Introduction
One of the most common gynecological malignancies is
cervical cancer, with its incidence ranking fourth among malignant
tumors globally, and it accounts for 85% of cancer cases in
developing countries; it is also the primary cause of
cancer-associated mortalities in these areas (1). Although the methods of prevention and
treatment of cervical cancer have improved proportionally with the
improvement of the overall standard of medical practice in recent
years, cervical cancer remains a major worldwide health problem. At
present, the primary treatment of patients with early cervical
cancer [stages IA2-IIA2, classified according to the International
Federation of Gynecology and Obstetrics (2)] is to perform a radical hysterectomy and
a pelvic lymphadenectomy, and if any pathological risk factors are
noted postoperatively, follow-up treatment is required. Malignant
tumors are primarily spread by direct spread, hematogenous
metastasis, lymph node metastasis and implantation. A previous
study has suggested that perineural invasion (PNI) is another
independent way of transmission into the lymphatic and circulatory
systems (3), and no lymphatic vessel
tissue exists in the epineurium, therefore tumor PNI is not
equivalent to lymph vascular space invasion (LVSI), which is
another pathological feature independent of LVSI (4). As early as 1835, Cruveilheir (5) first reported the PNI phenomenon, as
manifested by malignant tumors, and identified that head cancer and
neck cancer can easily penetrate into the nerves and form growths
in the brain. In subsequent studies, PNI was demonstrated in a
number of malignant tumors, including pancreatic cancer, colorectal
cancer, bladder cancer, prostate cancer and cholangiocarcinoma, and
it was revealed to negatively affect patient prognosis (6,7). Although
there are relatively few studies on PNI in cervical cancer, one
study has revealed an association between PNI and the prognosis of
patients with early cervical cancer, with the 5-year overall
survival (OS) time for patients with PNI-positive tumors being
significantly decreased (8), and this
may be a prognostic factor for these patients. On the other hand,
another study has indicated that the prognosis of patients with
cervical cancer was not affected by PNI, and that PNI was not
associated with recurrence and survival rate (9). Therefore, whether PNI-positivity affects
the prognosis of patients with cervical cancer remains unclear. The
aim of the present study was to investigate the association between
PNI and the clinical features and pathological parameters of
patients with cervical cancer, to analyze the effect of PNI on the
survival time of the patients, to assess whether PNI-positivity
affects the prognosis of patients with early cervical cancer, and
to provide a basis for the patient to select between nerve-sparing
radical hysterectomy or adjuvant therapy.
Patients and methods
Patient characteristics and clinical
pathology
Overall, 406 patients with early cervical cancer who
underwent radical hysterectomy and pelvic lymphadenectomy between
January 2007 and December 2014 at the Affiliated Hospital of
Jiangnan University (Wuxi, China), were included in the present
study. The patients had not received any treatment prior to
surgery. A radical hysterectomy is the basic surgical procedure for
treating cases of cervical cancer. At the Affiliated Hospital of
Jiangnan University, the type of surgery selected for each patient
depends on the clinical stage of the cancer, classified according
to the Querleu-Morrow classification system (10): Stage IA1, hysterectomy outside the
anadesma; Stage IA2, expanded hysterectomy, increasing 1–2 cm;
Stage IB or IIA, radical hysterectomy with excision of 3 cm
parametrium and 3 cm vagina. Pelvic lymph node dissection was
performed according to the Querleu-Morrow guidelines. The inclusion
criteria for the patients in the present study were: i) Diagnosis
with cervical cancer by two senior pathologists; ii) stage IA2-IIA2
cervical cancer, classified according to the International
Federation of Gynecology and Obstetrics (2009) (2); iii) a radical hysterectomy and pelvic
lymphadenectomy as a method of treatment; and iv) availability of
the clinical and pathological data. The recorded
clinicopathological parameters included the age of onset, evidence
of hypertension or diabetes, clinical stage, histological type,
tumor size, lymph node metastasis, depth of cervical invasion,
surgical margin, vascular invasion and PNI.
Research methods
The diagnosis and the recording of the clinical and
pathological features of the patients and samples were provided by
the Department of Pathology of the Affiliated Hospital of Jiangnan
University. Tumor tissue samples were taken from the cervix of the
patients during surgery and were fixed with 4% paraformaldehyde at
4°C for 24 h and paraffin-embedded. Tissue sections were stained
with hematoxylin and eosin as previously described (11), and the sections were evaluated by two
senior pathologists using a light microscope. In the cases of
differing evaluations, discrepancies were settled by the more
senior pathologist. The present study focused on PNI in the cervix,
and not on parametrial invasion. The definition of PNI-positivity
(12) was based on the location of
the tumor cells around the nerve fibers, with the cells being
present along the nerve into the epineurium, perineurium or
endoneurium of any layer, or gathered and wrapped around the nerve
for ≥33% of its diameter and spread along the expansion of the
tumor cells with local infiltration. All 406 patients were followed
up by telephone every 3 months between the time of diagnosis and
January 16, 2017, until the occurrence of tumor recurrence or
mortality. The follow-up content included: i) The general condition
of the patient; ii) questions of whether to have the regular
follow-up, the results of the follow-up and the last follow-up
time; iii) the timing of any recurrence or metastasis, and iv) the
patients who succumbed, so as to understand the reasons for tumor
recurrence and mortality. Prognostic indicators included the OS
time, defined as the time between the date of surgery or diagnosis
and the time of patient mortality or last follow-up, and the
disease-free survival (DFS) time, defined as the time between the
date of surgery and the time of the first recurrence in the patient
or last follow-up.
Statistical analysis
The statistical analyses of the data was performed
using the IBM SPSS Statistics software (version 20.0; IBM Corp.,
Armonk, NY, USA). The associations between PNI and other clinical
features and pathological indices were analyzed using a
χ2 test; a Kaplan-Meier analysis was performed to
calculate and compare the survival rate of the patients; the
log-rank test was used to determine whether the survival curve
showed significant differences; and a Cox proportional hazards
regression model was used to analyze the influence of the clinical
characteristics and pathological features on the patient
prognosis.
Results
Association between PNI and
clinicopathological features of patients with early cervical
cancer
Of the 406 cases of early cervical cancer included
in the present study, 41 cases were lost, with a follow-up rate of
89.90%. The incidence of PNI was 43/406 (10.59%). With respect to
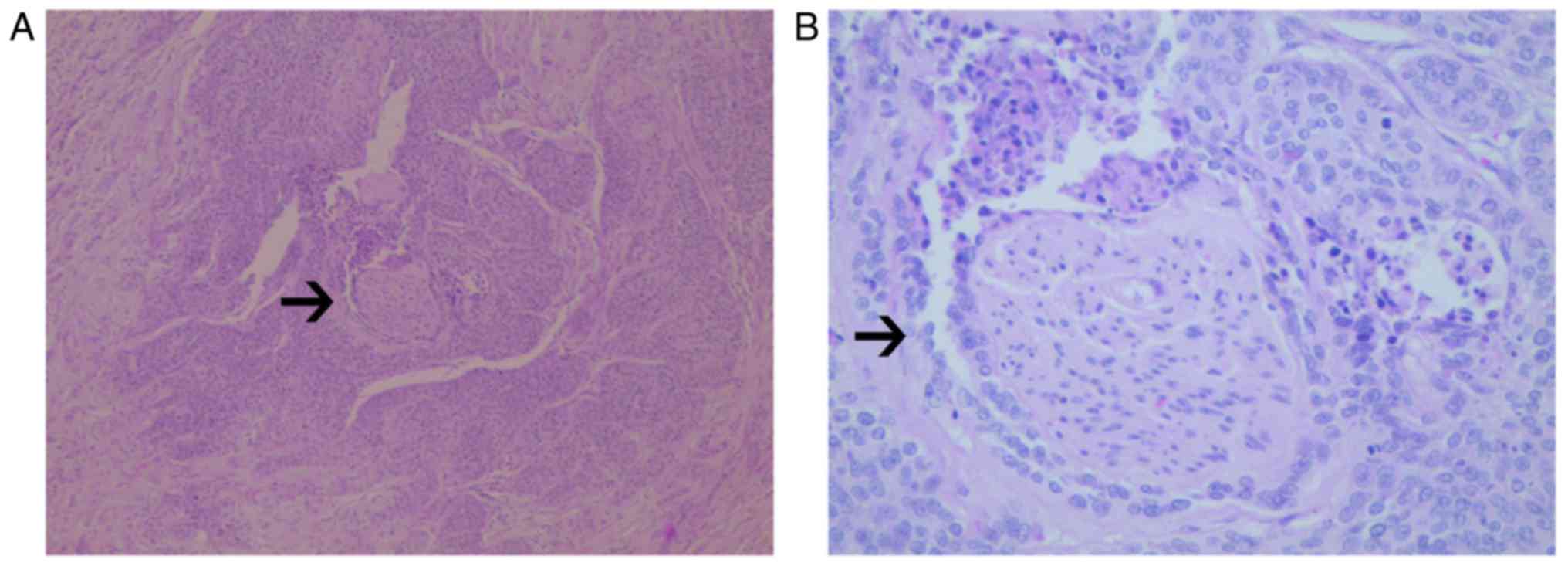
microscopic observations, in a number of the PNI-positive cases
there was a manifestation of tumor cells around the nerve and nerve
fibers, and local invasion and metastasis along its extension
(Fig. 1). The average age of the 363
patients without PNI was 48.59±9.61 years and that of the 43
patients with PNI was 47.37±9.27 years (P=0.118). The phenomenon of
PNI was significantly associated with hypertension (P<0.001),
but there was no association between PNI and diabetes (P=0.093) or
clinical stage (P=0.148). PNI was significantly associated with
lymph node metastasis (P<0.001), depth of cervical invasion
(P<0.001), surgical margin (P=0.002), and vascular invasion
(P<0.001), but not with tumor histological type (P=0.357) or
size (P=0.560) (Table I). These
results indicate that PNI is associated with risk factors that have
an effect on the prognosis of patients with cervical cancer.
 | Table I.Association between PNI and clinical
and pathological features. |
Table I.
Association between PNI and clinical
and pathological features.
| Parameter | PNI-negative | PNI-positive | P-value |
|---|
| Age, years | 48.59±9.61 | 47.37±9.27 | 0.118 |
| Hypertension |
|
| <0.001 |
|
Negative | 325 (89.5%) | 30 (69.8%) |
|
|
Positive | 38 (10.5%) | 13 (30.2) |
|
| Diabetes |
|
| 0.093 |
|
Negative | 344 (94.8%) | 38 (88.4%) |
|
|
Positive | 19 (5.2%) | 5 (11.6%) |
|
| Clinical stage |
|
| 0.148 |
| IA2 | 7 (1.9%) | 1 (2.3%) |
|
| IB1 | 206 (56.7%) | 16 (37.2%) |
|
| IB2 | 41 (11.3%) | 9 (20.9%) |
|
| IIA1 | 68 (18.7%) | 11 (25.6%) |
|
| Histological
type |
|
| 0.357 |
| Squamous
cell carcinoma | 321 (89.0%) | 41 (95.3%) |
|
|
Adenocarcinoma | 31 (8.5%) | 1 (2.3%) |
|
|
Adenosquamous carcinoma | 9 (2.5%) | 1 (2.3%) |
|
| Tumor size, cm |
|
| 0.560 |
| ≤4 | 244 (67.2%) | 27 (62.8%) |
|
|
>4 | 119 (32.8%) | 16 (37.2%) |
|
| Lymph node
metastasis |
|
| <0.001 |
|
Negative | 296 (81.5%) | 21 (48.8%) |
|
|
Positive | 67 (18.5%) | 22 (51.2%) |
|
| Depth of cervical
invasion |
|
| <0.001 |
|
<2/3 | 171 (47.1%) | 6 (14.0%) |
|
| ≥2/3 | 192 (52.9%) | 37 (86.0%) |
|
| Surgical margin |
|
| 0.002 |
|
Negative | 362 (99.7%) | 41 (95.3%) |
|
|
Positive | 1 (0.3%) | 2 (4.7%) |
|
| Vascular
invasion |
|
| <0.001 |
|
Negative | 251 (69.1%) | 15 (34.9%) |
|
|
Positive | 112 (30.9%) | 28 (65.1%) |
|
Effect of PNI on survival times of
patients with early cervical cancer
In the patients with early cervical cancer,
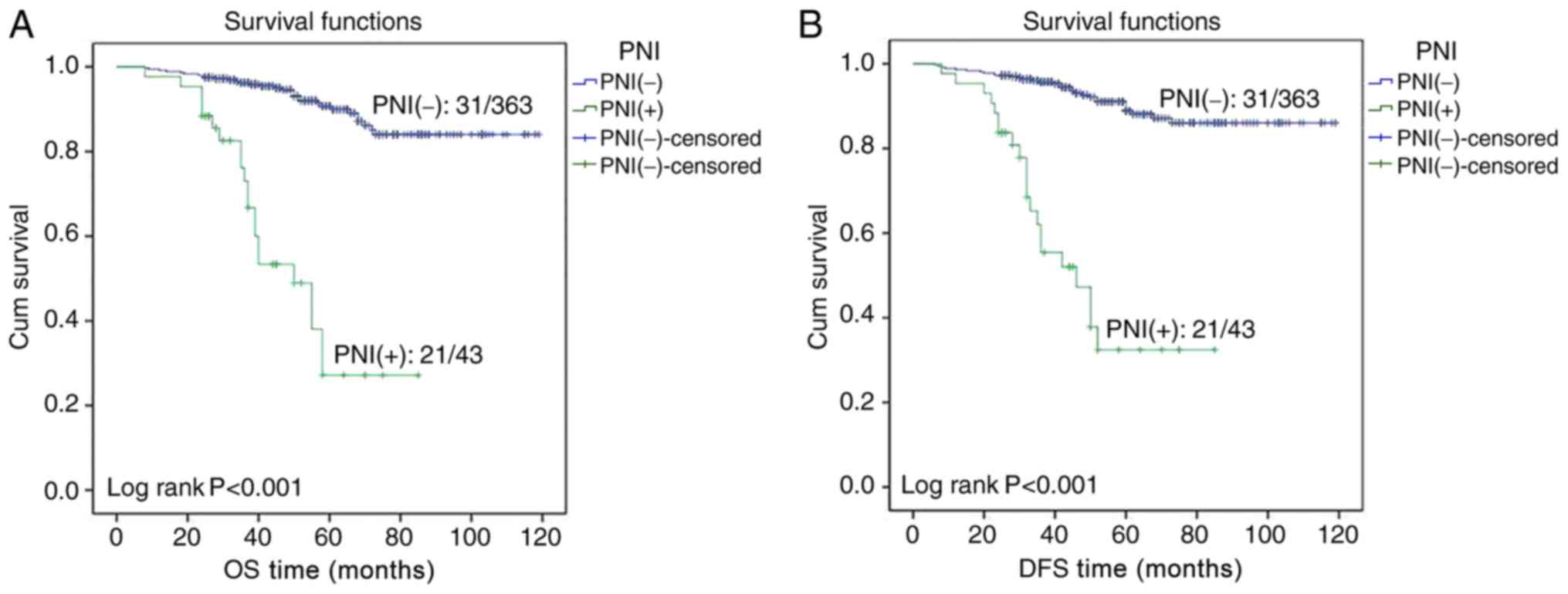
Kaplan-Meier survival curves indicated that the OS and DFS times of
PNI-positive patients were significantly lower compared with those
of patients without PNI (both P<0.001; Fig. 2). In the 2017 National Comprehensive
Cancer Network clinical practice guidelines (1), tumor size and lymph node metastasis are
risk factors for patients with cervical cancer. Therefore, tumor
size and lymph node metastasis were selected for further survival
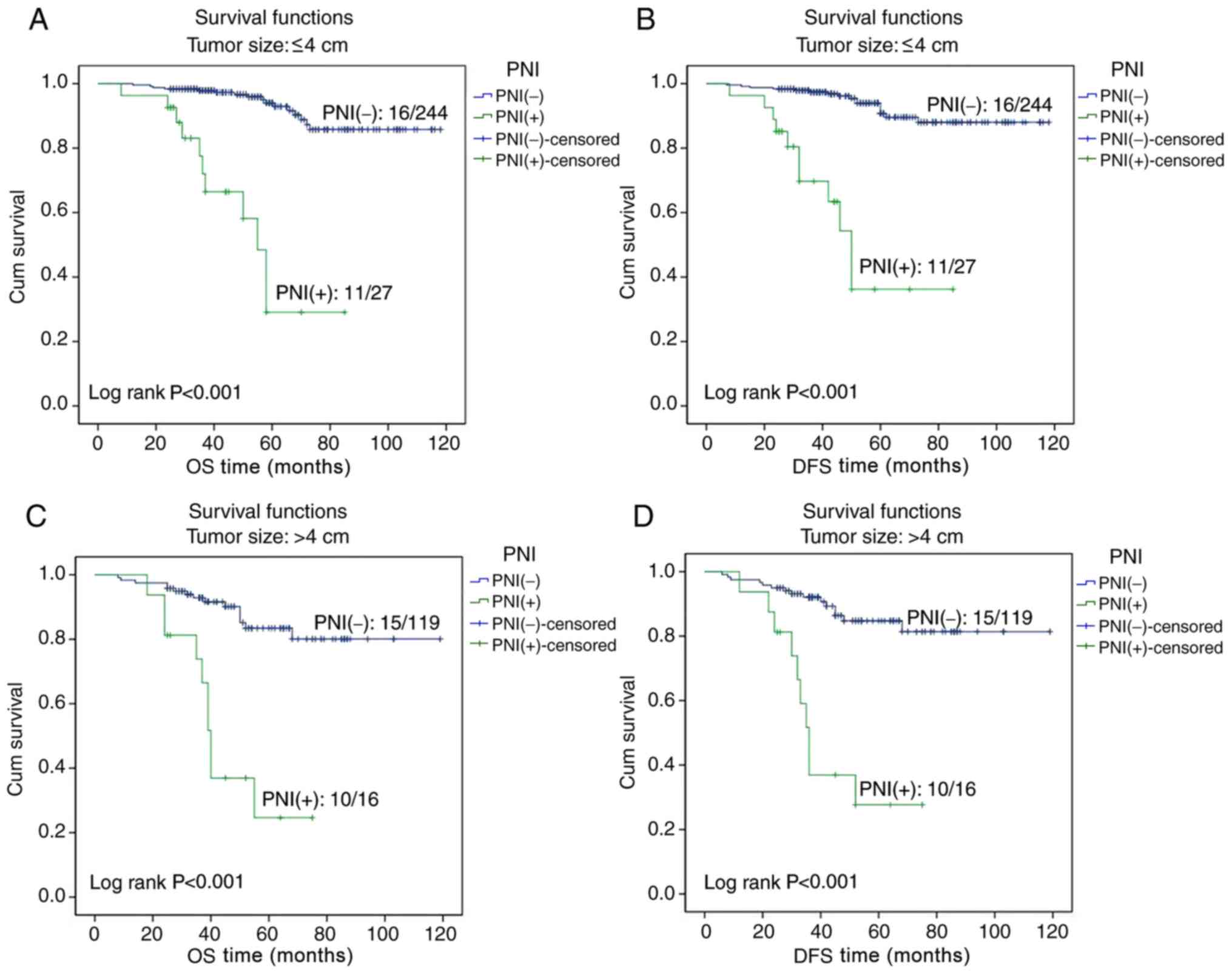
analysis. A survival analysis was performed for patients with
tumors of size ≤4 or >4 cm, and the Kaplan-Meier curves revealed
that the OS and DFS times in PNI-positive patients were
significantly lower compared with in PNI-negative patients for both
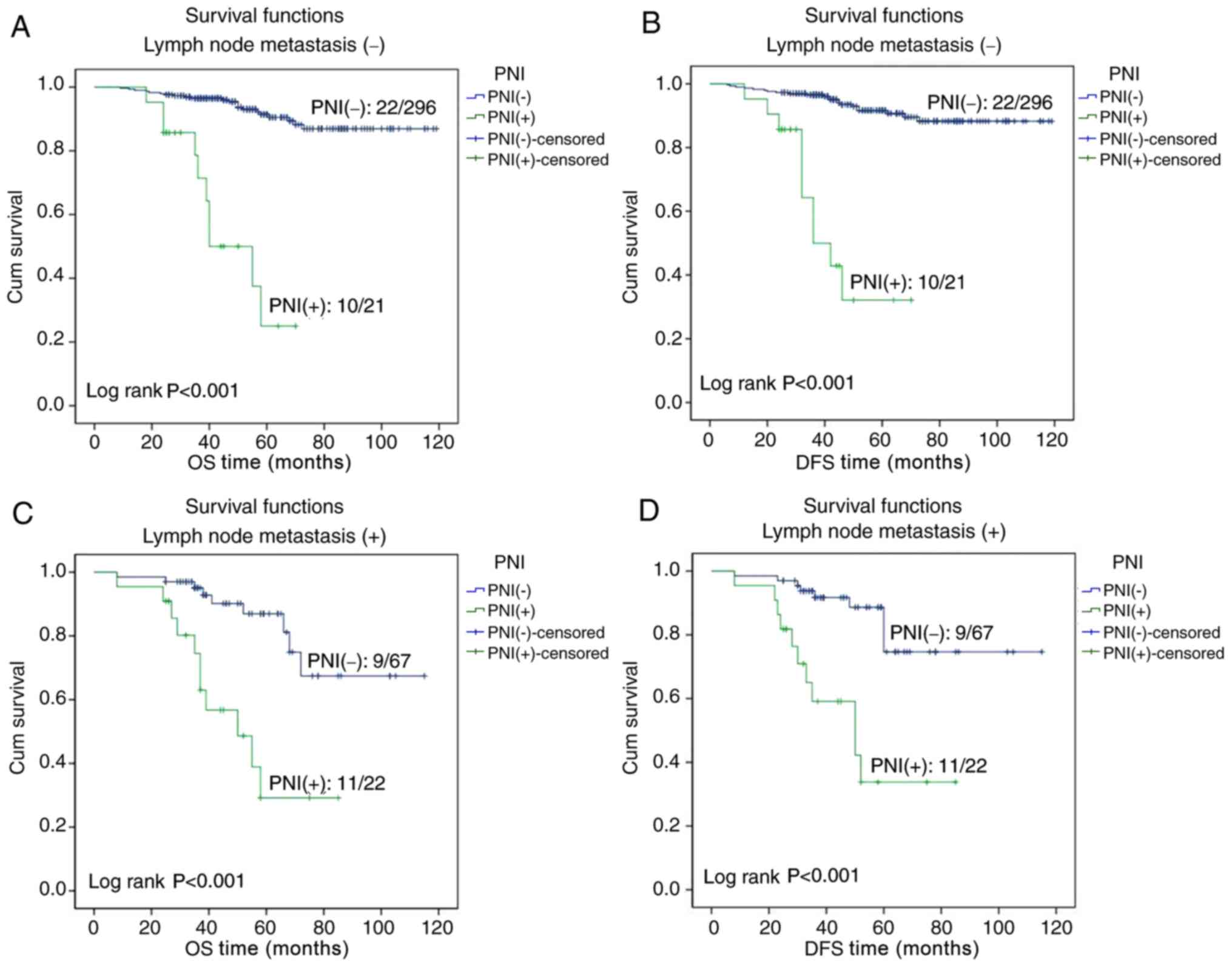
categories of tumor size (all P<0.001; Fig. 3). A survival analysis was also
performed for patients with or without lymph node metastasis, and
the Kaplan-Meier curves demonstrated that the OS and DFS times were
significantly less for PNI-positive compared with PNI-negative
patients, regardless of the lymph node metastasis status (all
P<0.001; Fig. 4).
A Kaplan-Meier survival analysis was used to
calculate the 3- and 5-year survival rates of patients, and
revealed that the 3- and 5-year OS rate for patients with PNI were
significantly lower compared with for patients without PNI (73.0
vs. 95.9, and 27.2 vs. 90.0%, respectively; P<0.001); the 3- and
5-year DFS rates for patients with PNI were also significantly less
compared with for patients without PNI (55.5 vs. 95.7, and 32.4 vs.
88.9%, respectively; P<0.001).
Univariate and multivariate analyses
of prognosis
The univariate Cox regression analysis revealed that
the OS time in patients with early cervical cancer was
significantly associated with tumor size (P=0.006), lymph node
metastasis (P=0.001), depth of cervical invasion (P=0.029) and PNI
(P<0.001), but was not associated with age (P=0.093),
hypertension (P=0.461), diabetes (P=0.754), clinical stage
(P=0.531), histological type (P=0.347), surgical margin (P=0.050)
or vascular invasion (P=0.244). The multivariate Cox regression
analysis revealed that age, clinical stage, tumor size and PNI were
independent risk factors for the OS time (P=0.004, P=0.007,
P=0.001, P<0.001, respectively) (Table II).
 | Table II.Univariate and multivariate survival
analyses for independent risk factors for overall survival
time. |
Table II.
Univariate and multivariate survival
analyses for independent risk factors for overall survival
time.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Age | 1.024 | 0.996–1.053 | 0.093 | 1.047 | 1.015–1.081 | 0.004 |
| Hypertension | 1.328 | 0.625–2.821 | 0.461 | 0.698 | 0.290–1.680 | 0.423 |
| Diabetes | 0.830 | 0.258–2.664 | 0.754 | 0.417 | 0.121–1.437 | 0.166 |
| Clinical stage | 0.923 | 0.717–1.187 | 0.531 | 0.653 | 0.479–0.890 | 0.007 |
| Histological
type | 1.314 | 0.743–2.322 | 0.347 | 1.553 | 0.847–2.849 | 0.155 |
| Tumor size | 2.144 | 1.244–3.698 | 0.006 | 2.832 | 1.538–5.218 | 0.001 |
| Lymph node
metastasis | 2.636 | 1.505–4.615 | 0.001 | 1.602 | 0.862–2.977 | 0.136 |
| Depth of cervical
invasion | 3.149 | 1.123–8.831 | 0.029 | 0.638 | 0.338–1.206 | 0.167 |
| Surgical
margin | 7.431 | 0.998–55.354 | 0.050 | 1.789 | 0.227–14.071 | 0.580 |
| Vascular
invasion | 1.390 | 0.798–2.419 | 0.244 | 0.762 | 0.401–1.446 | 0.406 |
| PNI | 9.267 | 5.266–16.307 | <0.001 | 14.621 | 6.974–30.652 | <0.001 |
With regard to the DFS time in these patients, the
univariate analysis revealed that it was significantly associated
with tumor size (P=0.008), lymph node metastasis (P=0.001), depth
of cervical invasion (P=0.026) and PNI (P<0.001), but not with
age (P=0.089), hypertension (P=0.442), diabetes (P=0.768), clinical
stage (P=0.553), histological type (P=0.392), surgical margin
(P=0.073) or vascular invasion (P=0.242). The multivariate analysis
revealed that age, clinical stage, tumor size and PNI were
independent risk factors for the DFS time (P=0.005, P=0.008,
P=0.001 and P<0.001, respectively) (Table III).
 | Table III.Univariate and multivariate survival
analyses for independent risk factor for disease-free survival
time. |
Table III.
Univariate and multivariate survival
analyses for independent risk factor for disease-free survival
time.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Age | 1.025 | 0.996–1.054 | 0.089 | 1.047 | 1.014–1.081 | 0.005 |
| Hypertension | 1.344 | 0.633–2.855 | 0.442 | 0.693 | 0.288–1.664 | 0.411 |
| Diabetes | 0.839 | 0.261–2.695 | 0.768 | 0.386 | 0.111–1.345 | 0.135 |
| Clinical stage | 0.927 | 0.720–1.192 | 0.553 | 0.654 | 0.478–0.895 | 0.008 |
| Histological
type | 1.285 | 0.724–2.282 | 0.392 | 1.521 | 0.826–2.803 | 0.178 |
| Tumor size | 2.084 | 1.209–3.592 | 0.008 | 2.734 | 1.479–5.052 | 0.001 |
| Lymph node
metastasis | 2.592 | 1.481–4.539 | 0.001 | 1.634 | 0.880–3.034 | 0.120 |
| Depth of cervical
invasion | 3.237 | 1.149–9.125 | 0.026 | 0.633 | 0.336–1.192 | 0.157 |
| Surgical
margin | 6.227 | 0.845–45.897 | 0.073 | 1.455 | 0.188–11.273 | 0.720 |
| Vascular
invasion | 1.393 | 0.800–2.424 | 0.242 | 0.746 | 0.395–1.411 | 0.368 |
| PNI | 9.273 | 5.269–16.321 | <0.001 | 14.923 | 7.147–31.162 | <0.001 |
Discussion
Around the nerve fibers, tumor cells can invade into
the epineurium, perineurium or endoneurium, and the phenomenon of
local invasion and metastasis along its extension is known as PNI.
The pathological characteristics of PNI are the existence of tumor
cells close to the nerve and surrounding >33% of the nerve
circumference, or in any layer of epineurium, perineurium or
endoneurium; this is defined as PNI-positivity (13).
A previous study has suggested that axonal migration
is a key factor in the occurrence of PNI; nerve growth factor
(NGF), brain-derived nerve growth factor (BDNF), and neurotropic
factors 3 and 4 are all neurotropins that are important in the
process of axonal growth (14). The
two primary NGF receptors are the neurotrophic receptor tyrosine
kinase (Trk) family (containing high-affinity receptors), and the
tumor necrosis factor receptor p75 (low-affinity). A number of
studies have demonstrated that the expression of NGF and its
receptors TrkA and p75 in pancreatic cancer cells and their
peripheral nerve cells increased, indicating that the expression of
NGF and its receptors TrkA and p75 in these tissues is associated
with the occurrence of PNI (15,16).
Regarding other nerve growth factors, an increase in the expression
of BDNF can promote the occurrence of PNI, and the proliferation
and invasion of tumor cells are associated with the high expression
of BDNF (12). TrkB is a BDNF
receptor in metastatic pancreatic ductal carcinoma, and an increase
in its expression is associated with the presence of PNI (12). A number of malignant tumors possess a
mechanism that involves PNI, but the number of studies on the
molecular mechanisms associated with PNI and cervical cancer is
relatively small, such that the phenomenon requires further
study.
Controversy remains as to whether PNI may be used as
a prognostic indicator of cervical cancer. Memarzadeh et al
(17) first conducted a clinical
study on PNI in cervical cancer parametrial tissue, and the results
revealed that high-risk factors for recurrence include large tumors
(>4 cm), PNI in the parametrial tissue, depth of cervical matrix
infiltration (>2/3) and lymphatic vessel invasion, whereas
survival regression analysis demonstrated that PNI can be a
prognostic risk factor for patients with early cervical cancer.
Ozan et al (18) reported
results on 36 IBl/IB2 stage postoperative cervical cancer cases,
and revealed that the recurrence and mortality rates of the
patients was not associated with PNI in the uterus. However, a
χ2 analysis demonstrated that PNI was associated with
risk factors such as vaginal and lymphatic invasion, suggesting
that PNI is an important factor affecting the prognosis of patients
with cervical cancer. A clinical study on PNI in patients with
cervical cancer indicated that the 5-year OS time in PNI-positive
patients was significantly decreased (8). The clinical stage of the tumor, pelvic
lymph node positivity, tumor grade and PNI were all revealed to be
independent prognostic factors affecting the OS time. However,
ElSahwi et al (9) recorded the
pathological characteristics of 192 patients with cervical cancer
and revealed that PNI was associated with the tumor size and
staging, but not with the recurrence or the survival rate. In 2013,
Cho et al (19) used a
retrospective analysis to evaluate whether PNI can be used as a
risk factor for selecting an adjuvant therapy, and demonstrated
that the occurrence of lymph node metastasis in PNI-positive
patients was significantly increased, and that the 5-year survival
rate was relatively lower in PNI-positive patients; this implied
that PNI is likely to be a novel risk factor for predicting cancer
recurrence. The results of the present study indicated that the
occurrence of PNI is associated with hypertension, lymph node
metastasis, depth of cervical invasion, surgical margin and
vascular invasion, suggesting that PNI is associated with the risk
factors that affect prognosis. The OS and DFS time of patients with
PNI were significantly decreased compared with those of patients
without PNI, indicating that PNI-positivity affects the survival
rate of the patient. Univariate and multivariate survival analyses
revealed that PNI is an important independent prognostic factor,
whereas the multivariate analysis indicated that age, clinical
stage and tumor size are also independent risk factors for OS and
DFS times. Collectively, these results suggest that the
aforementioned factors should be regarded more seriously.
If there is a high-risk pathological factor,
including lymph node metastasis, surgical margin, and parametrial
invasion, patients with early cervical cancer are advised to seek
follow-up treatment. Any case presenting with a high-risk factor is
recommended for postoperative pelvic irradiation with simultaneous
chemotherapy of cisplatin, and brachytherapy of the vagina. At
present, it is generally accepted that a parametrial invasion is an
indicator of poor prognosis, and its pathological description
necessitates treatment, but the existence of PNI is often ignored.
A number of studies have indicated that PNI is associated with
certain risk factors, as PNI-positive patients have a relatively
poor prognosis, indicating that PNI is a prognostic factor for
decreased survival time (8,18,19).
However, PNI, as a novel risk factor for cervical cancer, may
provide a guiding influence as to whether postoperative patients
should select adjuvant therapy. Therefore, further studies on PNI
are required, investigating its mechanism of action and clarifying
its prognostic significance, so as to allow it to receive the
appropriate attention in order to decrease the recurrence and
mortality rates in cervical cancer, and improve the DFS and OS
times of the patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Maternal and Child Health Research Project of Wuxi Municipal Health
and Family Planning Commission (grant no. FYKT201703) and the Wuxi
Key Medical Talents Cultivation Project (grant no. ZDRCPY014).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YW and MT designed the study. MT designed the
experiments and performed the majority of them. QL and JY were
responsible for patient's follow-up. LC and XY collected clinical
samples. XQ and JY evaluated the tissue sections. MT and YW wrote
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent
prior to participating in the study. The study was approved by the
Ethical Committee of the Affiliated Hospital of Jiangnan
University.
Patient consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PNI
|
perineural invasion
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
LVSI
|
lymph vascular space invasion
|
|
NGF
|
nerve growth factor
|
|
BDNF
|
brain-derived nerve growth factor
|
References
|
1
|
Zhou H, Liu Z and Lin ZQ: 2017 NCCN
Cervical Cancer Clinical Practice Guideline Interpretation. Chin J
Practical Gynecol Obstetrics. 1:100–107. 2017.(In Chinese).
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynecol
Obstet. 105:103–104. 2009. View Article : Google Scholar
|
|
3
|
Hassan MO and Maksem J: The prostatic
perineural space and its relation to tumor spread: An
ultrastructural study. Am J Surg Pathol. 4:143–148. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsson Y: Microenvironment of the
peripheral nervous system under normal and pathological conditions.
Crit Rev Neurobiol. 5:265–311. 1990.PubMed/NCBI
|
|
5
|
Cruveilheir J: Maladies des nerfs anatomic
pathologique ducorps humain 2nd edition. Paris, JB Bailliere. 3–8.
1835.(In French).
|
|
6
|
Cui L, Shi Y and Zhang GN: Perineural
invasion as a prognostic factor for cervical cancer: A systematic
review and meta-analysis. Arch Gynecol Obstet. 292:13–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang GN and Cui L: Cervical cancer
peripheral nerve invasion: Should pay attention to the pathological
prognostic factors. Cancer Prevention Treatment. 26:183–186.
2013.
|
|
8
|
Horn LC, Meinel A, Fischer U, Bilek K and
Hentschel B: Perineural invasion in carcinoma of the cervix
uteri-prognostic impact. J Cancer Res Clin Oncol. 136:1557–1562.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
ElSahwi KS, Barber E, Illuzzi J, Buza N,
Ratner E, Silasi DA, Santin AD, Azodi M, Schwartz PE and Rutherford
TJ: The significance of perineural invasion in early-stage cervical
cancer. Gynecol Oncol. 123:561–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Querleu D, Cibula D and Abu-Rustum NR:
2017 Update on the Querleu-morrow classification of radical
hysterectomy. Ann Surg Oncol. 24:3406–3412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luna LG: Hematoxylin and eosin staining
problems and solutions. J Histotechnol. 6:162013. View Article : Google Scholar
|
|
12
|
Liebig C, Ayala G, Wilks JA, Berger DH and
Albo D: Perineural invasion in cancer: A review of the literature.
Cancer. 115:3379–3391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marchesi F, Piemonti L, Mantovani A and
Allavena P: Molecular mechanisms of perineural invasion, a
forgotten pathway of dissemination and metastasis. Cytokine Growth
Factor Rev. 21:77–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liebig C, Ayala G, Wilks J, Verstovsek G,
Liu H, Agarwal N, Berger DH and Albo D: Perineural invasion is an
independent predictor of outcome in colorectal cancer. J Clin
Oncol. 27:5131–5137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Dang C, Ma Q, Chen W and Nagata
K: Predictors of systemic chemotherapy contraindication in
pancreatic cancer patients with distant metastasis.
Hepatogastroenterology. 54:254–259. 2007.PubMed/NCBI
|
|
16
|
Wood JN: Nerve growth factor and pain. N
Engl J Med. 363:1572–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Memarzadeh S, Natarajan S, Dandade DP,
Ostrzega N, Saber PA, Busuttil A, Lentz SE and Berek JS:
Lymphovascular and perineural invasion in the parametria: A
prognostic factor for early-stage cervical cancer. Obstet Gynecol.
102:612–619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozan H, Ozuysal S and Ediz B: Perineural
invasion in early-stage cervical carcinoma. Eur J Gynaecol Oncol.
30:379–383. 2009.PubMed/NCBI
|
|
19
|
Cho HC, Kim H, Cho HY, Kim K, No JH and
Kim YB: Prognostic significance of perineural invasion in cervical
cancer. Int J Gynecol Pathol. 32:228–233. 2013. View Article : Google Scholar : PubMed/NCBI
|