Introduction
The regression of cutaneous pigmented lesions,
benign or malignant, is a well-known phenomenon. The underlying
mechanism is not completely understood, but factors like host
immune system, UV radiation and trauma have been suggested.
Melanoma is a multifactorial disease witch demonstrates a very
complex interplay between identifiable risk factors such as UV
exposure, phenotype and genotype (1–3). According
to Bastian (4) melanoma risk increase
with the presence of multiple enlarged acquired nevi. Melanomas can
be epithelium-associated or not and can show different age
distribution and different relationships to UV exposure.
Melanoma is the tumor with the highest number of
reported somatic mutations across human cancer types (5). The most common one, that occurs in ~50%
of melanomas is BRAF mutation. His variant V600E is the most
prevalent one and has been associated with younger age at diagnosis
and location of primary tumor often on the trunk. BRAF V600E
mutation is also present in 70% of benign nevi (6).
After the discovery of BRAF mutations, new targeted
therapies like BRAF inhibitors have been introduced for the
treatment of melanoma. Although well tolerated when used in
clinical practice, they present some adverse events, cutaneous
adverse events being the most common ones. Under BRAF therapy
dynamic changes in melanocytic lesions like involution of nevi,
changes in pigmentation and size and appearance of new melanomas
have been described (7,8).
Although partial regression is relatively frequent
and occurs in 10–35% of cases, spontaneous complete regression with
documented histology is very rare and occurs only in 0.22–0.27% of
all melanoma cases (9).
Case report
A 58-year-old man presented in the Department of
Surgical in 2014 with a 6/4.5 cm subcutaneous tumor on the left
lateral chest wall of several months duration. Excision of the
lesion revealed a nodular, solid, dermo-hypodermic proliferation
with large atypical round, polygonal cells, high number of mitosis
(12/10 HPF) at pathological examination. It showed HMB45 and S100
positivity on immunohistochemical staining, concluding to an
in-transit metastasis of unknown primary melanoma.
The clinical examination showed in the left lumbar
region a suspicious elevated lesion, unknown to the patient, with
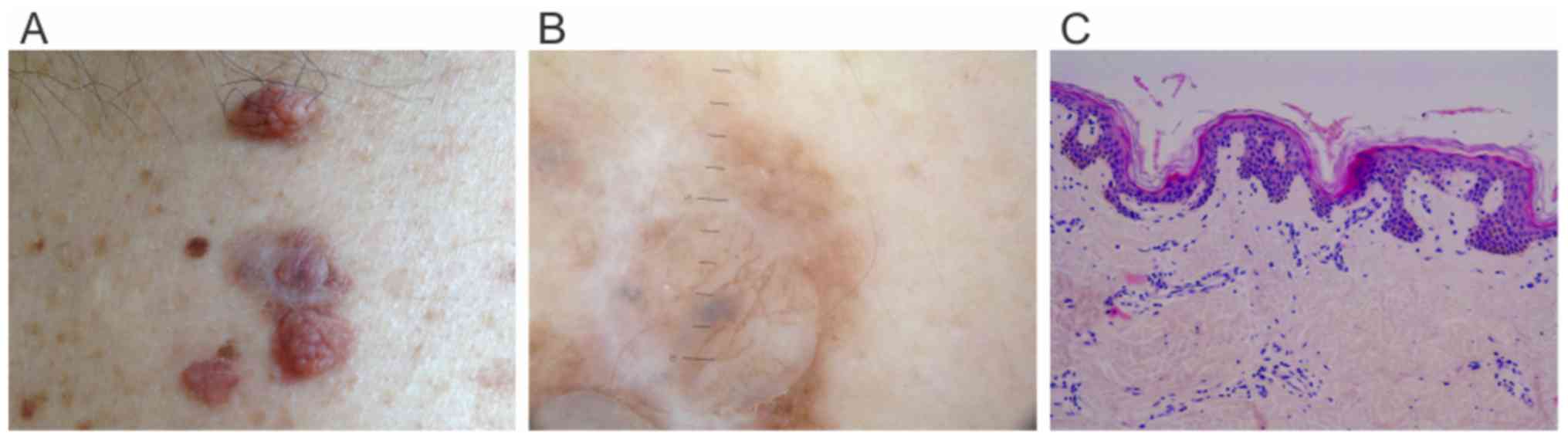
grayish appearance (Fig. 1A) and
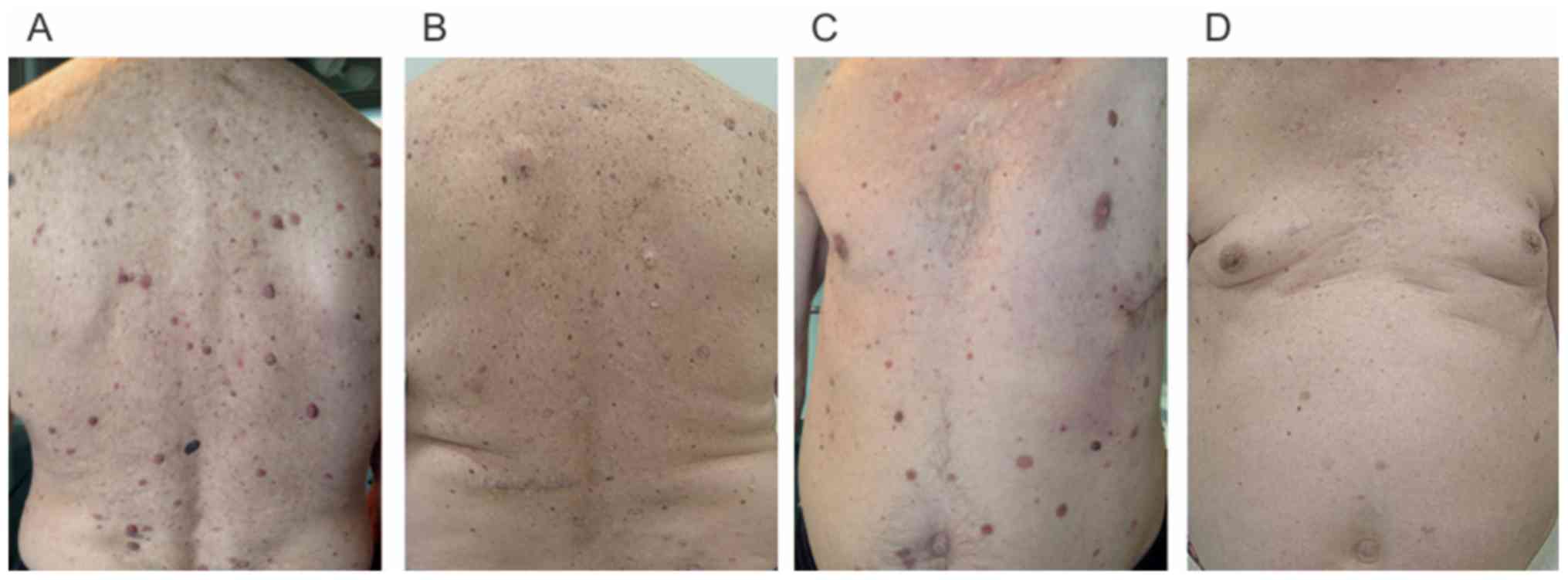
multiple enlarged papillomatous nevi on the trunk (Fig. 2A). Polarized dermoscopy revealed scar
like depigmentation, pigmented globules and white transverse
lighter bands between two papillomatous lesions (Fig. 1B). The clinical and dermoscopic aspect
of the lesion, the ipsilateral location indicated a regressed
tumor, most likely the primary melanoma. He also presented
left-sided axillary lymphadenopathy on clinical examination,
confirmed by the CT scan. Surgery was performed on the suspicious
lesion and axillary lymph nodes. Pathological examination of the
regressed lesion showed flat rete ridges, lamellar fibrosis,
increased vascularity, focal lymphocytic infiltrate with rare
melanophages and no signs of melanocytic proliferation (Fig. 1C). Four of the 26 axillary lymph nodes
removed showed tumoral cells with positivity for the HMB45 staining
concluding to a lymph node melanoma metastasis. After surgery the
patient showed rapid progression with detection of pleural
metastasis on the CT scan. The patient was diagnosed with a stage
IV melanoma with pleural metastasis and a completely regressed
primary tumor. BRAF V600E mutation was detected in the subcutaneous
lesion.
The patient started Vemurafenib treatment and showed
partial response on the pulmonary CT scan after 3 months of
therapy, but with numerous cutaneous adverse events: Multiple
keratoachantomas, warts and changes in nevi color. One month later
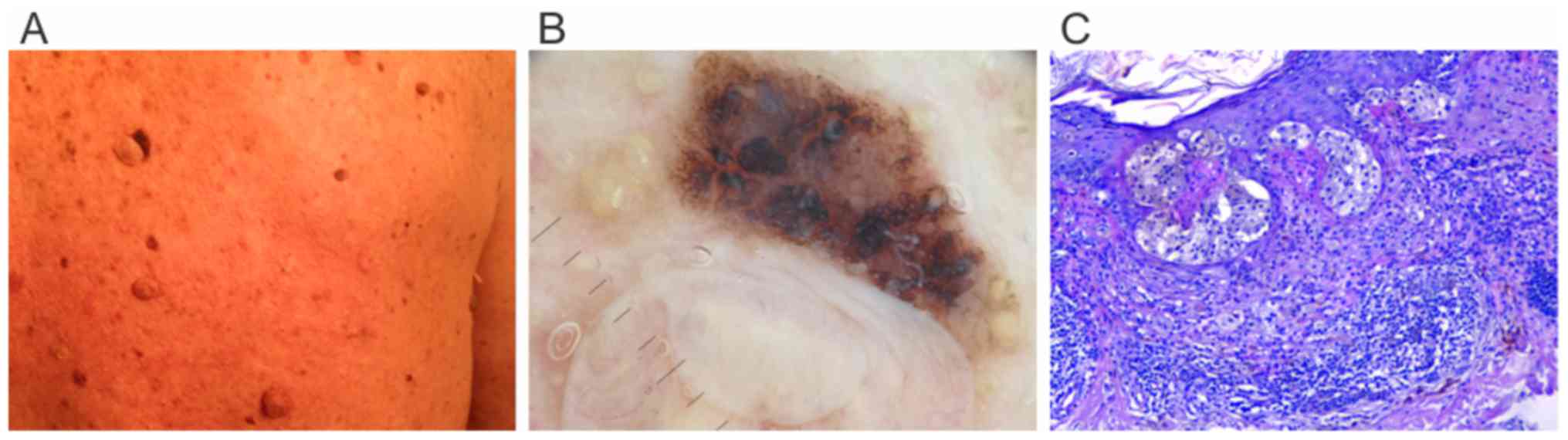
an atypical pigmented lesion (Fig. 3A and
B) at the base of a papillomatous nevus that changed from
baseline evaluation was excised. It turned out to be a 0.7-mm
Breslow index superficial spreading melanoma with less than 1
mitosis/mm2, a brisk lymphocytic infiltrate and no
regression present (Fig. 3C). In the
same time involution of some papillomatous nevi was observed, by
diminished size and lighter color. After other three months
Vemurafenib treatment was stopped because of insurance issues and
another BRAF inhibitor was started, Dabrafenib, which became
available for BRAF mutated melanoma patients in Romania at that
time.
After three years of BRAF inhibitor monotherapy all
his papillomatous lesions involuted (Fig.
2B and D). The patient had excellent systemic response with
stable disease and no cutaneous or systemic side effects from BRAF
inhibitor therapy. He is still under surveillance.
The present case is interesting because the
association of completely regressed melanoma with metastases, and
development of a new lesion under BRAF treatment.
Methods
We performed a search on PubMed using the terms
‘primary melanoma’, ‘spontaneous regression’, ‘complete regression’
to identify similar cases, restricting the papers to those
published in English or French. Additional articles using the
reference lists of the identified papers were noted. We found 50
cases of fully regressed melanomas which developed metastasis and
three cases of initially confirmed melanomas with complete
regression after partial incisional biopsy. Two cases of completely
regressed melanomas with no confirmed distant metastasis in spite
of extensive work up for two year follow-up were excluded from this
study (10,11). Age, sex, location of the tumor and
site of metastasis were noted for each case. We also recorded
information about the diagnosis of the primary tumor, if it was
retrospective or initial and pathologically confirmed. Dermoscopy
of complete regressed melanomas was reported only in 10 cases
(10–12).
We did not find in the literature any association
between completely regressed melanomas and BRAF mutational status.
Demographic and clinical information on the patients are summarized
in Table I.
 | Table I.Demographic and clinical information
on the patients. |
Table I.
Demographic and clinical information
on the patients.
| Author (year) | Ref. | No. of
patients | Age (years) | Sex | Location of primary
tumor | Sites of
metastasis | Diagnosis of
primary tumor |
|---|
| Dasgupta et
al, 1963 | (13) | 2 | 35 | M | Trunk | Ipsilateral
cervical and axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 49 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Smith et al,
1965 | (14) | 7 | 40 | M | Trunk | Ipsilateral
axillary | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 45 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 29 | F | Arm | Ipsilateral
epitrochlear & axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 27 | M | Arm | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 33 | M | Cervical | Ipsilateral
cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 31 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 44 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Todd et al,
1966 | (15) | 1 | 31 | M | Cervical | Ipsilateral
cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Milton et
al, 1967 | (16) | 1 | 53 | M | Cervical | Contralateral
cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Cochran et
al, 1970 | (17) | 2 | 41 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 50 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| McGovern, 1975 | (18) | 1 | 57 | M | Trunk | Supraclavicular
and. cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
| McDougal et
al, 1976 | (19) | 1 | 72 | F | Cervical | Ipsilateral
cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Whicker et
al, 1980 | (20) | 1 | 53 | F | Cervical | Ipsilateral
cervical lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Pelligrini,
1980 | (21) | 1 | 50 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Landthaler and
Braun-Falco, 1981 | (22) | 2 | 41 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 63 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Kessler et
al, 1984 | (23) | 1 | 40 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Rampen and Meijer,
1985 | (24) | 1 | 37 | M | Cervical | Brain | Retrospective/skin
examination/excisional biopsy |
| Paul and Müllhofer,
1990 | (25) | 1 | 60 | M | Trunk | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Bottger et
al, 1992 | (26) | 2 | 41 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 63 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Avril et al,
1992 | (27) | 7 | 40 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 57 | M | Trunk | Contralateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 42 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 68 | M | Arm | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 52 | M | Arm | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 33 | F | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 63 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Shai et al,
1994 | (9) | 1 | 53 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Sais et al,
1994 | (28) | 1 | 57 | F | Cervical | Ipsilateral parotid
gland, brain | Retrospective/skin
examination/excisional biopsy |
| High et al,
2005 | (11) | 4 | 68 | M | Trunk | Ipsilateral
axillary lymph nodes, brain | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 48 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 55 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 21 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Emanuel et
al, 2008 | (29) | 2 | 40 | F | Trunk | Regional lymph
nodes NOS | skin
examination/excisional biopsy first |
|
|
|
| 53 | M | Cervical | Soft tissue
NOS | Retrospective/skin
examination/excisional biopsy |
| Bories et
al, 2008 | (12) | 1 | 63 | M | Trunk | Bilateral axillary
lymph nodes | Retrospective/skin
examination/Excisional biopsy |
|
|
| 2 | 38 | F | Trunk | Ipsilateral
sus-clavicular lymph nodes | Retrospective/skin
examination/Excisional biopsy |
|
|
| 3 | 48 | M | Foot | Ipsilateral
inguinal lymph nodes and lung | Retrospective/skin
examination/Excisional biopsy |
|
|
| 4 | 36 | F | Cervical | Ipsilateral
cervical lymph nodes | Retrospective/skin
examination/Excisional biopsy |
|
|
| 5 | 63 | M | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/Excisional biopsy |
|
|
| 6 | 68 | F | Leg | Ipsilateral
inguinal lymph nodes, iliac nodes and bone | Retrospective/skin
examination/Excisional biopsy |
|
|
| 7 | 78 | F | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/Excisional biopsy |
| Margaritescu et
al, 2014 | (30) | 3 | 45 | F | Trunk | Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
|
|
|
| 45 | M | Trunk | Ipsilateral
axillary lymph nodes | skin
examination/Biopsy first |
|
|
|
| 40 | M | Trunk | Brain Ipsilateral
axillary lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Yamada et
al, 2016 | (31) | 1 | 65 | M | Leg | Ipsilateral
inguinal lymph nodes | Retrospective/skin
examination/excisional biopsy |
| Complete regression
of primary tumor after incisional biopsy |
| Dunn et al,
2008 | (32) | 1 | 39 | F | Trunk | No distant
metastasis | Initial partial
biopsy (SSM 1.8 mm growth Breslow thickness, vertical fase)
complete regression in 12 weeks (excision) |
| Menzies and
McCarthy, 2015 | (33) | 1 | 62 | M | Leg |
| Initial partial
biopsy (SSM 0.7 mm Breslow thickness) complete regression in 18
months on punch biopsy |
| Khosravi et
al, 2016 | (34) | 1 | 49 | M | Trunk | Multiple
metastasis | Initial partial
biopsy (ulcerated NM 8 mm Breslow thickness) complete regression in
4 weeks (excision) |
Discussion
Complete regression in melanoma is a rare event,
being reported only in 0.22–0.27% of all melanoma cases (9). Clinical and pathological presentation of
complete regressed melanomas have been extensively described
through the literature. Dermoscopic evaluation of completely
regressed melanoma is based only on a few case reports in the last
10 years (10–12). We did not find in the literature any
association between completely regressed melanomas and BRAF
mutational status. Nevi involution associated with melanoma was
reported in the literature as a result of melanoma development
followed by spontaneous regression of nevi or as a result of
targeted therapy with BRAF inhibitors (35,36).
Clinical presentation of completely regressed
melanoma. Completely regressed melanoma usually presents as a
hypopigmented macule with pink or scar-like appearance. In rare
cases, they can also appear as either hyperpigmented macules, or
with remnants of pigmentation as blue-gray discolorations (11). Our review showed that all melanomas
were epithelium associated (4), with
a male predominance representing 62.26% of all cases and a median
age of 48.5 years. Eighty-three percent of cases presented in
relation with low UV exposure in non-chronic sun damaged skin
(39,62% involving the trunk and 43.39% involving arms and legs) and
only 16.98% were located on chronic sun damaged skin (cervical), in
relation with high UV exposure. The diagnosis of regressed melanoma
was consistent with criteria of Smith and Stehlin and was
retrospective in all cases except one (14). Three pathologically confirmed
melanomas (two superficial spreading and one nodular melanoma)
completely regressed after incisional partial biopsy of primary
tumor (32–34).
Dermoscopic presentation of completely regressed
melanoma. There are no established dermoscopic criteria for the
diagnosis of complete regressed melanomas. In the literature we
found only 10 reported cases of completely regressed melanoma with
pathological confirmation and dermoscopic evaluation. Seven cases
are reported by Bories et al (12) who showed that the most frequent
dermoscopic features present in all cases were scar-like
depigmentation and pink background. Eighty-six percent of patients
in his serie had linear irregular vessels as defined by Argenziano
et al (37) and remnants of
pigmentation on dermoscopic evaluation. Forty-three percent of
patients in Bories et al (12)
serie had ‘peppering’ as defined by Zalaudek et al (38), white transverse lighter bands in
polarized dermoscopy and globular vessels. High et al
(11) recorded just ‘peppering’ and
dusty blue-gray coloration on dermoscopic evaluation, performed in
only two of his 38 melanoma cases reported. Pozzobon et al
(39) found that ‘peppering’ is a
dermoscopic feature frequently associated with regression in BRAF
mutated melanoma. They stated that this can be considered a
morphological consequence of BRAF mutated melanoma biological
behavior.
Our case presented on polarized dermoscopy:
Scar-like depigmentation, pigmented globules and white transverse
lighter bands, features associated with regression (Fig. 1B).
Scar-like depigmentation and white transverse
lighter bands on a pink background were observed in our patient
also at the level of some regressed papillomatous nevi.
Pathological analysis of completely regressed
melanoma. To confirm the diagnosis of a completely regressed
melanoma is often very difficult. The diagnostic approach is almost
always retrospective, with primary lesion being detected in
metastatic stage (11). There is only
one reported case of complete primary regressed melanoma detected
before metastatic stage (30).
According to Massi and LeBoit (40)
the important issue is to differentiate between a completely
regressed nevus and a completely regressed melanoma, and they
proposed some criteria. In a regressed nevus the epidermis is
normal, with preserved rete ridges, no tumor melanosis and a
lymphocytic infiltrate that leaves the nevus undisturbed. In
completely regressed melanoma the epidermis is rarely normal; it
can be atrophic or irregularly hyperplastic. There is dermal
fibrosis, sometimes with foci of tumor melanosis and a rich
lymphocytic infiltrate. We can also note the presence of an ectatic
superficial vasculature (Fig. 1C). A
challenging situation is when, like in our case, regression
involves a melanoma developed in a nevus (Fig. 1A and B).
Mutational status in completely regressed melanomas.
Across human cancer type, melanoma is the tumor with the highest
prevalence of somatic mutations (5).
Bastian (4) found that BRAF
mutational status was highly present in melanoma arising in
non-chronic sun damaged skin, but it can be present in a small
percentage in melanomas arising on chronic sun damaged skin or
glabrous skin. The most common somatic mutation in melanoma is BRAF
V600E, present in up to 90% of BRAF mutated melanomas (41,42). It is
associated with younger age at diagnosis and primary lesion located
often on the trunk (6). Partial
regression, found in 10–35% of primary melanoma cases, is a common
dermoscopic criteria associated with BRAF mutation (39). We present for the first time to our
knowledge a case of completely regressed primary melanoma which
harbors BRAF V600E mutation in the metastatic lesion and develops a
second primary BRAF wild-type melanoma under BRAF inhibitors.
BRAF mutation is present in nevi in a higher
percentage than in melanoma (70–82% in nevi vs. 50–60% in
melanomas) (43). The introduction of
BRAF inhibitor therapy in the treatment of melanoma improved
response rates and overall survival in patients with melanoma but
with nevi dynamic change as a common side effect. Nevi involution,
especially for the papillomatous compound type, increasing in size
and pigmentation for the flat lesions but also appearance of new
BRAF wild-type melanoma were reported (36,44). This
is due to paradoxical activation of MAPK pathway, especially with
BRAF inhibitor monotherapy, also associated with resistance to
therapy (45).
Spontaneous complete regression - possible
mechanisms. A lot of factors leading to complete regression in
melanoma were mentioned in the literature. There are exogenous
factors like surgical trauma, vaccines (BCG, rabies), UV exposure,
medication (BRAF inhibitors) and endogenous factors like pregnancy
(32,33,46–49). The
host immune system has an important role in tumor regression in
melanoma. The occurrence of regression in primary tumor after lymph
node or visceral metastases is highly related to an effective
immune response against tumor cells (46,49).
In conclusion, we present for the first time to our
knowledge a case of completely regressed BRAF V600E mutated
melanoma which showed nevi involution and developed a new BRAF
wild-type melanoma after therapy with BRAF inhibitors. On one hand,
this case favors the role of BRAF mutation in melanoma immune
pathogenesis, and on the other hand, shows the importance of
clinical and dermoscopic follow-up in melanoma patients. Our review
shows that the diagnosis of completely regressed melanoma is very
difficult, especially because there are no unifying concepts in
assessing complete regression dermoscopically and
pathologically.
Acknowledgements
Not applicable.
Funding
This manuscript was partially supported by the
Romanian Society of Dermato-oncology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GLE, AV, LU, RC were responsible for conception of
the manuscript, data analysis and contributed to writing the
manuscript. NB, CS, SS, EC, OF contributed to data acquisition and
the critical revision of the manuscript for important intellectual
content. All authors read and approved the final version of
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the ‘Iuliu Hatieganu’ University of Medicine and Pharmacy (no.
170/12.05.2014; Cluj-Napoca, Romania). All the participants gave
their consent to be included in the study.
Patient consent for publication
The patient gave his written consent for image and
data publication.
Competing interests
Rodica Cosgarea has received speaker fees from Roche
Pharma and Novartis Pharma. Grigore Lavinia Elena has received a
speaker fee from Novartis Pharma.
References
|
1
|
Mitchell JK and Leslie KS: Melanoma death
prevention: Moving away from the sun. J Am Acad Dermatol.
68:e169–e175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bastian BC: The molecular pathology of
melanoma: An integrated taxonomy of melanocytic neoplasia. Annu Rev
Pathol. 9:239–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al Australian Pancreatic Cancer Genome
Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq
Consortium; ICGC PedBrain, : Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sullivan RJ: Molecular diagnostics and
tumor mutational analysis. BRAF Targets in Melanoma-Biological
Mechanisms. Resistance and Drug Discovery; Springer, New York, NY:
pp. 47–72. 2015
|
|
7
|
Carlos G, Anforth R, Clements A, Menzies
AM, Carlino MS, Chou S and Fernandez-Peñas P: Cutaneous toxic
effects of BRAF inhibitors alone and in combination with MEK
inhibitors for metastatic melanoma. JAMA Dermatol. 151:1103–1109.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bolognia JL, Schaffer JV and Cerroni L:
Neoplasms of the skin. Dermatology. 4th. Elsevier; pp. 1989–2020.
2018
|
|
9
|
Shai A, Avinoach I and Sagi A: Metastatic
malignant melanoma with spontaneous and complete regression of the
primary lesion. Case report and review of the literature. J
Dermatol Surg Oncol. 20:342–345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ehrsam E, Kallini JR, Lebas D, Khachemoune
A, Modiano P and Cotten H: Fully regressive melanoma: A case
without metastasis. J Clin Aesthet Dermatol. 9:42–46.
2016.PubMed/NCBI
|
|
11
|
High WA, Stewart D, Wilbers CR, Cockerell
CJ, Hoang MP and Fitzpatrick JE: Completely regressed primary
cutaneous malignant melanoma with nodal and/or visceral metastases:
A report of 5 cases and assessment of the literature and diagnostic
criteria. J Am Acad Dermatol. 53:89–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bories N, Dalle S, Debarbieux S, Balme B,
Ronger-Savlé S and Thomas L: Dermoscopy of fully regressive
cutaneous melanoma. Br J Dermatol. 158:1224–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dasgupta T, Bowden L and Berg JW:
Malignant melanoma of unknown primary origin. Surg Gynecol Obstet.
117:341–345. 1963.PubMed/NCBI
|
|
14
|
Smith JL Jr and Stehlin JS Jr: Spontaneous
regression of primary malignant melanomas with regional metastases.
Cancer. 18:1399–1415. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Todd DW, Farrow GM, Winkelmann RK and
Payne WS: Spontaneous regression of primary malignant melanoma with
regional metastasis: Report of a case with photographic
documentation. Mayo Clin Proc. 41:672–676. 1966.PubMed/NCBI
|
|
16
|
Milton CW, Lane Brown MM and Gilder M:
Malignant melanoma with an occult primary lesion. Br J Surg.
54:651–658. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cochran AJ, Diehl V and Stjernswärd J:
Regression of primary malignant melanoma associated with a good
prognosis despite metastasis to lymph nodes. Rev Eur Etud Clin
Biol. 15:969–972. 1970.PubMed/NCBI
|
|
18
|
McGovern VJ: Spontaneous regression of
melanoma. Pathology. 7:91–99. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacDougal BA, Weeks PM and Wray RC Jr:
Spontaneous regression of the primary lesion of a metastatic
malignant melanoma. Plast Reconstr Surg. 57:355–358. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whicker JH, DeMarco PR and Fitzgibbons JF:
Spontaneous regression of a facial malignant melanoma. Arch
Otolaryngol. 106:50–51. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pellegrini AE: Regressed primary malignant
melanoma with regional metastases. Arch Dermatol. 116:585–586.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landthaler M and Braun-Falco O: Malignant
melanoma with unknown primary tumor. Case reports of 12 patients
with overview. Hautarzt. 32:339–344. 1981.(In German). PubMed/NCBI
|
|
23
|
Kessler E, Schwartz P and Antebi E:
Spontaneous regression of primary malignant melanoma with
metastases. Plast Reconstr Surg. 74:427–429. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rampen FH and Meijer J: Metastatic
melanoma of the brain after spontaneous regression of the primary.
Acta Neurol Scand. 72:222–224. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paul E and Müllhofer R: Metastasizing
malignant melanoma from unknown primary tumor. Hautarzt.
41:432–437. 1990.(In German). PubMed/NCBI
|
|
26
|
Bottger D, Dowden RV and Kay PP: Complete
spontaneous regression of cutaneous primary malignant melanoma.
Plast Reconstr Surg. 89:548–553. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Avril MF, Charpentier P, Margulis A and
Guillaume JC: Regression of primary melanoma with metastases.
Cancer. 69:1377–1381. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sais G, Marcoval J, Juggla A, Curco N and
Servitje O: Dermatomyositis and metastatic malignant melanoma, with
complete regression of the primary lesion. Br J Dermatol.
130:796–797. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emanuel PO, Mannion M and Phelps RG:
Complete regression of primary malignant melanoma. Am J
Dermatopathol. 30:178–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mărgăritescu I, Chiriţă AD and Vasilescu
F: Completely regressed primary cutaneous melanoma - difficulties
in diagnosis and classification. Rom J Morphol Embryol. 55 (Suppl
2):635–642. 2014.PubMed/NCBI
|
|
31
|
Yamada S, Nawata A, Yoshioka M, Hiraki T,
Higashi M, Hatanaka K and Tanimoto A: Complete regression of
primary cutaneous malignant melanoma associated with distant lymph
node metastasis: A teaching case mimicking blue nevus. BMC Res
Notes. 9:3662016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dunn GP, Lewis JS Jr, Sunwoo JB and
Uppaluri R: Spontaneous regression of cutaneous head and neck
melanoma: Implications for the immunologic control of neoplasia.
Head Neck. 30:267–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menzies SW and McCarthy WH: Complete
regression of primary cutaneous malignant melanoma. Arch Surg.
132:553–556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khosravi H, Akabane AL, Alloo A, Nazarian
RM and Boland GM: Metastatic melanoma with spontaneous complete
regression of a thick primary lesion. JAAD Case Rep. 2:439–441.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martín JM, Pinazo I, Monteagudo C,
Markovic J, Allende A and Jordá E: Spontaneous regression of
multiple melanocytic nevi after melanoma: Report of 3 cases. Am J
Dermatopathol. 36:e183–e188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McClenahan P, Lin LL, Tan JM,
Flewell-Smith R, Schaider H, Jagirdar K, Atkinson V, Lambie D, Prow
TW, Sturm RA, et al: BRAFV600E mutation status of involuting and
stable nevi in dabrafenib therapy with or without trametinib. JAMA
Dermatol. 150:1079–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Argenziano G, Zalaudek I, Corona R, Sera
F, Cicale L, Petrillo G, Ruocco E, Hofmann-Wellenhof R and Soyer
HP: Vascular structures in skin tumors: A dermoscopy study. Arch
Dermatol. 140:1485–1489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zalaudek I, Argenziano G, Ferrara G, Soyer
HP, Corona R, Sera F, Cerroni L, Carbone A, Chiominto A, Cicale L,
et al: Clinically equivocal melanocytic skin lesions with features
of regression: A dermoscopic-pathological study. Br J Dermatol.
150:64–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pozzobon FC, Puig-Butillé JA,
González-Alvarez T, Carrera C, Aguilera P, Alos L, Badenas C,
Grichnik JM, Malvehy J and Puig S: Dermoscopic criteria associated
with BRAF and NRAS mutation status in primary cutaneous melanoma.
Br J Dermatol. 171:754–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Massi G and LeBoit PE: Histological
Diagnosis of Nevi and Melanoma. Springer; Berlin, Heidelberg: pp.
385–398. 2014
|
|
41
|
Cheng L, Lopez-Beltran A, Massari F,
MacLennan GT and Montironi R: Molecular testing for BRAF mutations
to inform melanoma treatment decisions: A move toward precision
medicine. Mod Pathol. 31:24–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diaconeasa A, Boda D, Solovan C, Enescu
DM, Vîlcea AM and Zurac S: Histopathologic features of Spitzoid
lesions in different age groups. Rom J Morphol Embryol. 54:51–62.
2013.PubMed/NCBI
|
|
43
|
Pollock PM, Harper UL, Hansen KS, Yudt LM,
Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J,
et al: High frequency of BRAF mutations in nevi. Nat Genet.
33:19–20. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haenssle HA, Kraus SL, Brehmer F,
Kretschmer L, Völker B, Asper H, Kapp A and Gutzmer R: Dynamic
changes in nevi of a patient with melanoma treated with
vemurafenib: Importance of sequential dermoscopy. Arch Dermatol.
148:1183–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weeraratna AT: RAF around the edges - the
paradox of BRAF inhibitors. N Engl J Med. 366:271–273. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aung PP, Nagarajan P and Prieto VG:
Regression in primary cutaneous melanoma: Etiopathogenesis and
clinical significance. Lab Invest. 97:657–668. 2017. View Article : Google Scholar
|
|
47
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Copenh). 10:545–558.
2014.
|
|
49
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|