDDP
|
cisplatin
|
|
5-fu
|
5-fluorouracil
|
|
GFP
|
green fluorescent protein
|
|
MOI
|
multiplicity of infection
|
|
ANOVA
|
one-way analysis of variance
|
|
rHuEPO
|
recombinant human erythropoietin
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer-slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim
W, Kim YB, Lee G, Han SU and Cho YK: Dichloroacetate attenuates
hypoxia-induced resistance to 5-fluorouracil in gastric cancer
through the regulation of glucose metabolism. Exp Cell Res.
321:219–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown LM, Cowen RL, Debray C, Eustace A,
Erler JT, Sheppard FC, Parker CA, Stratford IJ and Williams KJ:
Reversing hypoxic cell chemoresistance in vitro using genetic and
small molecule approaches targeting hypoxia inducible factor-1. Mol
Pharmacol. 69:411–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S, Han Z, et al: Hypoxia-inducible factor-1
alpha contributes to hypoxia-induced chemoresistance in gastric
cancer. Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
7
|
Hao J, Song X, Song B, Liu Y, Wei L, Wang
X and Yu J: Effects of lentivirus-mediated HIF-1alpha knockdown on
hypoxia-related cisplatin resistance and their dependence on p53
status in fibrosarcoma cells. Cancer Gene Ther. 15:449–455. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nardinocchi L, Puca R, Sacchi A and
D'Orazi G: Inhibition of HIF-1alpha activity by
homeodomain-interacting protein kinase-2 correlates with
sensitization of chemoresistant cells to undergo apoptosis. Mol
Cancer. 8:12009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Hypoxia, clonal selection, and
the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol.
35:71–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8 (4
Suppl):S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayer A and Vaupel P: Hypoxia, lactate
accumulation, and acidosis: Siblings or accomplices driving tumor
progression and resistance to therapy? Adv Exp Med Biol.
789:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wafik SE: Impact of genetic targets on
cancer therapy. Springer; New York: 2013
|
|
13
|
Serakinci N, Fahrioglu U and Christensen
R: Mesenchymal stem cells, cancer challenges and new directions.
Eur J Cancer. 50:1522–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Lu Y, Huang W, Xu H, Chen X, Geng Q,
Fan H, Tan Y, Xue G and Jiang X: In vitro effect of
adenovirus-mediated human Gamma Interferon gene transfer into human
mesenchymal stem cells for chronic myelogenous leukemia. Hematol
Oncol. 24:151–158. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao P, Ding Q, Wu Z, Jiang H and Fang Z:
Therapeutic potential of human mesenchymal stem cells producing
IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer
Lett. 290:157–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo SH, Kim KS, Park SH, Suh YS, Kim SJ,
Jeun SS and Sung YC: The effects of mesenchymal stem cells injected
via different routes on modified IL-12-mediated antitumor activity.
Gene Ther. 18:488–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grisendi G, Bussolari R, Cafarelli L,
Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi
M, Barti-Juhasz H, et al: Adipose-derived mesenchymal stem cells as
stable source of tumor necrosis factor-related apoptosis-inducing
ligand delivery for cancer therapy. Cancer Res. 70:3718–3729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Shan H, Li D, Li ZR, Zhu KS and
Jiang ZB: The inhibitory effect of MSCs expressing TRAIL as a
cellular delivery vehicle in combination with cisplatin on
hepatocellular carcinoma. Cancer Biol Ther. 13:1175–1184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan WT, Verma CS, Lane DP and Gan SK: A
comparison and optimization of methods and factors affecting the
transformation of Escherichia coli. Biosci Rep. 33(pii):
e000862013.PubMed/NCBI
|
|
21
|
Ji RC: Hypoxia and lymphangiogenesis in
tumor microenvironment and metastasis. Cancer Lett. 346:6–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon AM, Bouchier-Hayes DJ, Condron CM
and Toomey D: Tumour hypoxia, chemotherapeutic resistance and
hypoxia-related therapies. Cancer Treat Rev. 29:297–307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bubnovskaya L, Osinsky D, Trachevsky V,
Naleskina L, Kovelskaya A and Gumenyuk L: Premorphological
alterations in gastric mucosa in patients with gastric cancer:
Hypoxia level assessed by 31P NMR spectroscopy. Exp Oncol.
36:271–275. 2014.PubMed/NCBI
|
|
24
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teicher BA: Hypoxia and drug resistance.
Cancer Metastasis Rev. 13:139–168. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura J, Kitajima Y, Kai K, Hashiguchi
K, Hiraki M, Noshiro H and Miyazaki K: HIF-1alpha is an unfavorable
determinant of relapse in gastric cancer patients who underwent
curative surgery followed by adjuvant 5-FU chemotherapy. Int J
Cancer. 127:1158–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Caprio G, Stokes C, Higgins JM and
Schonbrun E: Single-cell measurement of red blood cell oxygen
affinity. Proc Natl Acad Sci USA. 12:9984–9989. 2015. View Article : Google Scholar
|
|
29
|
Dicato M, Duham C, Berchem G and Ries F:
Clinical benefit from erythropoietin. Curr Opin Oncol. 12:297–302.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Väänänen HK: Mesenchymal stem cells. Ann
Med. 37:469–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Loebinger MR, Kyrtatos PG, Turmaine M,
Price AN, Pankhurst Q, Lythgoe MF and Janes SM: Magnetic resonance
imaging of mesenchymal stem cells homing to pulmonary metastases
using biocompatible magnetic nanoparticles. Cancer Res.
69:8862–8867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasportas LS, Kasmieh R, Wakimoto H,
Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza
RL, Weissleder R and Shah K: Assessment of therapeutic efficacy and
fate of engineered human mesenchymal stem cells for cancer therapy.
Proc Natl Acad Sci USA. 106:4822–4827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang B, Wu X, Mao Y, Bao W, Gao L, Zhou P,
Xie R, Zhou L and Zhu J: Dual-targeted antitumor effects against
brainstem glioma by intravenous delivery of tumor necrosis
factor-related, apoptosis-inducing, ligand-engineered human
mesenchymal stem cells. Neurosurgery. 65:610–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Corcione A, Benvenuto F, Ferretti E,
Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi
GL, Pistoia V and Uccelli A: Human mesenchymal stem cells modulate
B-cell functions. Blood. 107:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsimberidou AM: Targeted therapy in
cancer. Cancer Chemother Pharmacol. 76:1113–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|