Introduction
Esophageal cancer, which consists of two principal
subtypes, adenocarcinoma and esophageal squamous cell carcinoma
(ESCC), is the sixth leading cause of cancer-associated mortality
worldwide (1). Compared with
adenocarcinoma, ESCC is more common in East Asia, and ~50% of ESCC
cases occur in China (2–4). Due to the rapid progression and high
malignancy, the prognosis of patients with ESCC remains poor
(5).
Chemotherapy is the most common treatment for cancer
(1). Unfortunately, the majority of
chemotherapeutic drugs have a limited effect on ESCC due to side
effects and drug resistance (6).
Thus, there is an urgent need to develop safe, efficacious agents
with limited harmful effects for the treatment of ESCC.
Pemetrexed is a novel multi-targeted antifolate that
targets a number of crucial enzymes involved in folate metabolism
(7). Recent studies have reported
that pemetrexed is also able to act as an antitumor drug, and its
cytotoxicity has been demonstrated in advanced non-small cell lung
cancer and malignant pleural mesothelioma (8,9). However,
the effects of pemetrexed on human esophageal cancer and the
possible mechanisms of such effects remain to be elucidated.
Determining whether pemetrexed exhibits anticancer effects on ESCC
cells and understanding the underlying molecular mechanism are
important for developing better chemotherapeutics for ESCC.
Cell cycle arrest and apoptosis induced by cytotoxic
agents are critical in cancer treatment (10,11). The
intrinsic mitochondrial pathway is an important signaling cascade
associated with apoptosis. Members of the apoptosis regulator Bcl-2
(Bcl-2) family serve crucial roles in the regulation of apoptotic
processes in various cancer cells (12). Phorbol-12-myristate-13-acetate-induced
protein 1 (NOXA), a crucial pro-apoptotic protein in the Bcl-2
family, has been reported to be involved in chemotherapeutic
agent-induced apoptosis (13). NOXA
is able to interact with the anti-apoptotic Bcl-2 protein induced
myeloid leukemia cell differentiation protein Mcl-1 (Mcl-1),
interfering with the polymerization of apoptosis regulator BAX and
Bcl-2 homologous antagonist/killer to trigger the mitochondrial
apoptosis pathway (14). The
combination of NOXA and Mcl-1 may also facilitate the proteasomal
degradation of Mcl-1 and thereby strengthen intrinsic apoptosis
(15).
Previous studies have demonstrated that the
activation of the intrinsic mitochondrial apoptosis pathway is
associated with persistent endoplasmic reticulum (ER) stress
(16). Once inositol-requiring enzyme
1α (IRE1α) and other ER sensors are released from binding
immunoglobulin protein (Bip), downstream effectors, including
CCAAT-enhancer-binding protein homologous protein (CHOP) may
trigger pro-apoptotic signals by targeting numerous apoptotic genes
(17,18). However, whether the intrinsic
mitochondrial apoptosis pathway and ER stress are active following
treatment with pemetrexed in human ESCC cells remains unknown.
The present study investigated whether pemetrexed
exerted anticancer effects on ESCC cells. The role of the cell
cycle and the NOXA/Mcl-1 axis in the regulation of this effect was
also studied. The present study revealed the therapeutic potential
of pemetrexed for ESCC and enriched the understanding of this
cancer type.
Materials and methods
Antibodies and reagents
Pemetrexed (cat no. CDS024404), was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany), and was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) at a
concentration of 10 mmol/l (19). The
stock solutions were stored at −20°C and diluted to the desired
concentrations with growth medium prior to use. All the antibodies
were previously described (13).
Antibodies targeting caspase (casp)8 (cat no. 9746; 1:1,000
dilution), casp9 (cat no. 9502; 1:1,000 dilution), poly(ADP-ribose)
polymerase (PARP; cat no. 9542; 1:1,000 dilution), IRE1α (cat no.
3294; 1:2,000 dilution) and Bip (cat no. 3183; 1:1,000 dilution)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Antibodies targeting casp3 (cat no. NB100-56708; 1:1,000
dilution) and NOXA (cat no. OP180; 1:500 dilution) were purchased
from Imgenex (Novus Biologicals, LLC, Littleton, CO, USA) and
Calbiochem; Merck KGaA, respectively. Anti-actin antibody (cat no.
A5441; 1:20,000 dilution) was obtained from Sigma-Aldrich; Merck
KGaA. Antibodies against CHOP (cat no. sc-7351; 1:100 dilution) and
Mcl-1 (cat no. sc-12756; 1:1,000 dilution) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell lines and cell culture
The human ESCC cell lines Eca-109 and EC9706 were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and were grown in monolayer cultures at 37°C in a humidified
atmosphere consisting of 5% CO2 and 95% air. The cells
were cultured with RPMI-1640 medium containing 5% fetal bovine
serum (both Gibco; Thermo Fisher Scientific Inc., Waltham, MA,
USA).
Cell treatment and MTT assay
A total of 7×104 cells were seeded in
96-well microtiter plates and treated with 0, 0.625, 1.25, 2.5, 5
and 10 µM pemetrexed on the 2nd day. Following culturing with
chemotherapeutics for 24, 36 or 48 h, cells were subjected to the
MTT assay. Each sample was incubated with 20 µl (5 mg/ml) MTT
(Sigma-Aldrich; Merck KGaA) at 37°C for 4 h. Following incubation,
the solution was discarded and 100 µl DMSO was added. Cell
viability was determined by measuring the absorbance at 495 nm
using an ELISA Multiskan reader (Thermo Fisher Scientific,
Inc.).
Cell cycle analysis
The cell cycle was evaluated through DNA flow
cytometry analysis. Overall, 2×105 cells were seeded in
six-well plates and treated with different concentrations of
pemetrexed (0, 2.5, 5 and 10 µM) for 24 h. Following treatment,
cells were harvested and washed twice with ice-cold PBS and fixed
in 70% ethanol at −20°C overnight. Prior to analysis, cells were
washed with ice-cold PBS and incubated at 4°C with 5 µl propidium
iodide (PI; 100 µg/ml) and 5 µl RNase (50 µg/ml; both Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 30
min in the dark. The samples were analyzed using a FACScan flow
cytometer and the BD FACSuite™ flow cytometry software (version
1.0.5; both BD Biosciences, San Jose, CA, USA). Data analysis was
performed using FlowJo software (version 7.2.2; Tree Star, Inc. San
Carlos, CA, USA) (13).
Apoptosis analysis
Apoptosis was evaluated according to a previously
described protocol (13). The Annexin
V-fluorescein isothiocyanate (FITC)/PI apoptosis detection kit was
purchased from Nanjing Biobox Biotech (Nanjing, China). Following
treatment with different concentrations of pemetrexed (0, 2.5, 5
and 10 µM) for 36 h, 2×106 cells were harvested, washed
with pre-chilled PBS and resuspended in 500 µl binding buffer. A
total of 5 µl each of Annexin V-FITC and PI were added to each
sample, and the samples were incubated for 10 min in the dark at
room temperature. Analysis was performed by flow cytometry with the
aforementioned flow cytometer and software.
Western blot analysis
Whole-cell protein lysates were prepared and
analyzed by western blotting according to a previously described
protocol (13). Cells were treated
with different concentrations of pemetrexed (0, 2.5, 5 and 10 µM)
for 36 h, harvested and rinsed with pre-chilled PBS. Cell extracts
were lysed and centrifuged at 12,000 × g for 15 min at 4°C, and the
protein concentration was determined via the bicinchoninic acid
assay. Whole-cell protein lysates (40 µg) were separated by
SDS-PAGE on a 12% gel and transferred to Hybond-enhanced
chemiluminescence (ECL) membranes via electroblotting. Following
blocking with 5% skimmed milk at room temperature for 1 h, the
proteins were first probed with the appropriate primary antibodies
at 4°C overnight followed by secondary goat anti-rabbit/mouse
monoclonal antibodies (cat nos. SA00001-1 and SA00001-2; 1:8,000
dilution; ProteinTech Group, Inc., Chicago, IL, USA). Antibody
binding was detected using an ECL system (EMD Millipore, Billerica,
MA, USA), according to the manufacturer's protocol. The expression
levels of the proteins were quantified using ImageJ software
(version 1.6.0_24; National Institute of Health, Bethesda, MD,
USA).
Statistical analysis
SPSS statistical software (version 20.0; IBM Corp.,
Armonk, NY, USA) was used for all statistical analyses. The data
obtained represent the mean ± standard deviation of at least three
independent assays performed in duplicate or triplicate. A one-way
analysis of variance followed by a Least Significant Difference
post hoc test was performed for testing for all data. P<0.05 was
considered to indicate a statistically significant difference.
Results
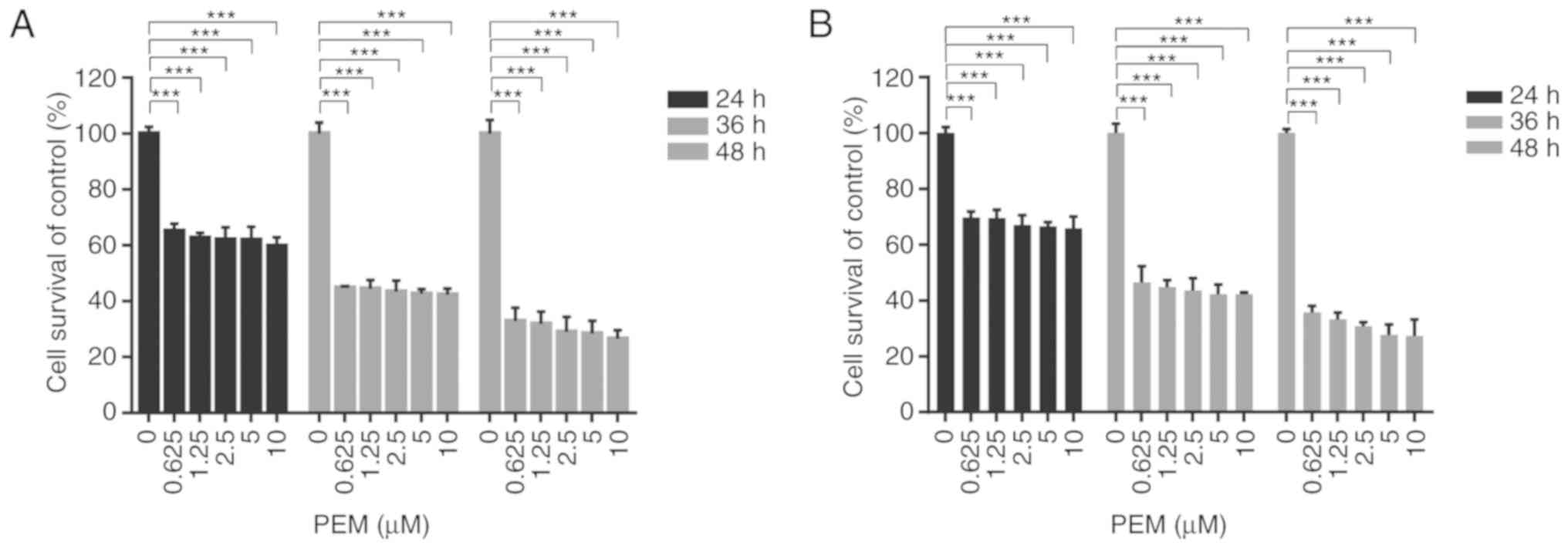
Pemetrexed inhibits the survival of
human ESCC cells
The cytotoxicity of pemetrexed in ESCC cells was
analyzed by treating Eca-109 and EC9706 cells with various
concentrations of pemetrexed for various times and performing an
MTT assay. The results demonstrated that pemetrexed may induce
survival inhibition in human ESCC cells. When the exposure time was
prolonged, the inhibitory effect was enhanced in Eca-109 and EC9706
cells (Fig. 1). These results
suggested that pemetrexed may effectively suppress the survival of
human ESCC cells.
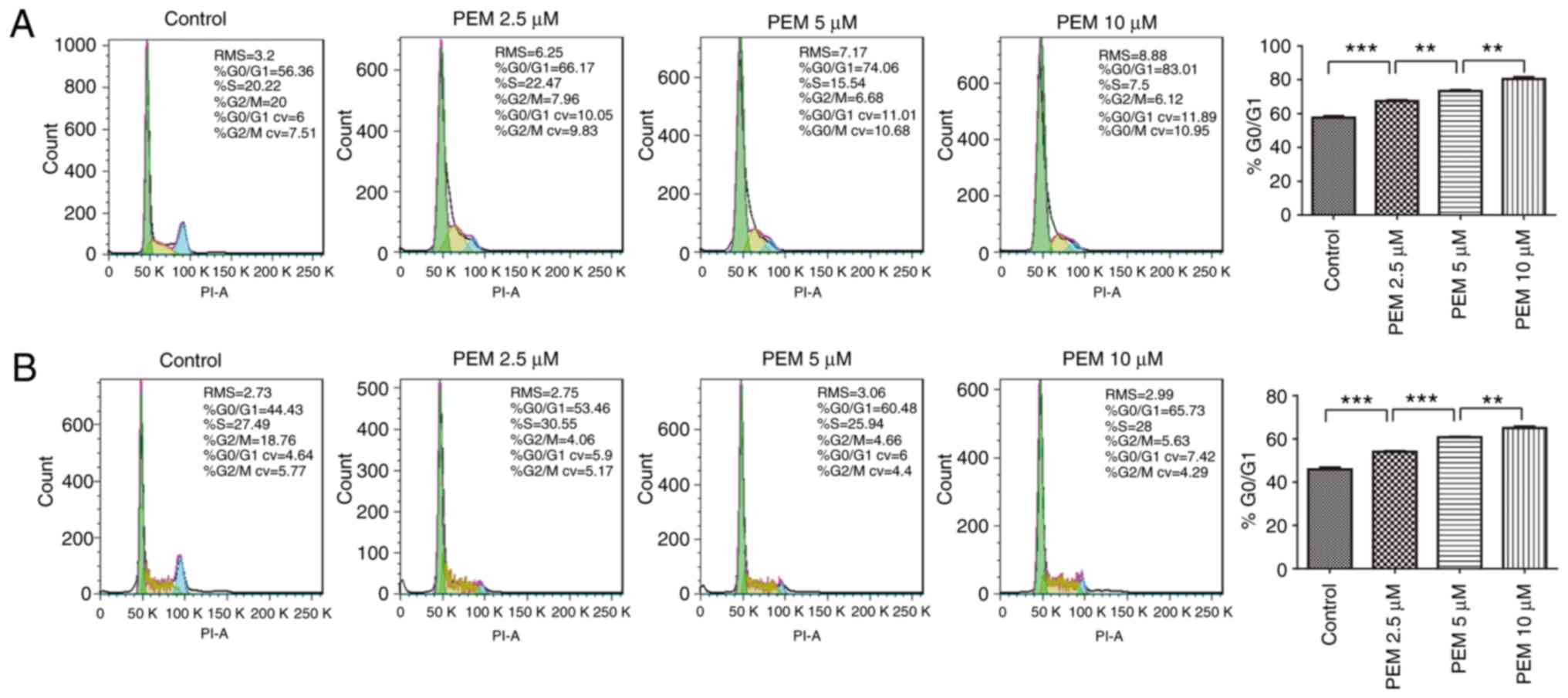
Pemetrexed induces
G0/G1 cell cycle arrest in ESCC cells
To determine the molecular mechanism by which
pemetrexed induced survival inhibition in human ESCC cells, the
ability of the agent to induce cell cycle arrest was analyzed by
DNA flow cytometry analysis. The results demonstrated that cell
cycle arrest was induced in a concentration-dependent manner; over
the range of drug treatments (0–10 µM), the frequency of
G1 cells increased from 57.63 to 80.34% in Eca-109 cells
(Fig. 2A). Similar results were
reported in EC9706 cells, in which the frequency of G1
cells increased from 45.94 to 65.05% (Fig. 2B). These results indicated that
exposure to pemetrexed may trigger G0/G1 cell
cycle arrest in human ESCC cells.
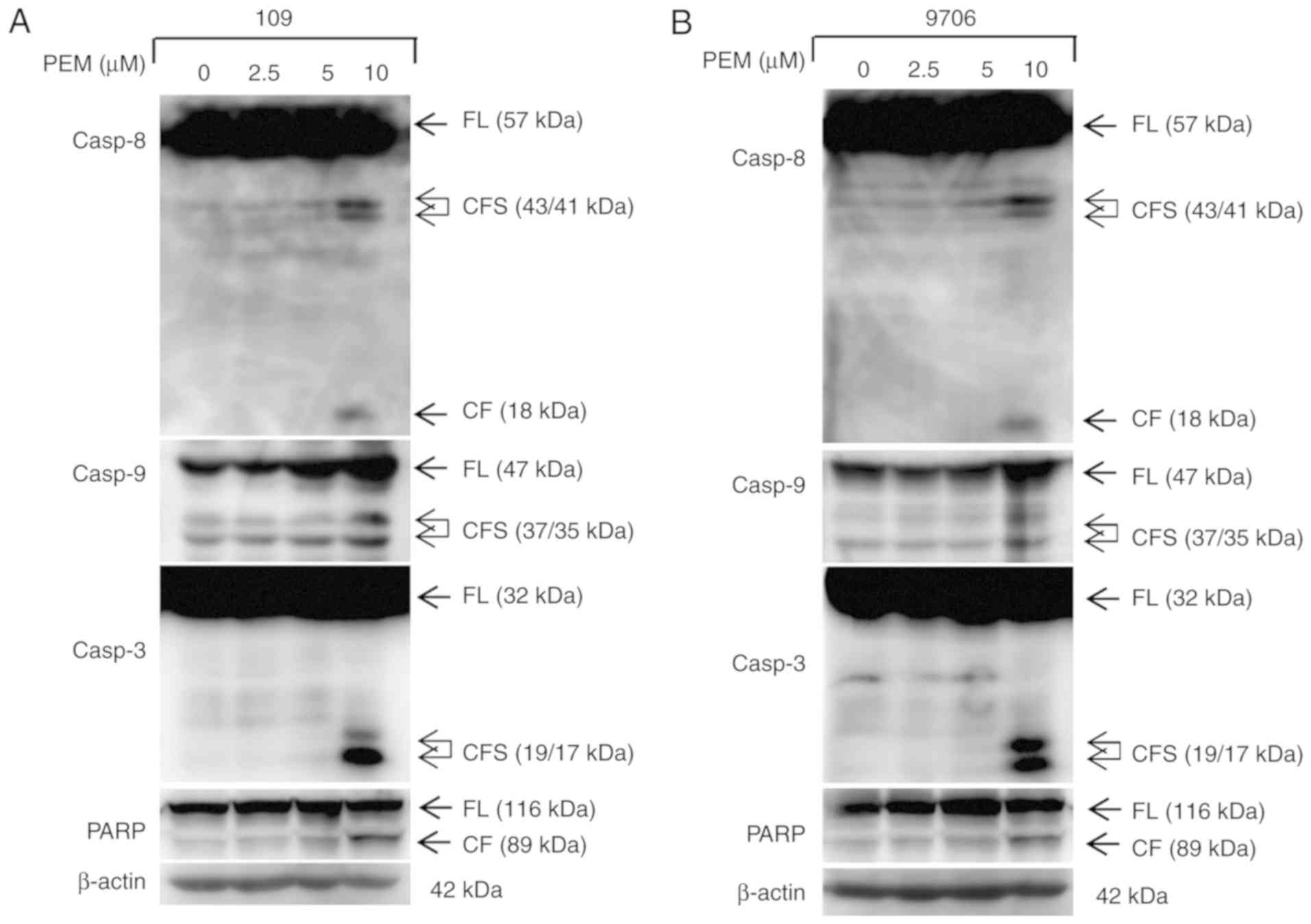
Pemetrexed induces apoptosis in human
ESCC cells
The present study also sought to determine whether
apoptosis was involved in the inhibition of survival induced by
pemetrexed. Therefore, an apoptosis assay was performed via Annexin
V/PI staining. The resulting data revealed that pemetrexed induced
apoptosis in a concentration-dependent manner in Eca-109 and EC9706
cells (Fig. 3). When treated with
pemetrexed (0–10 µM), the frequency of apoptosis was increased from
4.317 to 32.43% in Eca-109 cells and 9.795 to 43.48% in EC9706
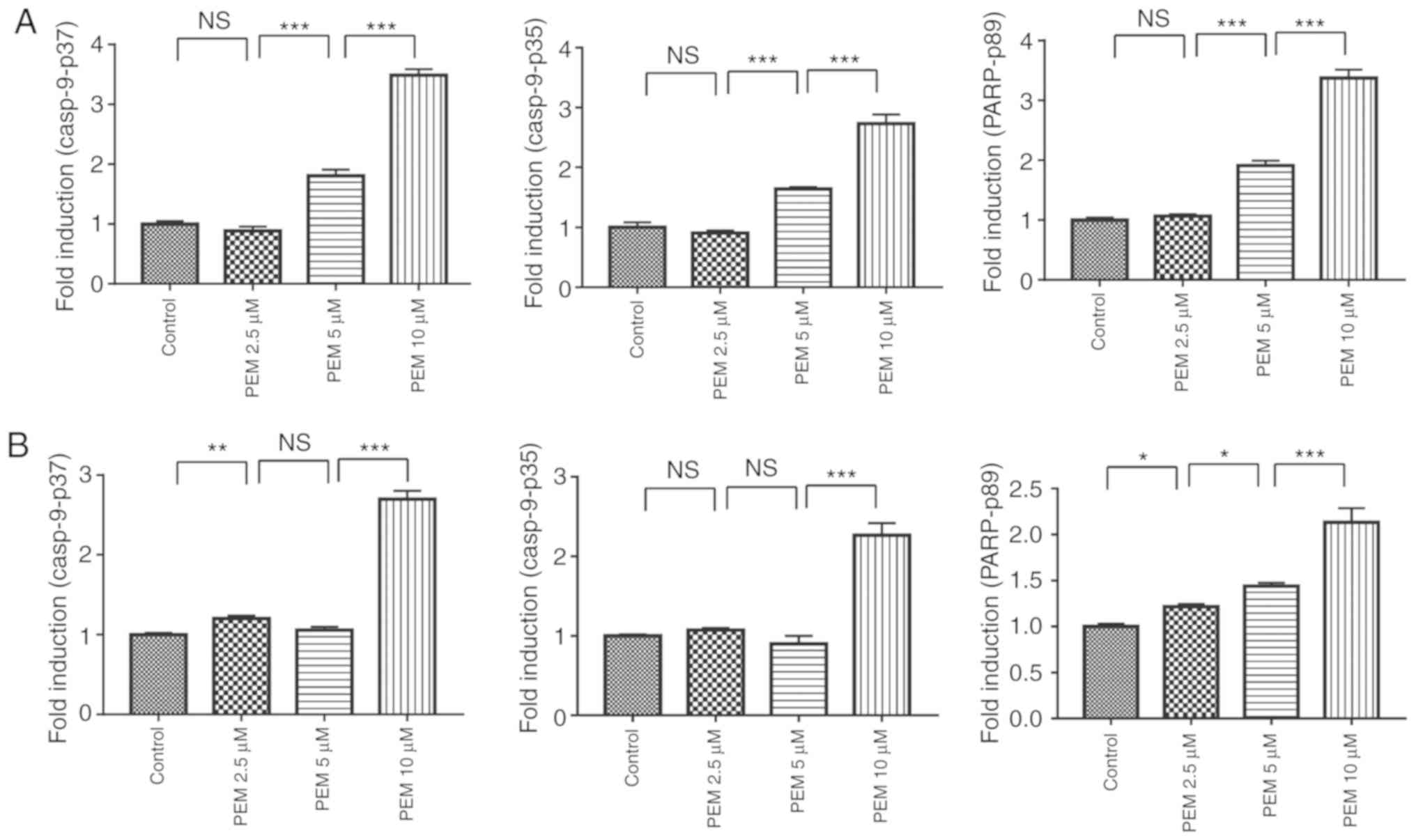
cells. Furthermore, the expression levels of apoptotic proteins
were detected via western blot analysis. The data demonstrated that
pemetrexed induced cleavage and activation of the apoptotic
proteins casp8, casp9, casp3 and PARP in a concentration-dependent
manner in human ESCC cells (Figs. 4
and 5). These results indicated that
exposure to pemetrexed may trigger apoptosis. In conclusion,
pemetrexed triggers G0/G1 cell cycle arrest
and apoptosis in human ESCC cells.
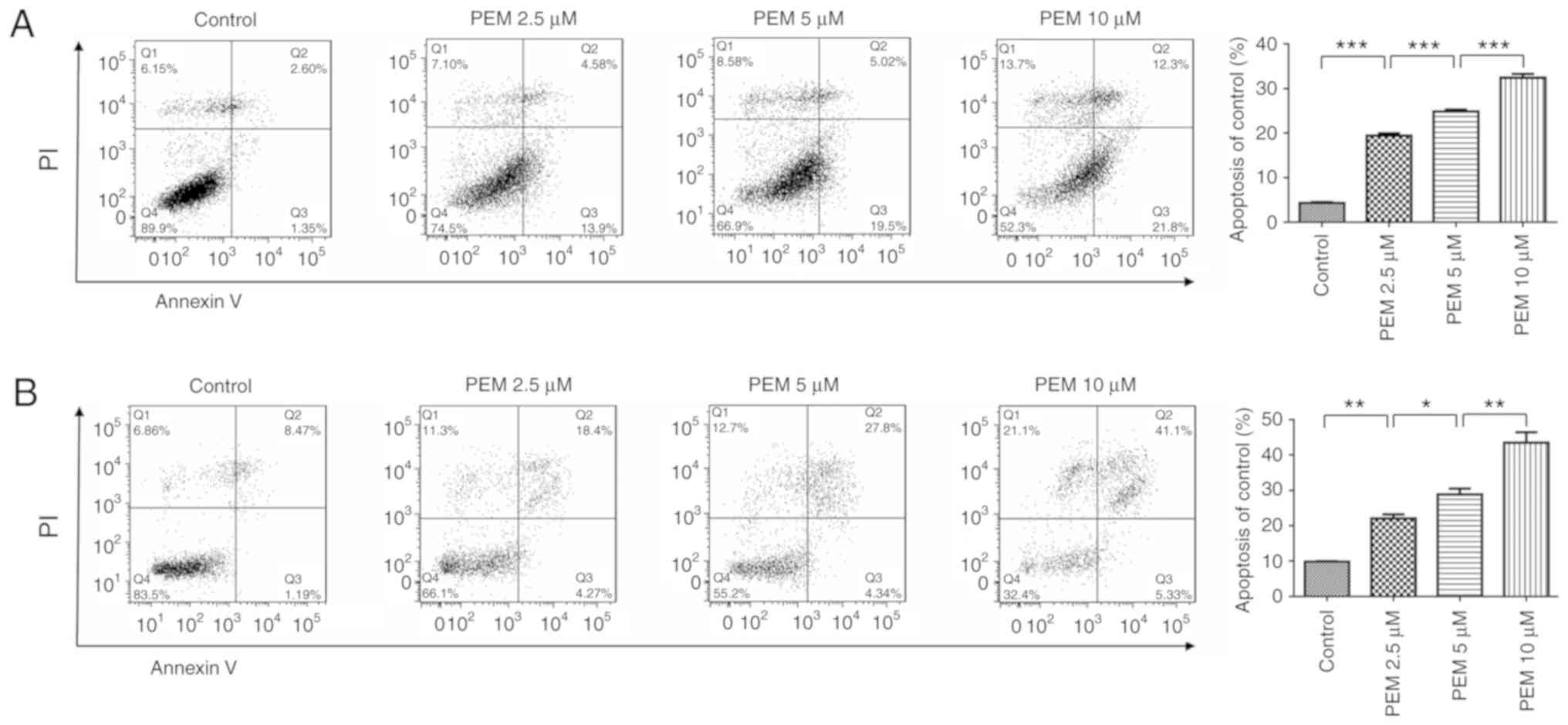 | Figure 3.Pemetrexed induces apoptosis in human
esophageal squamous cell carcinoma cells. (A) Eca-109 and (B)
EC9706 cells were treated with 0, 2.5, 5 or 10 µM pemetrexed and
incubated for 36 h. Following treatment, the cells were harvested
for apoptosis assays. The percentage of apoptotic cells was
quantified using FlowJo software and analyzed using SPSS software.
All data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01, ***P<0.001. Q1: (Annexin V−
FITC)−/PI+, necrotic cells. Q2: (Annexin
V−FITC)+/PI+, late apoptotic
cells. Q3: (Annexin V− FITC)+/PI−,
early apoptotic cells. Q4: (Annexin V−
FITC)−/PI−, normal control cells. PI,
propidium iodide; FITC, fluorescein isothiocyanate; PEM,
pemetrexed. |
Involvement of the NOXA/Mcl-1 axis in
pemetrexed-induced apoptosis
The NOXA/Mcl-1 axis has been reported to be involved
in chemotherapeutically-induced apoptosis in numerous types of
tumor cells (12,13). To characterize the molecular mechanism
of pemetrexed-induced apoptosis in human ESCC cells, the expression
levels of proteins following treatment with pemetrexed were
analyzed. Western blot analysis revealed that the expression of
NOXA was upregulated in a concentration-dependent manner following
treatment with pemetrexed. By contrast, the expression levels of
Mcl-1 decreased (Fig. 6A and B).
These results indicated that the NOXA/Mcl-1 axis may be involved in
pemetrexed-induced apoptosis in human ESCC cells. The expression
level of Bcl-2, which is another member of the Bcl-2 family, was
also detected and the western blotting results demonstrated that
Bcl-2 was downregulated following treatment with pemetrexed
(Fig. 6C and D). In summary,
pemetrexed may induce mitochondrial apoptosis in human ESCC
cells.
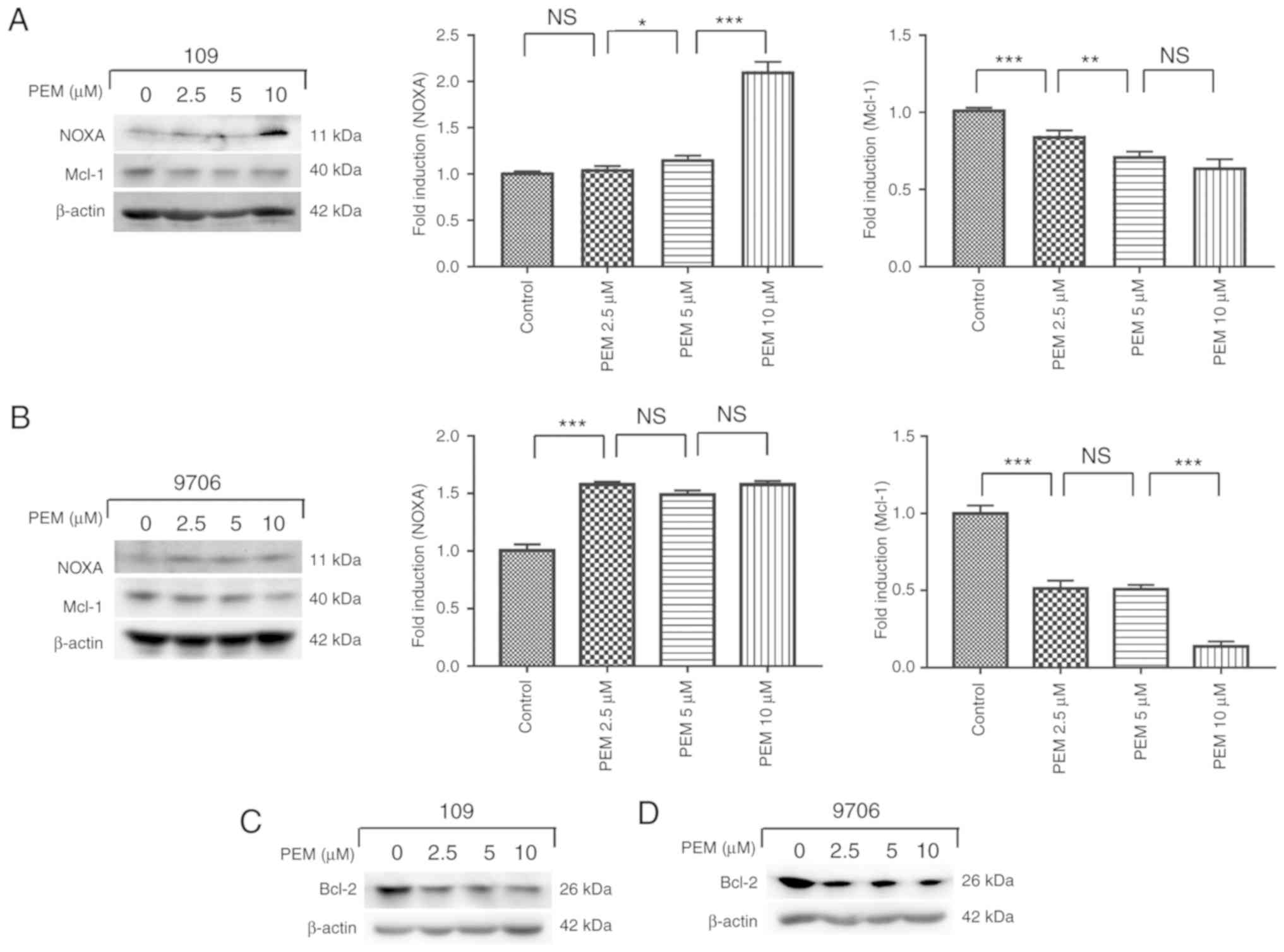 | Figure 6.Involvement of the NOXA/MCL-1 axis in
pemetrexed-induced apoptosis. (A and C) Eca-109 and (B and D)
EC9706 cells were treated with 0, 2.5, 5 or 10 µM pemetrexed and
incubated for 36 h. Following treatment, NOXA and Mcl-1 expression
was quantified via western blot analysis. All data are presented as
the mean ± standard deviation. *P<0.05, **P<0.01,
***P<0.001. PEM, pemetrexed; NOXA,
phorbol-12-myristate-13-acetate-induced protein 1; Mcl-1, induced
myeloid leukemia cell differentiation protein Mcl-1; Bcl-2,
apoptosis regulator Bcl-2; NS, not significant. |
Pemetrexed triggers ER stress in human
ESCC cells
The ER response has been reported to be activated by
chemotherapeutics while inducing apoptosis in cancer cells
(12,20). To determine whether pemetrexed
triggers ER stress in human ESCC cells, a number of relevant
proteins in the ER stress pathway were examined. The results
illustrated that the marker proteins IRE1α, Bip and CHOP were
upregulated in a concentration-dependent manner following treatment
with pemetrexed (Fig. 7). These
results indicated that pemetrexed may trigger ER stress in human
ESCC cells.
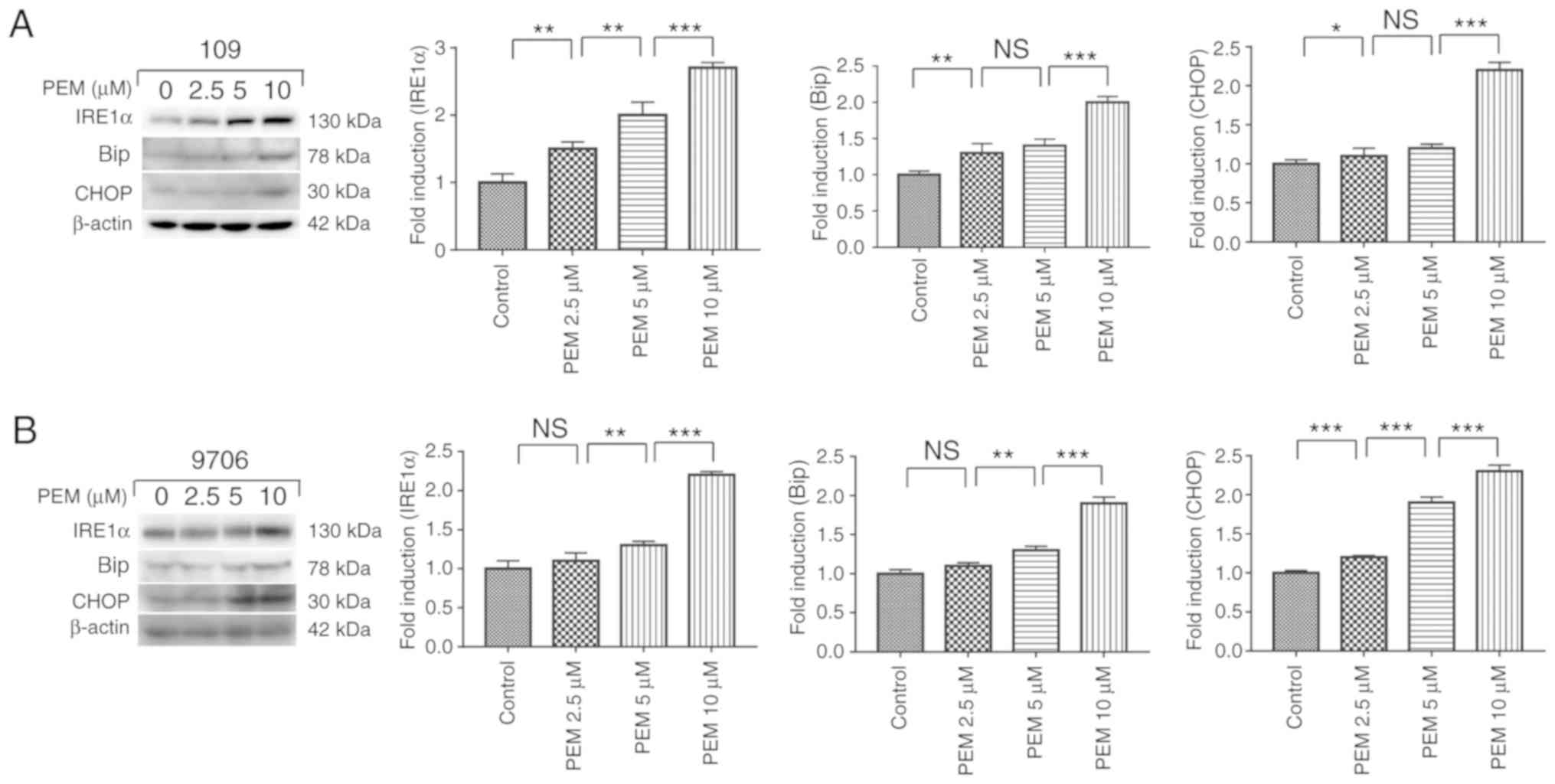 | Figure 7.Pemetrexed triggers endoplasmic
reticulum stress in human esophageal squamous cell carcinoma cells.
(A) Eca-109 and (B) EC9706 cells were treated with 0, 2.5, 5 or 10
µM pemetrexed and incubated for 36 h. Following treatment, the
protein expression of IRE1α, Bip and CHOP was quantified via
western blot analysis. All data are presented as the mean ±
standard deviation, *P<0.05, **P<0.01, ***P<0.001. NS, not
significant; PEM, pemetrexed; IRE1α, inositol-requiring enzyme 1α;
CHOP, CCAAT-enhancer-binding protein homologous protein; Bip,
binding immunoglobulin protein. |
Discussion
Esophageal cancer is the third leading cause of
cancer-associated mortality in China (21). The incidence and mortality rates of
esophageal cancer in China have increased in recent years (21,22). ESCC
is the predominant histological subtype of esophageal cancer. Due
to its high malignancy and the inadequate efficacy of conventional
therapy, ESCC frequently has an unfavorable prognosis (23,24). Thus,
the development of effective novel agents and treatments is of the
utmost importance.
Pemetrexed is a clinically available multi-target
antifolate cytotoxic agent (19). It
is able to inhibit the synthesis of purine and pyrimidine by
blocking dihydrofolate reductase, thymidylate synthase and
glycinamide ribonucleotide formyltransferase (25). Recently, pemetrexed has been reported
to exhibit antitumor effects in various solid tumors (19,25). The
present study aimed to determine whether pemetrexed was able to
exert anticancer effects in ESCC and to elucidate the underlying
molecular mechanism.
In the present study, the cytotoxicity of pemetrexed
against ESCC cells was analyzed, and it was reported that
pemetrexed displayed a time-dependent inhibitory effect on the
survival of ESCC cells. Subsequently, it was demonstrated that
pemetrexed induced G0/G1-phase cell cycle
arrest and apoptosis in a concentration-dependent manner in human
ESCC cells. These results indicated that pemetrexed may exert
anticancer effects by inducing G0/G1-phase
cell cycle arrest and apoptosis in human ESCC cells. It has also
been demonstrated that pemetrexed combined with cisplatin displays
an effective synergistic effect in a wide variety of solid tumors
(12,26,27).
However, how this combination may exert a beneficial synergistic
effect in human ESCC cells, and the underlying molecular mechanism,
requires further research.
The results of the present study revealed that
necrosis was also increased following treatment with pemetrexed.
Cell death occurs via necrosis in addition to apoptosis. Research
has reported that cell apoptosis and necrosis may be led by ER
stress and mitochondrial membrane permeability (28,29). In
the present study, persistent ER stress and increasing
mitochondrial membrane permeability may be involved in the
induction of necrosis. The molecular mechanism and effect of
necrosis in pemetrexed-induced survival inhibition requires further
investigation.
To understand the underlying molecular mechanism by
which pemetrexed induces apoptosis in human ESCC cells, the
expression levels of apoptosis-associated proteins were analyzed.
NOXA and Mcl-1 are key proteins in intrinsic mitochondrial
apoptosis. It was reported that the expression levels of the
pro-apoptotic protein NOXA was upregulated in a
concentration-dependent manner following treatment with pemetrexed,
while the expression levels of the anti-apoptotic protein Mcl-1
decreased. The results indicated that pemetrexed may induce
intrinsic mitochondrial apoptosis via the NOXA/Mcl-1 axis in human
ESCC cells. The expression levels of Bcl-2 were also measured and
the western blotting results demonstrated that Bcl-2 was
downregulated following treatment with pemetrexed. In summary,
pemetrexed may induce mitochondrial apoptosis in human ESCC cells.
In addition to intrinsic mitochondrial apoptosis, extrinsic death
receptor apoptosis is another key apoptotic signaling pathway.
However, further research is required to determine whether
pemetrexed induces extrinsic death receptor apoptosis in human ESCC
cells.
A number of chemotherapeutics activate an ER
response while inducing apoptosis in cancer cells (12,20). To
determine whether pemetrexed triggers ER stress in human ESCC
cells, a number of relevant proteins in the ER stress pathway were
investigated. The results demonstrated that the marker proteins
IRE1α, Bip and CHOP were upregulated in a concentration-dependent
manner following treatment with pemetrexed, which indicates that
pemetrexed triggers ER stress in human ESCC cells. Cyclic
AMP-dependent transcription factor ATF-3 (ATF3) is also a key
protein marker of ER stress (30).
ATF3 has been reported to act as a suppressor of CHOP in a number
of cancer types, whereas it may promote the expression of CHOP in
others (31,32). Previous studies have reported that the
expression of ATF3 is downregulated in certain ESCCs, and this
decreased expression is negatively correlated with a poor prognosis
in vivo, and with cell growth and invasion in vitro
(33,34). However, further research is required
to determine whether ATF3 is involved in pemetrexed-induced ER
stress in human ESCC cells.
In conclusion, the results of the present study
demonstrated that pemetrexed was able to inhibit cell survival and
induce G0/G1-phase cell cycle arrest and
apoptosis in human ESCC cells. The NOXA/Mcl-1 axis may be involved
in intrinsic apoptosis induced by pemetrexed. Furthermore,
pemetrexed induced an ER stress response while activating
apoptosis. The present study provided important mechanistic
insights into pemetrexed as a potential cancer treatment and
enhanced the current understanding of human ESCC.
Acknowledgements
The authors would like to thank the staff of the
Central Research Laboratory of the Second Hospital of Shandong
University (Jinan, China) for their technical assistance and
support.
Funding
The present study was supported by the Shandong
Natural Science Foundation (grant no. ZR2016HP43) and the key
research and development plan of Shandong Province (grant no.
2015GSF118037).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
XZ and HZ participated in every step of the design
project and in specific experiments. XL also participated in the
design of this study and most of the experiments, and was the
writer of this manuscript. HS helped in the experiments and
modified the language of the manuscript. FK participated in the
quantification of proteins and provided advice for the revision of
the manuscript. YG cultured the cells for this study. YC, LZ and DG
performed the statistical analysis. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lagergren J and Lagergren P: Recent
developments in esophageal adenocarcinoma. CA Cancer J Clin.
63:232–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang P, Zhao J, Hou L, Yang L, Wu K and
Zhang L: Vitamin E succinate induces apoptosis via the PI3K/AKT
signaling pathways in EC109 esophageal cancer cells. Mol Med Rep.
14:1531–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown LM, Devesa SS and Chow WH: Incidence
of adenocarcinoma of the esophagus among white Americans by sex,
stage, and age. J Natl Cancer Inst. 100:1184–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He J, Zhou M, Chen X, Yue D, Yang L, Qin
G, Zhang Z, Gao Q, Wang D, Zhang C, et al: Inhibition of SALL4
reduces tumorigenicity involving epithelial-mesenchymal transition
via Wnt/β-catenin pathway in esophageal squamous cell carcinoma. J
Exp Clin Cancer Res. 35:982016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mariette C, Robb WB, Piessen G and Adenis
A: Neoadjuvant chemoradiation in esophageal cancer. Lancet Oncol.
16:1008–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seiwert TY, Connell PP, Mauer AM, Hoffman
PC, George CM, Szeto L, Salgia R, Posther KE, Nguyen B, Haraf DJ
and Vokes EE: A phase I study of pemetrexed, carboplatin, and
concurrent radiotherapy in patients with locally advanced or
metastatic non-small cell lung or esophageal cancer. Clin Cancer
Res. 13:515–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raizer JJ, Rademaker A, Evens AM, Rice L,
Schwartz M, Chandler JP, Getch CC, Tellez C and Grimm SA:
Pemetrexed in the treatment of relapsed/refractory primary central
nervous system lymphoma. Cancer. 118:3743–3748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JP, Lee EQ, Nayak L, Doherty L,
Kesari S, Muzikansky A, Norden AD, Chen H, Wen PY and Drappatz J:
Retrospective study of pemetrexed as salvage therapy for central
nervous system lymphoma. J Neurooncol. 115:71–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Yang Y, Fan C, Di S, Hu W, Jiang
S, Li T, Ma Z, Chao D, Feng X, et al: Pterostilbene inhibits the
growth of human esophageal cancer cells by regulating endoplasmic
reticulum stress. Cell Physiol Biochem. 38:1226–1244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo YX, Lin ZM, Wang MJ, Dong YW, Niu HM,
Young CY, Lou HX and Yuan HQ: Jungermannenone A and B induce ROS-
and cell cycle-dependent apoptosis in prostate cancer cells in
vitro. Acta Pharmacol Sin. 37:814–824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Kong F, Wang L and Zhang H: c-FLIP
and the NOXA/Mcl-1 axis participate in the synergistic effect of
pemetrexed plus cisplatin in human choroidal melanoma cells. PLoS
One. 12:e01841352017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Liu X and Su L: Parthenolide
induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung
cancer cells. J Exp Clin Cancer Res. 33:32014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Lv Z, Zou J, Liu X, Ma J, Wang J,
Sa N, Jing P and Xu W: Afatinib down-regulates MCL-1 expression
through the PERK-eIF2alpha-ATF4 axis and leads to apoptosis in head
and neck squamous cell carcinoma. Am J Cancer Res. 6:1708–1719.
2016.PubMed/NCBI
|
|
15
|
Hauck P, Chao BH, Litz J and Krystal GW:
Alterations in the Noxa/Mcl-1 axis determine sensitivity of small
cell lung cancer to the BH3 mimetic ABT-737. Mol Cancer Ther.
8:883–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Yang Y, Chen H, Dan J, Cheng J,
Guo S, Sun X, Wang W, Ai Y, Li S, et al: The predominant pathway of
apoptosis in THP-1 macrophage-derived foam cells induced by
5-aminolevulinic acid-mediated sonodynamic therapy is the
mitochondria-caspase pathway despite the participation of
endoplasmic reticulum stress. Cell Physiol Biochem. 33:1789–1801.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Kwon EJ and Kim DH: Calumenin has
a role in the alleviation of ER stress in neonatal rat
cardiomyocytes. Biochem Biophys Res Commun. 439:327–332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chow SE, Kao CH, Liu YT, Cheng ML, Yang
YW, Huang YK, Hsu CC and Wang JS: Resveratrol induced ER expansion
and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma
cells. Apoptosis. 19:527–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su L, Liu G, Hao X, Zhong N, Zhong D, Liu
X and Singhal S: Death receptor 5 and cellular FLICE-inhibitory
protein regulate pemetrexed-induced apoptosis in human lung cancer
cells. Eur J Cancer. 47:2471–2478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng H, Zheng R, Zhang S, Zuo T, Xia C,
Zou X and Chen W: Esophageal cancer statistics in China, 2011:
Estimates based on 177 cancer registries. Thorac Cancer. 7:232–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quanjun D, Qingyu Z, Qiliang Z, Liqun X,
Jinmei C, Ziquan L and Shike H: Effect and mechanism of PAR-2 on
the proliferation of esophageal cancer cells. Eur Rev Med Pharmacol
Sci. 20:4688–4696. 2016.PubMed/NCBI
|
|
24
|
Ma YC, Ke Y, Zi X, Zhao F, Yuan L, Zhu YL,
Fan XX, Zhao NM, Li QY, Qin YH and Liu HM: Induction of the
mitochondria-mediated apoptosis in human esophageal cancer cells by
DS2, a newly synthetic diterpenoid analog, is regulated by Bax and
caused by generation of reactive oxygen species. Oncotarget.
7:86211–86224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Wang Y, Han S, Xing B, Li H, Zhu Y,
Zhou S, Wang X, Xu J and Tao R: Efficacy and safety of pemetrexed
on recurrent primary central nervous system lymphomas in China: A
prospective study. Onco Targets Ther. 10:2595–2600. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolukbas S, Manegold C, Eberlein M,
Bergmann T, Fisseler-Eckhoff A and Schirren J: Survival after
trimodality therapy for malignant pleural mesothelioma: Radical
Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and
radiotherapy. Lung Cancer. 71:75–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JC, Hirsh V, Schuler M, Yamamoto N,
O'Byrne KJ, Mok TS, Zazulina V, Shahidi M, Lungershausen J, Massey
D, et al: Symptom control and quality of life in LUX-Lung 3: A
phase III study of afatinib or cisplatin/pemetrexed in patients
with advanced lung adenocarcinoma with EGFR mutations. J Clin
Oncol. 31:3342–3350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Liu Z, Gou Y, Qin Y, Xu Y, Liu J
and Wu JZ: Apoptosis and necrosis induced by novel realgar quantum
dots in human endometrial cancer cells via endoplasmic reticulum
stress signaling pathway. Int J Nanomedicine. 10:5505–5512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arakawa S, Nakanomyo I, Kudo-Sakamoto Y,
Akazawa H, Komuro I and Shimizu S: Identification of a novel
compound that inhibits both mitochondria-mediated necrosis and
apoptosis. Biochem Biophys Res Commun. 467:1006–1011. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu L, Su L and Liu X: PKCδ regulates death
receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis
in human lung cancer cells. Mol Cancer Ther. 11:2174–2182. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolfgang CD, Chen BP, Martindale JL,
Holbrook NJ and Hai T: Gadd153/Chop10, a potential target gene of
the transcriptional repressor ATF3. Mol Cell Biol. 17:6700–6707.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu G, Su L, Hao X, Zhong N, Zhong D,
Singhal S and Liu X: Salermide up-regulates death receptor 5
expression through the ATF4-ATF3-CHOP axis and leads to apoptosis
in human cancer cells. J Cell Mol Med. 16:1618–1628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan H, Zhang H, Xie J, Chen B, Wen C, Guo
X, Zhao Q, Wu Z, Shen J, Wu J, et al: A novel staging model to
classify esophageal squamous cell carcinoma patients in China. Br J
Cancer. 110:2109–2115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie JJ, Xie YM, Chen B, Pan F, Guo JC,
Zhao Q, Shen JH, Wu ZY, Wu JY, Xu LY and Li EM: ATF3 functions as a
novel tumor suppressor with prognostic significance in esophageal
squamous cell carcinoma. Oncotarget. 5:8569–8582. 2014. View Article : Google Scholar : PubMed/NCBI
|