Introduction
Hepatoblastoma (HB) is the most common malignant
liver tumor in children under 3 years of age (1). Epidemiological data indicate that the
incidence of HB has increased in European countries, Japan and the
USA in the last 30 years (2). HB is
usually associated with constitutional genetic abnormalities,
malformations and familial cancer syndromes (3,4). Overall
survival rates of patients with HB was relatively low (20–30%) a
few decades ago; however, the survival rates of these patients has
increased to 70–80% because of the use of neoadjuvant and adjuvant
chemotherapy (2). Thus, novel
chemotherapeutic treatments are critical for HB.
Cyclin-dependent kinase (CDK) inhibitors induce cell
cycle arrest and promote apoptosis of tumor cells (5). Among all small-molecule CDK inhibitors,
alsterpaullone (Alp;
9-nitro-7,12-dihydroindolo[3,2-d][1]-benzazepin-6(5H)-one;
Fig. 1A) is a paullone derivative
confirmed to have increased potency in enzymatic and
anti-proliferative assays compared with other derivatives (6,7). Alp
inhibits a variety of tumors (5,8–11) and it selectively inhibits CDK1,
resulting in cell cycle arrest at G2/M phase (7,12). In
addition, Alp can activate caspase-9 via perturbation of the
mitochondrial membrane potential, thereby inducing apoptosis in
cancer cells (8,9,13). Studies
indicate that Alp also inhibits glycogen synthase kinase-3β
(14–16).
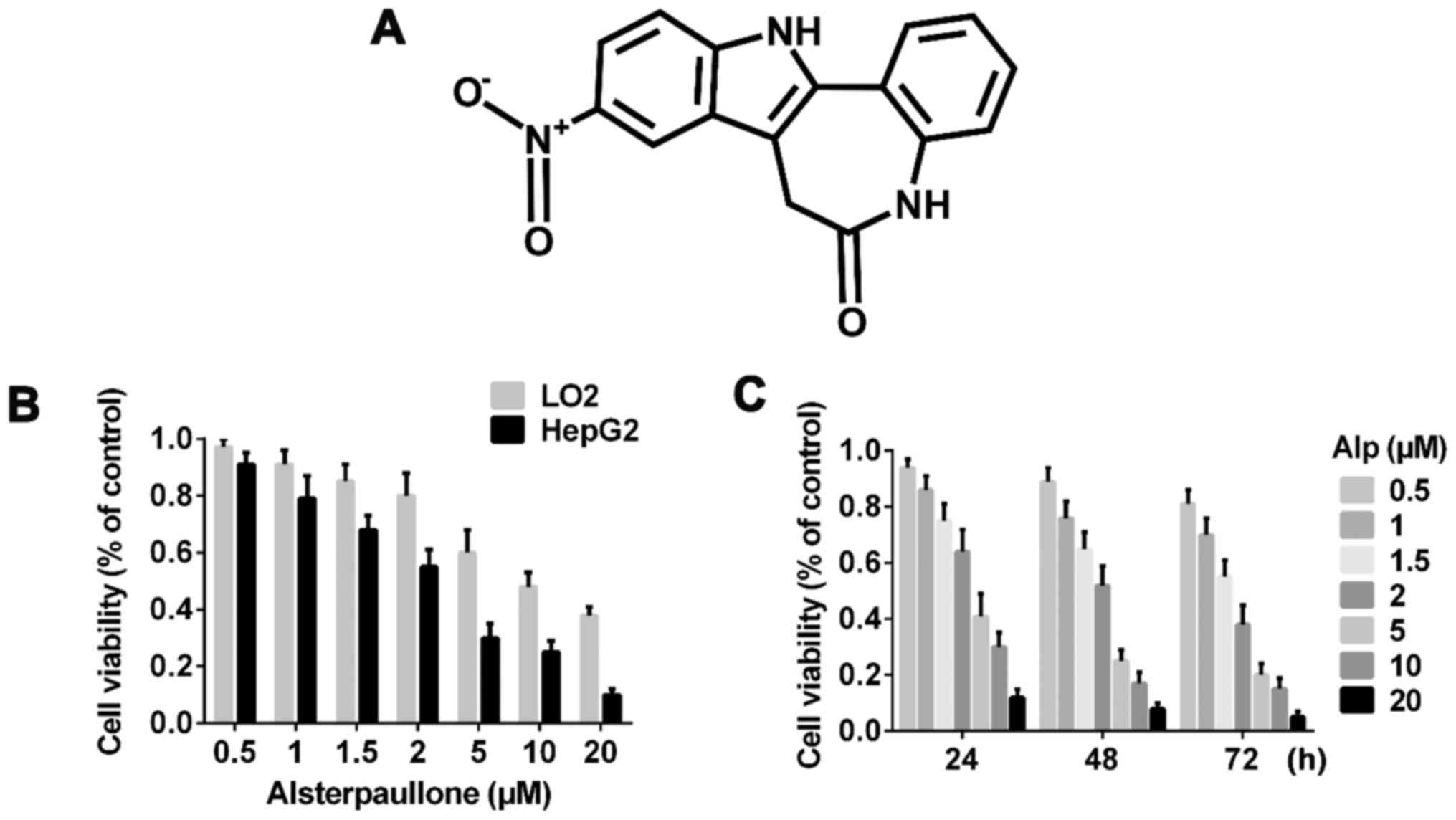 | Figure 1.Alp time- and dose-dependently
inhibits the proliferation of LO2 and HepG2 cells. (A) Chemical
structure of Alp. (B) Viability analysis of LO2 and HepG2 cells
incubated with various concentrations of Alp for 24 h, as assayed
using a Cell Counting kit-8 assay. (C) Viability of HepG2 cells
incubated with 0, 0.5, 1, 1.5, 2, 5, 10 or 20 µM Alp for 24, 48 or
72 h. The half-maximal inhibitory concentration of Alp for HepG2
cells was determined to be 3.095, 2.333 and 1.681 µM at 24, 48 and
72 h, respectively. Results are presented as the mean ± standard
deviation (n=3). Results for each concentration and time point are
significantly different (P<0.05) from those for all other
concentrations and time points. Alp, alsterpaullone. |
To the best of our knowledge, the present study is
the first to investigate the effect of Alp in the HB HepG2 cell
line in an in vivo and in vitro model, and it was
identified that Alp inhibited HepG2 cell proliferation via
apoptosis and downregulated B-cell lymphoma 2 (Bcl-2), upregulated
Bcl-2-associated X protein (Bax) and p38 mitogen-activated protein
kinase (p38MAPK), and activated caspase-3 and −9. Thus,
Alp may be useful for treating HB.
Materials and methods
Animals
A total of 27 male nude mice (4–6-week-old, 18–20 g)
were purchased from the Experimental Animal Center of The Second
Military Medical University (Shanghai, China) and housed in a
continuous air laminar chamber in a specific pathogen-free animal
facility in the Institute of Immunology of The Second Military
Medical University with the following conditions: 28°C, normal
pressure, humidity of 40–60%, and 10 h of light and 14 h without
light daily. All animals had free access to water and food. All
treatments complied with the animal welfare guidelines of Chinese
Official Guiding Opinions on the Treatment of Experimental Animals
and the Secondary Military Medical University. Experimental
protocols for animal experiments were approved by the ethics
committee of The Secondary Military Medical University.
Chemicals and reagents
Alp (purity 99%) was dissolved in dimethylsulfoxide
(DMSO; both Sigma Chemical Co.; Merck KGaA, Darmstadt, Germany) to
concentrations of 10 µM and 1 mg/ml and stored separately at 4°C.
The final concentration of DMSO in experimental culture medium was
consistently <0.1%. A Cell Counting kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). A Fluorescein Isothiocyanate (FITC)-Annexin
V/Propidium Iodide (PI) Apoptosis Detection kit and Hoechst
33342/PI were purchased from BD Biosciences (Franklin Lakes, NJ,
USA) and Signalway Antibody LLC (College Park, MD, USA). Antibodies
against cleaved caspase-3 (cat. no. sc-271759) and caspase-9 (cat.
no. sc-133109) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), antibodies against Bcl-2 (cat. no. BS1511), p38
(cat. no. BS3566), p-p38 (cat. no. BS4635) and Bax (cat. no.
BS6420) were from Bioworld Technology, Inc. (St. Louis Park, MN,
USA) and antibody against GAPDH (cat. no. SAB2108266) was from
Sigma Chemical Co.; Merck KGaA. SB202190 (a p38-specific inhibitor;
cat. no. 559388) was from EMD Millipore, Billerica, MA, USA. Cell
lysis buffer was from Cell Signaling Technology, Inc. (Danvers, MA,
USA), Protease Inhibitor Cocktail Tablets (cat. no. 617586) were
from EMD Millipore and a Pierce™ Bicinchoninic Acid (BCA) Protein
assay kit was from Thermo Fisher Scientific, Inc.
In vitro cell culture
A normal liver LO2 cell line and an HB HepG2 cell
line were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). LO2 and HepG2 cells were cultured in
DMEM containing 10% FBS and incubated at 37°C under 5%
CO2.
CCK-8 assay
To determine the cytotoxicity of Alp towards the two
cell lines at various concentrations for various durations of
treatment, a CCK-8 kit was used, according to the manufacturer's
protocol. Briefly, each well of 96-well plate was seeded with
5×103 cells and cells were cultured until they reached
70–80% confluence. Alp (0, 0.5, 1, 1.5, 2, 5, 10 or 20 µM) was
applied to cells and controls were treated with DMEM containing 10%
FBS and 0.1% DMSO. Cells were incubated for 24, 48 or 72 h, and 10
µl CCK-8 solution was added to each well prior to incubation at
37°C for 40 min. Sample absorbance was determined at 450 nm using
an Epoch microplate spectrophotometer (BioTek Instruments, Inc.,
Winooski, VT, USA) and the half-maximal inhibitory concentrations
(IC50) were calculated.
Fluorescence staining
Hoechst 33342/PI double staining was performed to
measure cytotoxicity of Alp in the two cell types. Cells
(1×105 cells/well) were seeded on individual coverslips
and incubated in 6-well plates. DMSO (0.1%) or Alp (1, 4 or 8 µM)
was added to wells prior to incubation at 37°C for 24 h. The
protocol was carried out as described previously (17). Cells for each treatment were stained
with Hoechst 33342 dye for 20 min and subsequently stained with PI
for 15 min. Following three PBS washes, cells on coverslips were
mounted on glass slides and observed under a fluorescence
microscope (magnification, ×200).
Annexin V/PI double staining
To determine the effect of Alp on HepG2 cell
viability, Alp (2, 4 or 8 µM) was applied to HepG2 cells for 24 h
and 0.1% DMSO was used for controls. An FITC-Annexin V/PI Apoptosis
Detection kit was used to determine levels of apoptosis. HepG2
cells (2×105 cells/well) were seeded in individual wells
of 6-well plates and treated as aforementioned. Cells were
harvested and 100 µl 1X binding buffer was used to resuspend cells,
followed by labeling with 5 µl FITC/annexin V and 5 µl PI at room
temperature for 15 min in the dark. To each sample, 400 µl 1X
binding buffer was added prior to analysis by flow cytometry
(Accuri™ C6 instrument; BD Biosciences).
Colony formation assay
Colony formation is the basic ability of tumor cells
(18). A cell colony formation assay
was performed on HepG2 cells to verify the effect of Alp on HB
cells. HepG2 cells were plated into 6 cm plates at 5×102
cells/well and were incubated at 37°C for 24 h. The culture medium
was removed, and fresh DMEM containing 10% FBS and 0.1% DMSO, or 2,
4 or 8 µM Alp was added. After 24 h of incubation at 37°C, the
medium was replaced with DMEM containing 10% FBS without DMSO or
Alp. After 14 days of incubation at 37°C, cells were washed twice
with PBS, fixed in 2 ml 4% paraformaldehyde for 30 min, stained
with 1 ml 0.1% crystal violet for 20 min and washed three times
with double-distilled water. The images of the plates were captured
using a digital camera, and colonies with <50 cells were
discounted. Each experiment was performed three times.
Western immunoblotting
HepG2 cells were treated with 0.1% DMSO and various
concentrations (2, 4 or 8 µM) of Alp for 24 h. The cells were
washed twice with ice-cold PBS and lysed with cell lysis buffer
supplemented with protease inhibitor. Protein concentrations were
determined using BCA assays. Equal amounts (30 µg) of protein from
each sample were separated by SDS-PAGE (10% polyacrylamide gels),
transferred electrophoretically onto a nitrocellulose membrane, and
incubated in blocking buffer containing 5% bovine serum albumin
(Bio-Light Co., Ltd., Shanghai, China) in TBST for 1 h at room
temperature. Nitrocellulose membranes were incubated with primary
antibody overnight at 4°C, washed and incubated with secondary
antibody for 120 min at room temperature. Both
peroxidase-conjugated AffiniPure goat anti-rabbit (cat. no.
BS13278) and peroxidase-conjugated AffiniPure goat anti-mouse (cat.
no. BS12478) secondary antibodies were purchased from Bioworld
Technology, Inc. (St. Louis Park, MN, USA). Proteins were detected
using chemiluminescence (Tanon Science and Technology Co., Ltd.,
Shanghai, China). The following antibody dilutions were used:
Anti-GAPDH at 1:2,000; anti-cleaved caspase-3 and −9 at 1:200;
anti-Bcl-2, -Bax, -p38 and -phospho (p)-p38 at 1:1,000. Both
secondary antibodies of rat and mouse were used at 1:2,000. HepG2
cells were pretreated (or not) with 25 µM SB202190 for 45 min prior
to treatment (or not) with 8 µM Alp for 24 h. Results were analyzed
and quantified using ImageJ (version 1.49v; National Institutes of
Health, Bethesda, MD, USA).
In vivo tumorigenic experiments
To verify the effect of Alp on HepG2 cells in an
in vivo model, a tumorigenic animal model was established
using male nude mice. To establish experimental animals,
5×106 HepG2 cells were injected (subcutaneously) singly
into the backs of nude mice and tumors were allowed to reach ~3×3
mm. The optimal Alp dose was 3 mg/kg according to the results of
the pre experiment (Alp dose 0, 0.5, 1, 1.5, 3, 5 mg/kg) and this
was administered to animals (intraperitoneally) once daily for 2
weeks. Nude control mice were given the same volume of 40%
propylene glycol (Sigma Chemical Co.; Merck KGaA). In total, 9 mice
were used in each group. At the end of the experimental period,
animals were sacrificed using CO2 (the flow rate was 250
ml/min and the volume of the cage was 1,000 cm3) and
tumors were collected and analyzed.
Statistical analysis
GraphPad Prism software (version 6.0; GraphPad
Software, San Diego, CA, USA) was used for statistical analyses and
figure preparation. Experimental data are presented as the mean ±
standard deviation. Student's t-test was used to compare two groups
and analysis of variance followed by a Dunnett's multiple
comparisons test was used when more than three groups were
analyzed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity of Alp in LO2 and HepG2
cells
Fig. 1B indicates that
Alp exhibited significantly less inhibitory effect on LO2 normal
liver cells compared with on HepG2 HB cells and these inhibitory
effects were dose- and time-dependent. Fig. 1C presents the half-maximal inhibitory
concentration data for 24, 48 and 72 h of incubation with Alp.
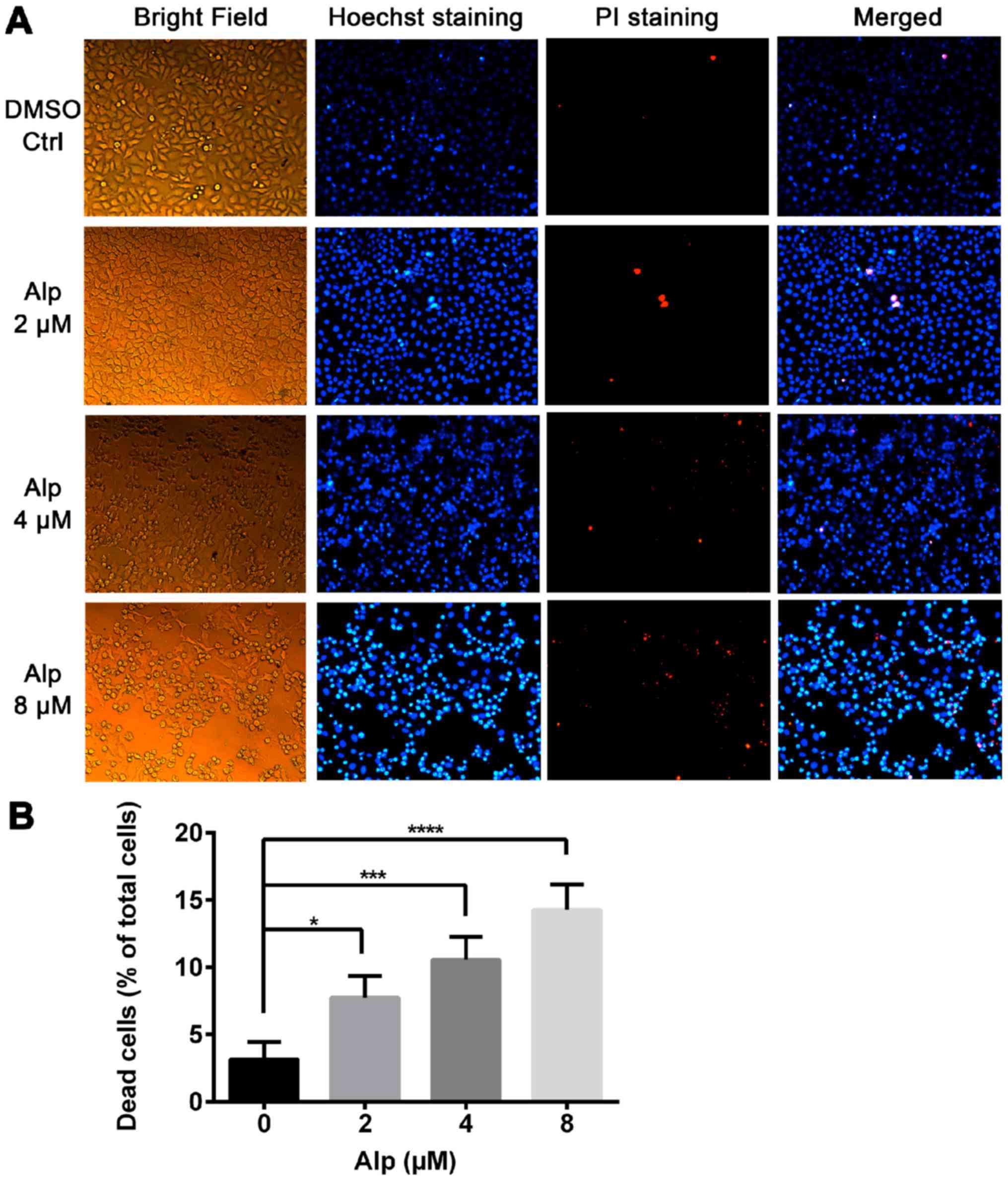
Fig. 2A indicates that normal nuclei
fluoresced blue following Hoechst 33342 staining and dead cells
fluoresced red following PI staining. Cell morphology was altered
with increasing Alp concentration. Alp significantly increased
HepG2 cell death in a dose-dependent manner (Fig. 2B). Annexin V and PI double staining
data presented in Fig. 3 indicated
that that proportion of apoptotic HepG2 cells increased
significantly with increasing Alp concentration, to a maximum at 8
µM Alp (P<0.05).
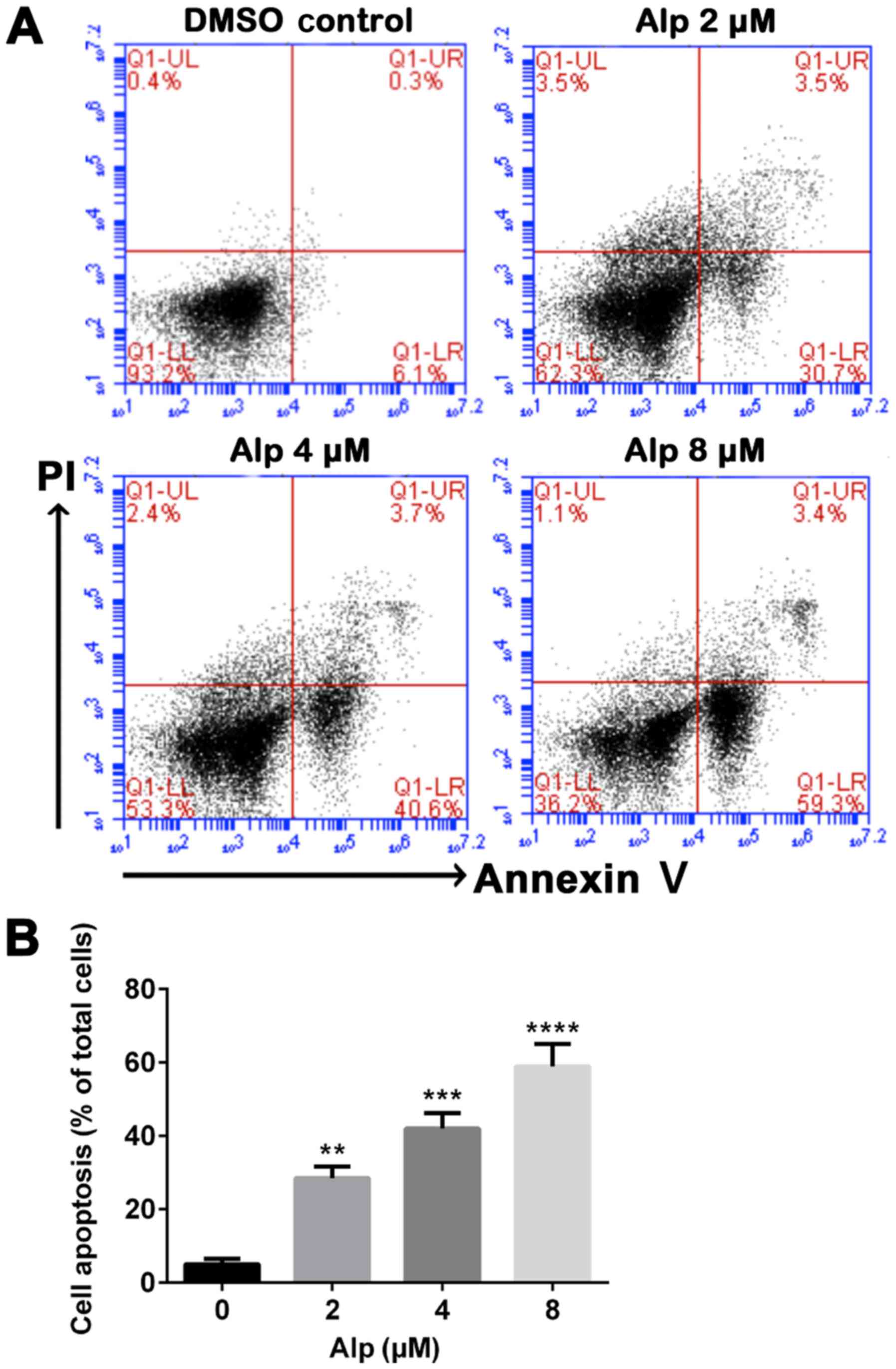 | Figure 3.Alp induces dose-dependent apoptosis
of HepG2 cells. (A) HepG2 cells were incubated with increasing
concentrations of Alp for 24 h, prior to analysis of cell apoptosis
by flow cytometry. (B) Quantification of apoptotic HepG2 cells
after 24 h of treatment with Alp at various concentrations. Results
are presented as the mean ± standard deviation for at least three
independent experiments. **P<0.01, ***P<0.001,
****P<0.0001 vs. control. Alp, alsterpaullone; DMSO,
dimethylsulfoxide; PI, propidium iodide; UL, upper left; UR, upper
right; LL, lower left; LR, lower right; Q, quadrant. |
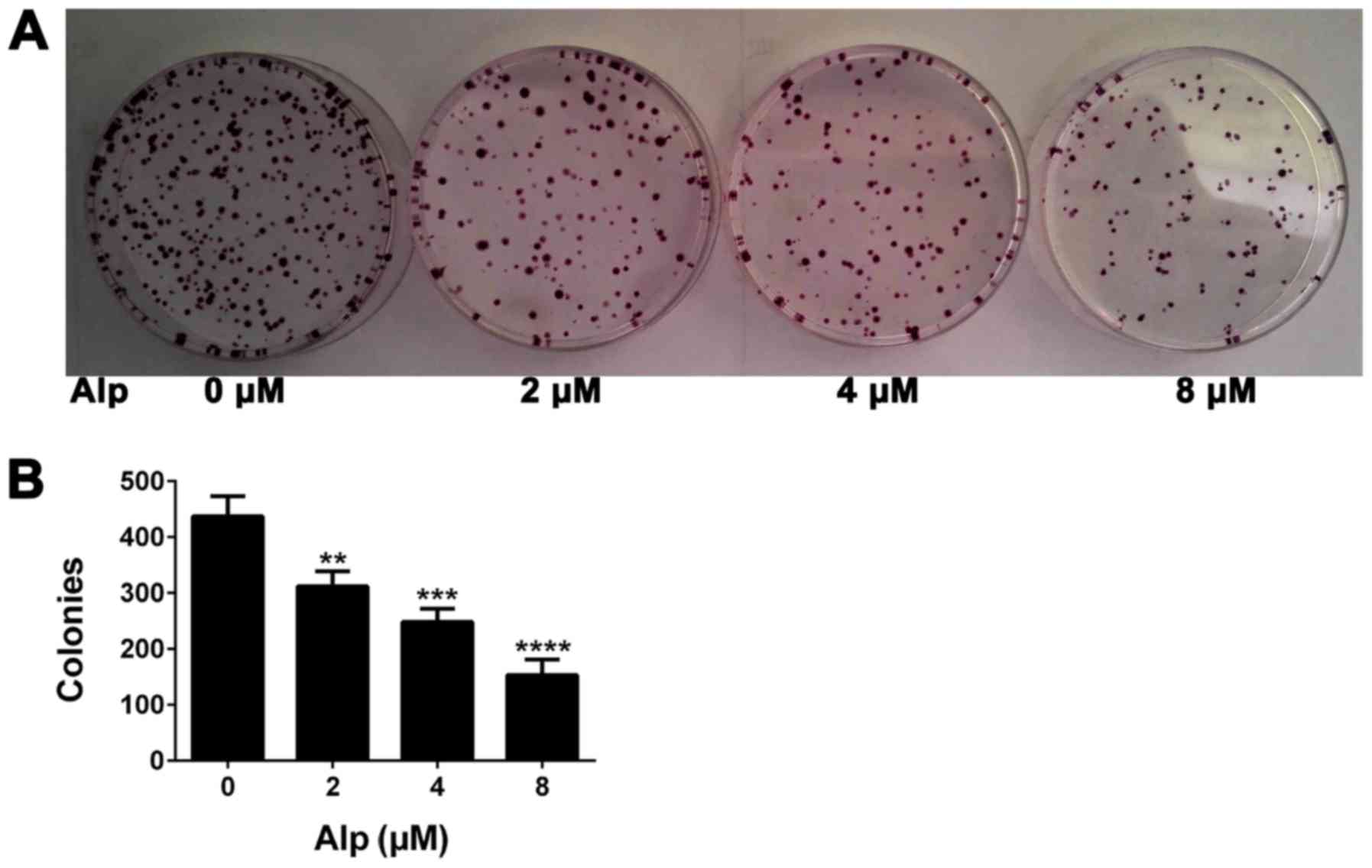
Alp decreases colony formation in
HepG2 cells
Fig. 4 presents the
results of a colony formation assay. The number of colonies of
HepG2 cells treated with Alp at concentrations of 0, 2, 4 and 8 µM
was 437±36, 312±27, 248±24 and 153±28, respectively, representing a
significant decrease with increasing concentrations of Alp
(P<0.05).
Alp induces the activation of
caspase-3 and −9 in HepG2 cells
To elucidate whether Alp-induced apoptosis was
associated with caspase-3 or −9, levels of these enzymes were
determined and, as presented in Fig.
5, caspase-3 and −9 cleavage in HepG2 cells was dose-dependent.
Cleaved caspase-3 and −9 expression increased in HepG2 cells
following 8 µM Alp treatment. Bax expression also increased,
whereas Bcl-2 expression decreased following Alp treatment.
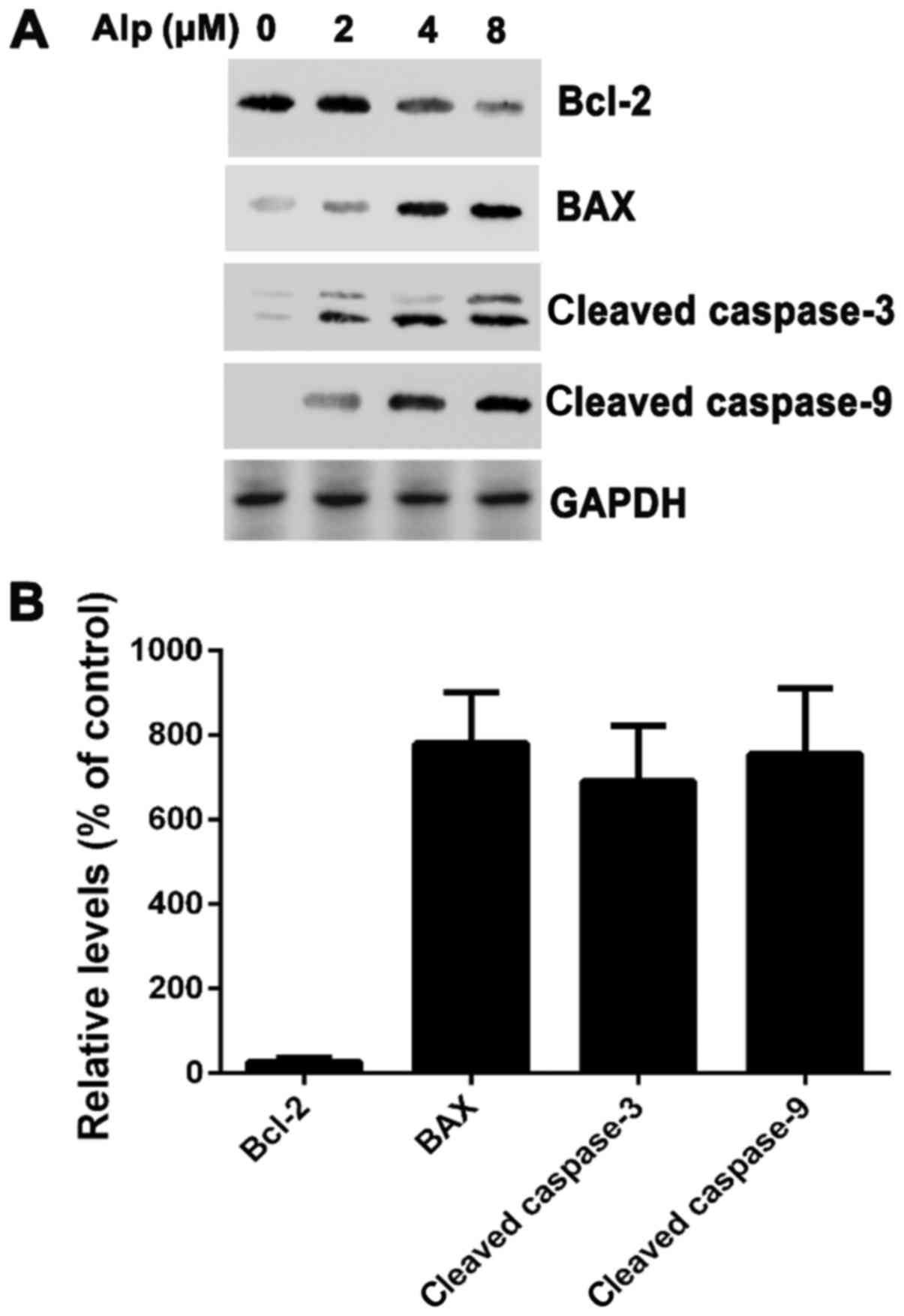 | Figure 5.(A) HepG2 cells were treated with 2, 4
or 8 µM Alp for 24 h and subjected to western blotting. Bcl-2 was
suppressed in Alp-treated HepG2 cells, whereas activation of
caspase-3, caspase-9 and Bax was increased. (B) Following treatment
with 8 µM Alp for 24 h, the relative expression levels of Bax,
cleaved caspase-3 and cleaved caspase-9 in HepG2 cells were
increased 7.8±1.2-, 6.9±1.3- and 7.5±1.5-fold, respectively. The
relative expression level of Bcl-2 was decreased 0.27±0.09-fold.
Results are presented as the mean ± standard deviation (n=3). Alp,
alsterpaullone; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
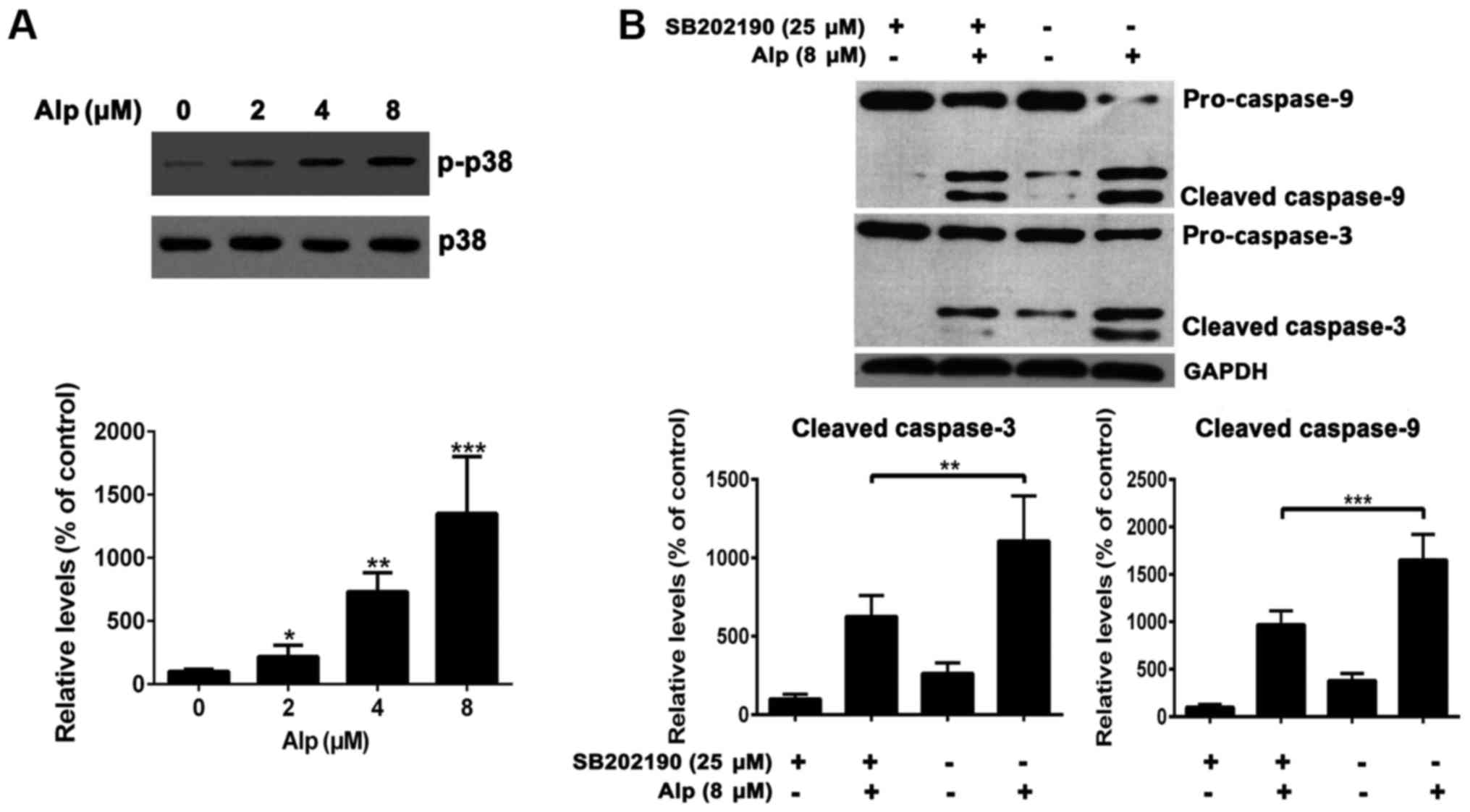
Alp induces HepG2 apoptosis via p38
pathways
Fig. 6A indicates
that, whereas total p38 expression levels in HepG2 cells were
consistent, phospho-p38 levels increased with increasing
concentrations of Alp. HepG2 cells treated with 25 µM SB202190, a
p38MAPK inhibitor, prior to 8 µM Alp treatment for 24 h
exhibited weak casapase-3 and −9 activity (Fig. 6B).
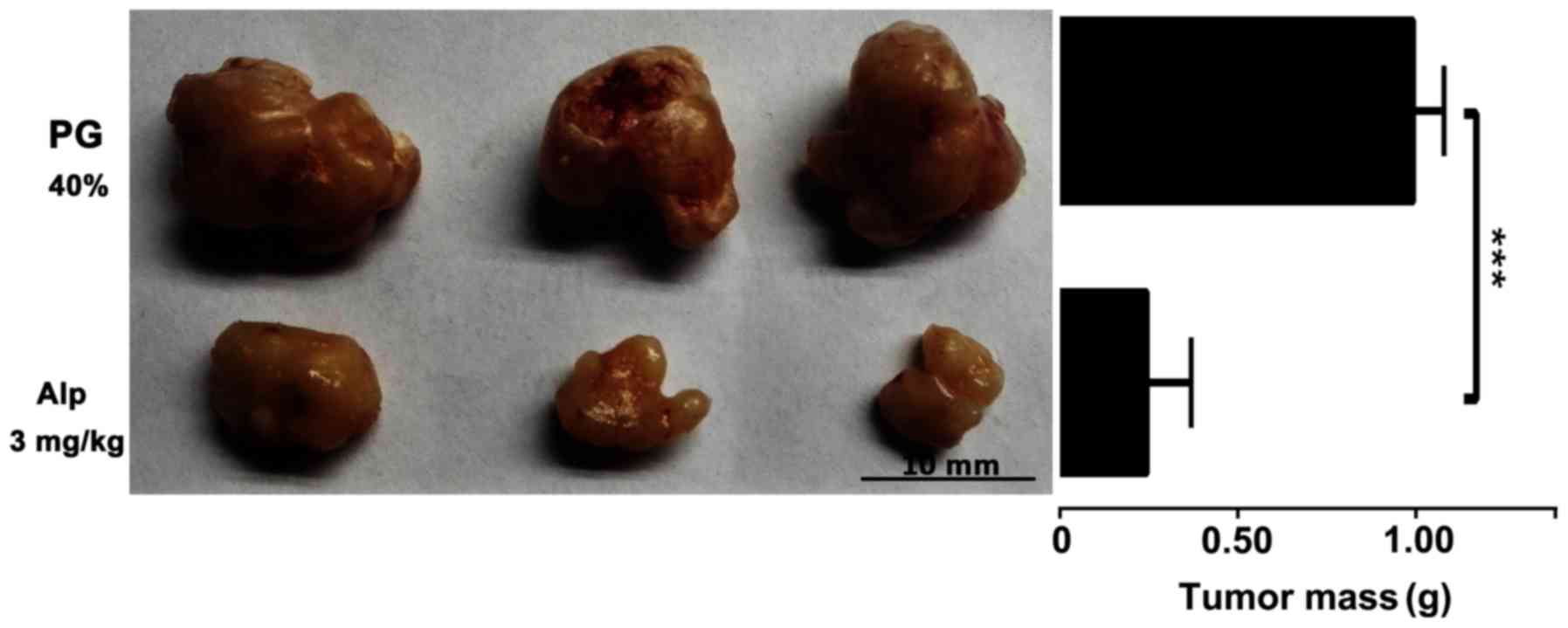
Inhibitory effect of Alp on HB
xenograft tumor growth
HB xenografts isolated from nude mice following
sacrifice were weighed and images were captured. Fig. 7 presents representative images of HB
xenografts and tumor weights from different treatment groups.
Alp-treated animals had smaller tumors compared with those of
PG-treated controls.
Discussion
HB is an embryonal tumor (3). Children with HB require complete
surgical resection, but ~50% of tumors are unresectable at
diagnosis (19). Currently, prognosis
is dismal with a low survival rate (20–30%) (18). The use of pre-operative chemotherapy,
surgical resection and post-operative chemotherapy has increased
the survival rate to 70–80% (20).
Therefore, novel chemotherapeutic drugs for HB are urgently
required. Modulation of CDKs is considered to be critical to the
treatment and prevention of human malignancies (6). Alp, a potent CDK modulator, competes
with ATP for the CDK-binding site (9,10),
suggesting promise as a novel chemotherapeutic agent. Previous
studies have identified antitumor effects of Alp in breast cancer,
leukemia cells and cervical cancer (5,8,10,11). In
the present study, HepG2 cells were selected to determine the
antitumor effects of Alp in HB. The HepG2 cell line, which has been
widely used in biological fields, was originally classified as a
cell line from hepatocellular carcinoma. Recently, HepG2 was
reidentified as a HB-derived cell line (1). The results of the present study reveal
for the first time, to the best of our knowledge, that Alp
inhibited HepG2 cells in vitro and in vivo, offering
significant cytotoxicity towards HepG2 cells in a dose- and
time-dependent manner. In a model of tumorigenicity using HepG2
cells in nude mice, Alp significantly decreased tumor weight at 3
mg/kg. These results suggested that Alp exhibits significant
antitumor effects in hepatic neoplasms.
Inhibition of cell proliferation is critical to
apoptosis and blockade of cell cycle progression. Lahusen et
al (8) first reported that Alp
induced cell cycle arrest and also apoptosis in a Jurkat cell line.
Additional studies indicated that Alp could induce apoptosis in
various cell lines (5,8,10,11), but the molecular mechanism by which
Alp induces apoptosis in HB cells is unclear. Previous results
indicate that apoptotic proteins participate in Alp-mediated HepG2
cell death, and that caspases and Bcl-2 family members may be
critical to this apoptotic process (21,22). Bcl-2
family proteins either suppress or promote apoptosis (23): Bcl-2 is an important anti-apoptotic
protein, whereas Bax belongs to a pro-apoptotic subfamily (24). It was identified in the present study
that Bax expression increased and Bcl-2 expression decreased
following Alp treatment. Furthermore, caspases are indicators of
apoptosis, and also critical regulators of initial apoptotic
processes. Among the 14 caspases identified in mammals, caspase-9
and −3 are essential for apoptosis (21,22,25).
Caspase-9, as an initiator caspase, can initiate a cascade of
increasing caspase activity by processing and activating effector
caspases (22). In addition,
caspase-3, as an effector caspase, can cleave and inactivate
certain vital cellular proteins by proteolysis (22,26). Of
note, Alp was identified to induce apoptosis by activating
caspase-9 and −3, as well as by increasing the expression of
cleaved caspase-9 and −3 in HepG2 cells.
MAPKs are involved in cell death (27), and activation of the MAPK signaling
pathway serves a central function in HB proliferation and cell
cycle progression (28). The
p38MAPK cascade is known to be involved in the apoptotic
pathway of human HB cell lines and, compared with non-tumorous
liver tissue, human HB cells have less p38MAPK activity,
suggesting that attenuation of p38MAPK activity causes
resistance to apoptosis in human HB cells. p38MAPK
activation has been identified to be associated with an apoptotic
response induced by several anticancer agents (29). However, to the best of our knowledge,
there have been no studies of the association between Alp, the MAPK
signaling pathway and HB proliferation. Thus, p38MAPK
activation was investigated in Alp-treated HepG2 cells. Results
indicate that SB202190 (a p38MAPK-specific inhibitor)
significantly inhibited the expression of caspase-3 and −9 induced
by Alp, indicating that p38MAPK is critical for
Alp-induced apoptosis in HepG2 cells.
In summary, Alp inhibits the growth of HB in
vivo and in vitro by inducing apoptosis via increasing
caspase expression and modulating Bcl-2/Bax protein expression.
Finally, the p38MAPK pathway may be involved in HB
apoptosis induced by Alp. Thus, Alp may hold promise as a novel
chemotherapeutic agent for treating HB.
Acknowledgements
The authors acknowledge Dr Meng Guo and Dr Fang Liu
of the National Key Laboratory of Medical Immunology and Institute
of Immunology (Shanghai, China) for the technical guidance of the
colony formation assay and in vivo tumorigenic
experiments.
Funding
This study was supported by the National Natural
Science Funds of China (grant no. 81471575).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GD designed the study, performed the experiments,
supervised the project, drafted the manuscript, and reviewed and
edited the final manuscript. PY collected the data, performed the
experiments, drafted the manuscript, and reviewed and edited the
final manuscript. NZ collected the data, performed data analysis,
performed the experiments and drafted the manuscript. JD performed
the experiments and data analysis, and drafted the manuscript. CX
and XZ performed data analysis and visualization of the data.
Ethics approval and consent to
participate
Experimental protocols for animal experiments were
approved by the ethics committee of The Secondary Military Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ismail H, Broniszczak D, Kaliciński P,
Dembowska-Bagińska B, Perek D, Teisseyre J, Kluge P, Kościesza A,
Lembas A and Markiewicz M: Changing treatment and outcome of
children with hepatoblastoma: Analysis of a single center
experience over the last 20 years. J Pediatr Surg. 47:1331–1339.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czauderna P, Lopez-Terrada D, Hiyama E,
Häberle B, Malogolowkin MH and Meyers RL: Hepatoblastoma state of
the art: Pathology, genetics, risk stratification, and
chemotherapy. Curr Opin Pediatr. 26:19–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finegold MJ, Lopez-Terrada DH, Bowen J,
Washington MK and Qualman SJ: College of American Pathologists:
Protocol for the examination of specimens from pediatric patients
with hepatoblastoma. Arch Pathol Lab Med. 131:520–529.
2007.PubMed/NCBI
|
|
4
|
Cohen MM Jr: Beckwith-Wiedemann syndrome:
Historical, clinicopathological, and etiopathogenetic perspectives.
Pediatr Dev Pathol. 8:287–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui C, Wang Y, Wang Y, Zhao M and Peng S:
Alsterpaullone, a cyclin-dependent kinase inhibitor, mediated
toxicity in HeLa cells through apoptosis-inducing effect. J Anal
Methods Chem. 2013:6020912013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schultz C, Link A, Leost M, Zaharevitz DW,
Gussio R, Sausville EA, Meijer L and Kunick C: Paullones, a series
of cyclin-dependent kinase inhibitors: Synthesis, evaluation of
CDK1/cyclin B inhibition, and in vitro antitumor activity. J Med
Chem. 42:2909–2919. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin CM and O'Leary JJ: Histology of
cervical intraepithelial neoplasia and the role of biomarkers. Best
Pract Res Clin Obstet Gynaecol. 25:605–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lahusen T, De Siervi A, Kunick C and
Senderowicz AM: Alsterpaullone, a novel cyclin-dependent kinase
inhibitor, induces apoptosis by activation of caspase-9 due to
perturbation in mitochondrial membrane potential. Mol Carcinog.
36:183–194. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rivest P, Renaud M and Sanderson JT:
Proliferative and androgenic effects of indirubin derivatives in
LNCaP human prostate cancer cells at sub-apoptotic concentrations.
Chem Biol Interact. 189:177–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soni DV and Jacobberger JW: Inhibition of
cdk1 by alsterpaullone and thioflavopiridol correlates with
increased transit time from mid G2 through prophase. Cell Cycle.
3:349–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faria CC, Agnihotri S, Mack SC, Golbourn
BJ, Diaz RJ, Olsen S, Bryant M, Bebenek M, Wang X, Bertrand KC, et
al: Identification of alsterpaullone as a novel small molecule
inhibitor to target group 3 medulloblastoma. Oncotarget.
6:21718–21729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takai N, Kira N, Ishii T, Nishida M, Nasu
K and Narahara H: Novel chemotherapy using histone deacetylase
inhibitors in cervical cancer. Asian Pac J Cancer Prev. 12:575–580.
2011.PubMed/NCBI
|
|
13
|
Arion VB, Dobrov A, Göschl S, Jakupec MA,
Keppler BK and Rapta P: Ruthenium- and osmium-arene-based paullones
bearing a TEMPO free-radical unit as potential anticancer drugs.
Chem Commun (Camb). 48:8559–8561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Overington JP, Al-Lazikani B and Hopkins
AL: How many drug targets are there? Nat Rev Drug Discov.
5:993–996. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imming P, Sinning C and Meyer A: Drugs,
their targets and the nature and number of drug targets. Nat Rev
Drug Discov. 5:821–834. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berman HM, Westbrook J, Feng Z, Gilliland
G, Bhat TN, Weissig H, Shindyalov IN and Bourne PE: The protein
data bank. Nucleic Acids Res. 28:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min J, Huang K, Tang H, Ding X, Qi C, Qin
X and Xu Z: Phloretin induces apoptosis of non-small cell lung
carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol Rep.
34:2871–2879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe K: Current chemotherapeutic
approaches for hepatoblastoma. Int J Clin Oncol. 18:955–961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katzenstein HM, London WB, Douglass EC,
Reynolds M, Plaschkes J, Finegold MJ and Bowman LC: Treatment of
unresectable and metastatic hepatoblastoma: A pediatric oncology
group phase II study. J Clin Oncol. 20:3438–3444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown J, Perilongo G, Shafford E, Keeling
J, Pritchard J, Brock P, Dicks-Mireaux C, Phillips A, Vos A and
Plaschkes J: Pretreatment prognostic factors for children with
hepatoblastoma-results from the International Society of Paediatric
Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 36:1418–1425. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar S and Lavin MF: The ICE family of
cysteine proteases as effectors of cell death. Cell Death Differ.
3:255–267. 1996.PubMed/NCBI
|
|
22
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oltvai ZN and Korsmeyer SJ: Checkpoints of
dueling dimers foil death wishes. Cell. 79:189–192. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strasser A, O'Connor L and Dixit VM:
Apoptosis signaling. Annu Rev Biochem. 69:217–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park WH and Kim SH: MAPK inhibitors
augment gallic acid-induced A549 lung cancer cell death through the
enhancement of glutathione depletion. Oncol Rep. 30:513–519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esmaeili MA, Farimani MM and Kiaei M:
Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling
pathways, ROS-mediated pathway and mitochondrial dysfunction in
hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem. 397:17–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui Y, Lu P, Song G, Liu Q, Zhu D and Liu
X: Involvement of PI3K/Akt, ERK and p38 signaling pathways in
emodin-mediated extrinsic and intrinsic human hepatoblastoma cell
apoptosis. Food Chem Toxicol. 92:26–37. 2016. View Article : Google Scholar : PubMed/NCBI
|