Introduction
Lung cancer is the second most common malignant
tumour. However it causes more deaths than breast, prostate and
colon cancer combined (1). Hungary
has the highest mortality rates of lung cancer in the world
regarding both men and women. Hungary, unlike other developed
countries, records a growing number of new cases. While the
incidence hasn't increased over the last few years in men, it
continuously does in women (2).
Survival rates remain poor in non-small cell lung
cancer (NSCLC) with 49% 5-year survival rate with early (stage IA)
NSCLC and 1% 5-year survival rate in stage IV. One reason for such
poor survival is that more than 50% of patients are diagnosed with
advanced disease (3).
Although many advances have been made in the
treatment of unresectable (stage IIIB), metastatic (stage IV) or
recurrent NSCLC, such as the introduction of targeted therapy for
specific oncogenic drivers (EGFR, ALK mutations etc.),
platinum-based chemotherapy (with or without radiotherapy) still
remains the first choice in most cases.
Targeted therapies showed superior survival data,
demonstrated improved response rates and are associated with less
toxicity. Druggable mutations for EGFR and ALK mutation, however,
only occur in 25 and 5%, respectively (4,5). Vascular
endothelial growth factor (VEGF) is a key factor to endothelial
cell growth and one of the most important regulators of
angiogenesis. Increased expression of VEGF can be demonstrated in
most solid tumours including NSCLC (6). In many cases, VEGF overexpression is
associated with an increased risk of relapse and metastasis
(7–10). According to preclinical studies,
anti-VEGF monoclonal antibodies are capable of inhibiting the
growth of human tumour xenografts both in monotherapy and in
combination with chemotherapy (11–14).
Bevacizumab (BEV) (Avastin®; Genentech/Roche, San
Francisco, CA, USA) is a humanized monoclonal antibody that acts by
binding and neutralizing the VEGF-A isoform, thus preventing VEGF
ligand-receptor binding. It has demonstrated its efficacy in
colorectal (15,16), ovarian (17), breast (18,19) and
renal cancer (20,21). This was the first antivascular drug to
be licensed for the treatment of NSCLC.
According to a phase II study (22), BEV treatment in combination with
chemotherapy in NSCLC was more effective than chemotherapy alone.
The combination was also well tolerated, however, the incidence of
lung haemorrhage increased. In a post hoc multivariate analysis,
squamous cell histology was identified as an independent risk
factor for bleeding (23).
Consequently, patients with squamous cell histology were excluded
from most of the clinical trials of BEV in NCSLC.
Subsequent to the above Phase II study, the Eastern
Cooperative Oncology Group (ECOG) E4599 trial was initiated
(24). This study, which was the
first published Phase III randomized trial of an antiangiogenesis
agent in combination with chemotherapy in patients with advanced
NSCLC, randomized chemotherapy-naive patients with predominantly
non-squamous cell histology were included. In the BEV treatment
arm, following completion of chemotherapy, single-agent BEV was
continued until disease progression. Results showed that the
addition of BEV was associated with a significant improvement in
the median overall survival (OS) compared with chemotherapy alone.
Progression-free survival (PFS) was also significantly
improved.
A second Phase III trial (Avastin® in
Lung; AVAiL), evaluating BEV in combination with cisplatin and
gemcitabine (25) (another commonly
used and efficacious regimen in NSCLC) was originally initiated
with a primary end point of OS. However, after the positive OS
results of E4599, the study design was amended so as to change the
primary end point from OS to PFS. Patients were randomly assigned
to receive cisplatin 80 mg/m2 and gemcitabine 1250
mg/m2 for up to six cycles plus low-dose BEV (7.5
mg/kg), high-dose BEV (15 mg/kg) or placebo every 3 weeks until
disease progression. PFS was significantly prolonged with BEV.
Interestingly, according to the final efficacy analysis, OS was
>13 months in all treatment groups, which was the longest OS
reported for advanced non-squamous NSCLC in a clinical trial
setting, although it did not yield a statistically significant
prolongation with either BEV dose (26).
As a result of the above trials, BEV in combination
with platinum-based chemotherapy was approved for the first-line
treatment of patients with advanced NSCLC by the European Medicines
Agency (EMA) in August 2007.
Although BEV was approved with platinum-based
chemotherapy in NSCLC in 2007, so far no Hungarian data have been
available. The AVALANCHE study (ClinicalTrials.gov, identifier: NCT03170284) was
undertaken to assess the clinical outcomes of first-line BEV
combined with standard platinum-based regimens in Hungarian
clinical practice.
Patients and methods
Study design
AVALANCHE (ClinicalTrials.gov, identifier: NCT03170284) was a
multi-centre single-arm observational study designed to assess the
efficacy and safety of BEV therapy in patients with advanced,
unresectable, metastatic or recurrent nsNSCLC (other than
predominantly squamous cell histology) in the routine oncology
practice in Hungary. Further objective of the study was to assess
and identify possible treatment-related prognostic factors.
Patients
This study was originally projected to enrol 150
patients from 40 Hungarian study centres. Fortunately, however, due
to the high number of patients recruited by some centres, nearly
300 patients were enrolled.
Patients with histology or cytology proven
unresectable advanced, metastatic or recurrent (stage IIIB/IV)
NSCLC other than predominantly squamous cell histology were
included in the present study. There were 143 male (50.5%) and 135
female (47.7%) patients and no data on gender was available in 5
patients (1.8%) (Table I).
 | Table I.Patient demographics and
treatment. |
Table I.
Patient demographics and
treatment.
|
Characteristics | No. of patients, n
(%) |
|---|
| Evaluable patient
population | 283 (99.6) |
| Patient population
evaluable in terms of PFS | 252 (88.7) |
| Patient population
evaluable in terms of OS | 250 (88) |
| Age (years) |
|
|
Mean | 58.16±9.032 |
|
Men | 58.30±8.986 |
|
Women | 58.02±9.113 |
| Gender |
|
|
Male | 143 (50.5) |
|
Female | 135 (47.7) |
| No
data | 5 (1.8) |
| Histologic
type |
|
|
Adenocarcinoma | 271 (95.8) |
|
Bronchoalveolar carcinoma | 11 (3.9) |
|
Squamous cell carcinoma | 1 (0.4) |
| Stage |
|
| III
B | 52 (18.4) |
| IV | 226 (79.9) |
| No
data | 5 (1.8) |
| Previous
treatment |
|
|
Previous surgery | 64 (22.6) |
|
Adjuvant/neoadjuvant
chemotherapy | 18 (6.4) |
|
Radiotherapy | 18 (6.4) |
| Chemotherapeutic
agent during study |
|
|
Paclitaxel | 132 (46.6) |
|
Gemcitabine | 111 (39.2) |
|
Docetaxel | 18 (6.4) |
|
Vinorelbine | 2 (0.7) |
|
Other | 7 (2.5) |
| No
data | 13 (4.6) |
| Reported reasons
for ending the study |
|
|
Progression of primary
disease | 172 (60.8) |
|
Deterioration of symptoms | 4 (1.4) |
| Loss of
contact with the patient | 7 (2.5) |
| Adverse
event associated with | 13 (4.6) |
| BEV
treatment |
|
Patient's decision | 17 (6.0) |
|
Mortality | 16 (5.7) |
|
Other | 45 (15.9) |
| No
data | 9 (3.2) |
The exclusion criteria were the following: i)
hypersensitivity to the active substance or to any of the
excipients of Avastin®; ii) hypersensitivity to products
derived from Chinese hamster ovary (CHO) cells or to other
recombinant human or humanized antibodies; iii) pregnancy and iv)
presence of untreated central nervous system metastases. The
present study was done in accordance with the Declaration of
Helsinki, Good Clinical Practice International Conference on
Harmonisation Tripartite Guidelines, laws and regulations of the
participating institutes' country. The present study was approved
by the Hungarian Ethics Committee and Health Authority. All
patients provided written informed consent.
Treatment
Eligible patients received first-line BEV with
cisplatin or carboplatin in accordance to the approved and
reimbursed BEV indication in Hungary (BEV 7.5 mg/kg, every 3 weeks
with any platinum-doublet for up to 6 cycles) then non-progressors
proceeded to receive BEV until disease progression or unacceptable
toxicity. The maintenance therapy regimen was 7,5 mg/kg every 3
weeks until PD or intolerable toxicity. The third component of the
combination chemotherapy was one of the following: paclitaxel,
gemcitabine, docetaxel or vinorelbine. Based on the therapeutic
protocol, patients were followed up until the first progression of
their primary disease, or death, or withdrawal of consent, or loss
of contact with the patient, or closure of the study, whichever
occurred first.
Progression-free and OS
Investigators seemed to be frequently using PFS and
time-to-progression (TTP) interchangeably in clinical trials in the
early 2000s (27). The protocol of
our study defined TTP as the time elapsed from the date of
enrolment until the first documented progression or the death of
the patient from any cause which is in accordance with the current
definition of PFS. To avoid confusion, PFS will be used hereinafter
for the denomination of the primary endpoint of the study.
Progression was determined by the investigator at the routine
clinical practice follow-up examinations. PFS was calculated from
the start of BEV treatment.
Secondary endpoints included best tumour response
(complete remission (CR), partial remission (PR), stable disease
(SD), progressive disease (PD)), OS (based on retrospective
analysis) and indicators of safety (serious and non-serious adverse
events). Objective response rate (ORR) was calculated from patients
experiencing complete or partial remission.
Basic demographic data, basic vital parameters,
primary disease-related historical data, ECOG performance status,
data related to BEV treatment, results of the staging assessments
as well as the patient's comorbidities and concomitant treatments
were recorded in an electronic case-report form.
Following the closure of the study, data for the
assessment of the PFS were available for 252 patients. As per the
amended protocol, the secondary endpoint (OS) was retrospectively
analysed based on data from 250 patients.
During the treatment period regular monitoring
visits were conducted to ensure high-quality data collection. Data
related to BEV treatment, blood pressure, body weight, concomitant
treatments and adverse events were registered.
The following data were recorded at the
end-of-treatment visit: End date of BEV treatment, reason for
ending treatment, ECOG status, best tumour response observed during
treatment, concomitant treatments administered during BEV treatment
and adverse events observed during BEV treatment.
Statistical analysis
Continuous variables were compared with Student's
t-tests if the sample distribution was normal or with Mann-Whitney
U test if the sample distribution was asymmetric. Categorical data
were compared using Fisher's exact probability and χ2
tests. PFS (primary study endpoint) and OS in the total population
were analysed using Kaplan-Meier curves. Both PFS and OS were
assessed separately in subgroups according to gender, age, ECOG
status, the platinum derivate used, the use of maintenance therapy
and weather prior surgical intervention was done. Log-rank test was
used for comparison between the above mentioned groups.
PFS was defined as the time elapsed from the start
of BEV treatment until the first documented progression or the
death of the patient from any cause. For study subjects who had not
shown progression and had not died by the closure of the study, the
data were censored at the date of the last contact.
OS was defined as the time elapsed from the date of
enrolment until the death of the patient from any cause. Regarding
subjects who had not died by the closure of the study, the OS data
were analysed retrospectively after the end of the study in the
knowledge of their dates of death. Otherwise, data were censored at
the date of the last contact.
P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were conducted
using Statistica 8.0 (StatSoft, Inc., Tulsa, OK, USA) software
program.
Results
Baseline characteristics of the
patients
A total of 284 patients with corresponding diagnosis
were identified at the Hungarian study sites, and were subsequently
enrolled into the study between 17th June 2008 and 3rd May 2011,
out of which data of 283 patients were evaluable. From among the 41
study centres originally involved, no patients were enrolled at 16
sites, thus in fact 25 centres participated actively. The highest
number of patients enrolled at one centre was 36, whereas the
smallest was 1. One patient did not comply with all the inclusion
and exclusion criteria: The patient's histological diagnosis was
squamous cell carcinoma; therefore evaluable patient population was
283. Central localization of the primary tumour was reported in 61
patients (21.6%) and cavitated tumour in 4 patients (1.4%) in the
total patient population.
The study population had to be reduced to 252 in
case of PFS and 250 regarding OS. In case of PFS 31 patients and in
case of OS 33 patients had to be excluded from the data assessment
due to missing or incomplete information. These information could
not be recovered retrospectively.
The demographic characteristics of the enrolled and
evaluable patients are summarized in Table I.
Treatment
Prior to enrolment, 64 patients (22.6%) had
undergone surgical intervention, 18 patients (6.4%) had received
adjuvant/neoadjuvant chemotherapy, and 18 patients (6.4%) had
received radiotherapy (Table I).
Patients received cisplatin (N=148, 52.3%) or
carboplatin (N=124, 43.8%) treatment in accordance with the
protocol in approximately half-and-half proportion during the
study. No data are available for 11 patients (3.9%). The other
components of the combination chemotherapy are shown in Table I.
The vast majority of patients (N=262, 92.6%)
received BEV in 3-weekly cycles. A treatment of different cycle
frequency was applied in two patients (0.7%), and no data were
available for 19 patients (6.7%). The median number of BEV
treatment cycles in the retrospectively evaluated patient
population was 6.
The most common reason for ending the study was
documented as progression of the primary disease in more than half
of the study subjects (60.8%). Patient's decision, patient's death,
adverse event related to BEV therapy, loss to follow-up, and
symptom deterioration accounted for ending the study in 6.0, 5.7,
4.6, 2,5 and 1.4% of the cases, respectively. Other reasons behind
ending the study occurred in 15.9%; no data were available in 3.2%
of cases.
Efficacy analysis
PFS
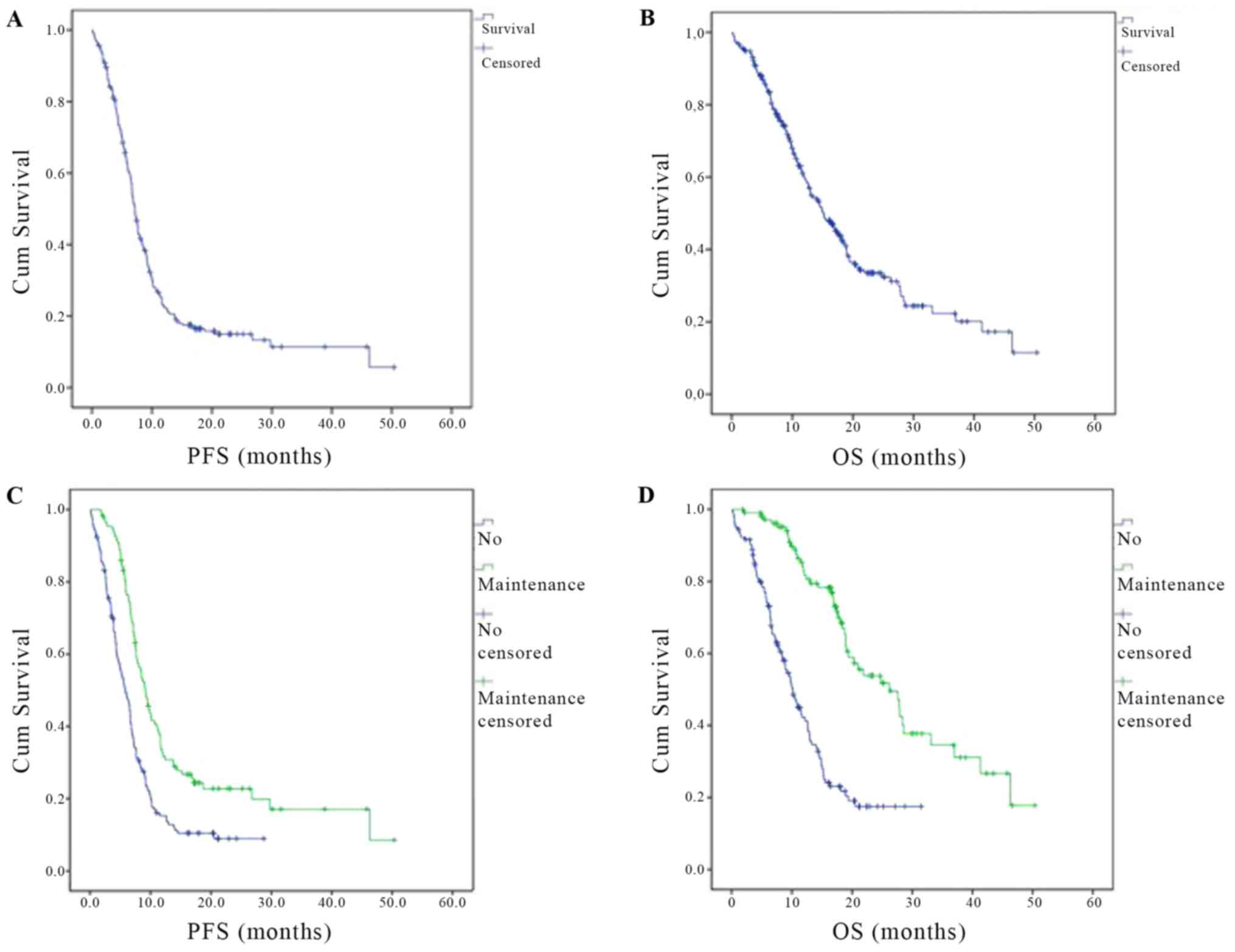
The PFS in the total study patient population was
7.162±0.282 (CI95%: 6.609–7.715) months (Fig. 1A). The subgroup-analysis of PFS by
gender showed that the survival time with BEV treatment was longer
in women (median: 7.589±0.647, CI95%: 6.321–8.858
months) than in men (median: 6.669±0.375, CI95%:
5.934–7.405 months). This difference, however, was not significant
(P=0.542).
The median PFS was higher in patients with an ECOG
status of 0 at enrolment (median: 7.326±0.535, CI95%:
6.278±8.375 months) than in patients with a baseline ECOG status of
1 (median: 6.702±0.597 months, CI95%: 5.531–7.873
months). However, the difference between the two groups was not
remarkable (P=0.123).
Similarly, PFS was not significantly influenced by
the localization of the tumour (central vs. non-central,
P=0.813).
Interestingly, the median PFS in patients who had
undergone surgical intervention prior to enrolment (median:
8.411±0.947, CI95%: 6.554–10.267 months) was notably
higher (P=0.017) compared with patients with no such prior
intervention (median: 6.834±0.265, CI95%: 6.314–7.353
months). In contrast, neither adjuvant/neoadjuvant chemotherapy
(P=0.165) nor radiotherapy (P=0.165) applied prior to enrolment had
a significant impact on median PFS.
The platinum derivative used had no significant
influence on median PFS, either (P=0.199).
Nearly 10% of the patient population with evaluable
data were over 70 years of age at the time of enrolment. The median
PFS was not significantly different between patients under or above
70 years of age (P=0.541).
Of note, median PFS was significantly higher
(P<0.001) in patients receiving BEV maintenance therapy (median:
9.166±0.601, CI95%: 7.988–10.345 months) compared with
those who received no maintenance therapy (median: 5.815±0.574,
CI95%: 4.690–6.940 months) (Fig. 1C).
Secondary endpoints
Tumour response
Disease control was achieved in a remarkable 86.5%
with CR in 2.3%, and PR in 44.4% of the cases with evaluable data.
PD was recorded in 13.5% of evaluable cases and sufficient data was
not available in 32.6% (Table
II).
 | Table II.Best tumor response reached during
the first-line treatment. |
Table II.
Best tumor response reached during
the first-line treatment.
| Response | N | Patient population
with evaluable data (n=133), (%) | Total patient
population (n=216), (%) |
|---|
| Complete
remission | 3 | 2.3 | 1.5 |
| Partial
remission | 59 | 44.4 | 29.9 |
| Stable disease | 53 | 39.8 | 26.9 |
| Progressive
disease | 18 | 13.5 | 9.1 |
| Not assessable | 83 | − | 32.6 |
OS
The median OS in the total study population was
15.179±1.377 months (CI95%: 12.480–17.877) (Fig. 1).
As with PFS, we performed subgroup-analysis of OS by
gender, ECOG status, prior surgical procedure and chemotherapy.
Results can be seen on Table
III.
 | Table III.Subgroup analysis of OS. |
Table III.
Subgroup analysis of OS.
|
| Gender | ECOG status | Prior surgery | Prior
chemo-/radiotherapy | Platinum derivative
used |
|---|
|
|
|
|
|
|
|
|---|
| Variable | Male | Female | ECOG 0 | ECOG 1 | Yes | No | Chemotherapy | Radiotherapy | Cisplatin | Carboplatin |
|---|
| Median OS | 12.583 | 17.511 | 18.891 | 13.306 | 26.218 | 13.306 | Not
significant | Not
significant | 16.953 | 12.977 |
|
|
|
|
|
|
|
| P=0.237 | P=0.237 |
|
|
| CI 95% | 9.544–15.622 | 14.320–20.703 | 14.869–22.914 | 10.385–16.227 | 18.721–33.714 | 11.373–15.239 |
|
| 13.475–20.431 | 9.661–16.294 |
| P-value | 0.071 | 0.004 | 0.001 |
|
| 0.006 |
The localization of the tumour had no impact on OS
(P=0.992) in the patient population studied.
Surprisingly, we found a tendency towards a higher
median OS for patients over 70 years of age (18.398±3.869 months,
CI95%: 10.815–25.982 months) compared with patients
younger than 70 years (15.014±1.329 months, CI95%:
12.410–17.619 months), although this difference remained not
significant (P=0.638).
A remarkably longer (P<0.001) OS was observed in
patients receiving BEV maintenance therapy (median: 26.218±3.946
months, CI95%: 18.484–33.952 months) than in those
without maintenance BEV therapy (median: 10.152±0.975 months,
CI95%: 8.240–12.064 months) (Fig. 1D).
Safety and adverse events
As per the protocol, possible adverse events (AE)
encountered during the study were recorded in the Case Report Form.
Data on AE were recorded from the start of treatment until the end
of treatment.
During the study, a total of 157 AEs were reported
for 59 patients, 14 of which were serious (sAE) (Table IV).
 | Table IV.Summary of the adverse events
reported in the present study. |
Table IV.
Summary of the adverse events
reported in the present study.
| Adverse event | n (%) |
|---|
| Anemia | 23 (14.7) |
|
Thrombocytopenia | 14 (9) |
| Neutropenia | 12 (7.7) |
| Hypertension | 7 (4.5) |
| Nausea | 7 (4.5) |
| Epistaxis | 6 (3.9) |
| Chest pain | 5 (3.2) |
| Acute
bronchitis | 4 (2.6) |
| Weight loss | 4 (2.6) |
| Bone pain | 3 (2) |
| Diarrhea | 3 (2) |
| Pulmonary
embolism | 3 (2) |
| Hemoptysis | 3 (2) |
| Hyponatremia | 3 (2) |
| Deep vein
thrombosis | 3 (2) |
| Hoarseness | 3 (2) |
| Cough | 2 (1.3) |
| Fever | 2 (1.3) |
| Respiratory
infection | 2 (1.3) |
| Obstipation | 2 (1.3) |
| Pneumonia | 2 (1.3) |
| Pyuria | 2 (1.3) |
| Tachycardia | 2 (1.3) |
| Throat pain | 2 (1.3) |
| Lung abscess | 1 (0.7) |
|
Agranulocytosis | 1 (0.7) |
| Acute osteomyelitis
(jaw) | 1 (0.7) |
| Allergic
dermatitis | 1 (0.7) |
| Allergic
reaction | 1 (0.7)) |
| Hip pain
(right-sided) | 1 (0.7) |
| Decubitus | 1 (0.7) |
| Dermatitis
(forehead, back) | 1 (0.7) |
| Dermatitis
(generalized) | 1 (0.7) |
| Cholesterol
increased | 1 (0.7) |
| Exsiccosis | 1 (0.7) |
| Ulcer (in the
mouth, tongue) | 1 (0.7) |
| Gastroesophageal
reflux disease | 1 (0.7) |
| Weakness | 1 (0.7) |
| Vomiting | 1 (0.7) |
| Abdominal pain | 1 (0.7) |
| Ileus | 1 (0.7) |
| Ischemic cerebral
vascular lesions | 1 (0.7) |
| Arthralgia | 1 (0.7) |
| Swelling of
arm | 1 (0.7) |
| Hand swelling | 1 (0.7) |
| Leg swelling | 1 (0.7) |
|
Laryngotracheitis | 1 (0.7) |
| Febrile
neutropenia | 1 (0.7) |
| Prostration | 1 (0.7) |
| Leukopenia | 1 (0.7) |
| Breast
swelling | 1 (0.7) |
| Esophageal
ulcer | 1 (0.7) |
| Duodenal ulcer | 1 (0.7) |
| Suffusion without
trauma | 1 (0.7) |
| Dizziness | 1 (0.7) |
| Thrombosis (left
femoral vein) | 1 (0.7) |
| Uremia | 1 (0.7) |
| Urticaria | 1 (0.7) |
| Iron
deficiency | 1 (0.7) |
| Bleeding following
superficial injury | 1 (0.7) |
| Clear-cell renal
carcinoma | 1 (0.7) |
| Numbness (of the
soles) | 1 (0.7) |
Of all the adverse events, 63 (40.1%) events
resolved without sequelae, the investigators reported improvement
for 61 cases (38.9%) and the event resolved with remaining symptoms
in 7 cases (4.5%). 2 AEs (1.3%) had not resolved, 14 AEs (8.9%)
persisted unchanged from observation until the last follow-up of
the patient, 5 AEs (3.2%) led to the death of the patient, and the
outcome was unknown for 4 AEs (2.5%).
Of the above-mentioned AEs, 14 were categorized as
sAE, which were the following: Anaemia (3 cases), pulmonary
embolism (3 cases), haemoptysis (2 cases), deep vein thrombosis (2
cases), hypertension (1 case), neutropenia (1 case),
thrombocytopenia (1 case), uraemia (1 case). 5 of these (two cases
of pulmonary embolism, haemoptysis, hypertension and uraemia) led
to the death of the patient.
During the study period, 16 (5.6%) of the 283
enrolled and evaluable patients died. The investigators reported
the cause of death as disease progression in 11 cases (3.8%), while
a serious adverse event was behind the death of the patient in 5
cases (1.7%).
In summary, the participating investigators did not
encounter and report on any new information on the safety profile
of BEV. Indeed, the rate of reported adverse events falls behind
the rate expected based on literature data.
Discussion
Various randomised trials showed superior survival
data and acceptable safety results with the use of BEV in NSCLC
(24,25,28,29). Most
of these trials, however, were not concluded in an unselected,
real-world environment. Of note, there are still several questions
yet to be answered regarding the drug's safety, efficacy and
optimal treatment protocol. The AVALANCHE observational cohort
study (OCS) provided an opportunity to examine the safety and
efficacy of BEV in combination with chemotherapy in a real-life
setting in Hungarian everyday practice.
Generally the results of observational studies
cannot be directly compared with those of a randomized study.
However, the indicators of effectiveness in the AVALANCHE study
(which included a higher variety of patients) are consistent with
those of several randomized trials shown in Table V.
 | Table V.Baseline patient characteristics and
effectiveness of Bevacizumab with First-Line Chemotherapy for
nsNSCLC in the AVALANCHE OCS, ARIES OCS, the Phase IV SAiL Study,
and the Phase III Clinical Trials E4599 and AVAiL. |
Table V.
Baseline patient characteristics and
effectiveness of Bevacizumab with First-Line Chemotherapy for
nsNSCLC in the AVALANCHE OCS, ARIES OCS, the Phase IV SAiL Study,
and the Phase III Clinical Trials E4599 and AVAiL.
|
| Baseline patient
characteristics |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
|
|
| Gender (%) | ECOG status
(%) | Stage (%) | Results |
|---|
|
|
|
|
|
|
|
|---|
| Trial | Age (years) | Female | Male | 0 | 1 | 2 | IIIB | IV | Recurrent | Chemotherapy
regimen used | Median follow-up
(months) | Median PFS
(months) | Median OS
(months) | ORRc (%) |
|---|
| AVAiL |
| 7.5 mg/kg |
| (n=345
ITT) | <65: 70.95% | 35.55 | 64.45 | 39.73 | 60.27 | 0 | 14.88 | 77.02 | 8.1 | Gemcitabine +
Cisplatin | ≥7 for PFS, ≥12.5
for OS | 6.5 | 13.4
(11.1–15.1) | 34.6 |
| (n=307
PP) | >65: 29.05% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| AVAiL |
| 15 mg/kg |
| (n=351
ITT) | <65: 69.92% | 36.67 | 63.33 | 40.1 | 59.9 | 0 | 15.9 | 76.64 | 7.3 | Gemcitabine +
Cisplatin | ≥7 for PFS, ≥12.5
for OS | 6.7 | 13.6
(11.8–15.8) | 37.8 |
| (n=285
PP) | >65: 30.08% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| E4599 |
|
(n=434) |
|
| <70: 76%;
>70: 24% | 48 | 52 | 47 | 53 | 0 | 14 | 86 | 0 | Paclitaxel +
carboplatin | 19 | 6.2 | 12.3
(11.3–13.7) | 34.9 |
| SAIL |
|
(n=2,212) | 58.8
(24–86)a | 40 | 60 | 37 | 57 | 6 | 20 | 80 | 0 | Investigator's
choice | 12.5 (SD:
7.1–12.5) | 7.8 (7.5–8.1) | 14.6
(13.8–15.3) | 51.5 |
| ARIES |
|
(n=1,967) | >65: 51.5%;
>75: 18.8% | 46.7 | 53.3 | 36 | 48.7 | 9.3 | Locally advanced
=16.6b | Metastatic
=83.4b | Investigator's
choice | 12.5 (SD:
0.2–65.5) | 6.6 (6.3–6.9) | 13 (12.2–13.8) | 49 |
|
| AVALANCHE |
|
(n=283) |
58.16±9.032b | 49.5 | 50.5 | 33 | 67 | 0 | 19.2 | 80.8 | 0 | Investigator's
choice | n.a. | 7.162±0.282
(6.609–7.715) | 15.179
(12.480–17.877) | 46.7 |
The median PFS and OS in our study were longer than
in the AVAiL (25,26), the E4599 (24) or the ARIES (28) studies. These OS outcomes are also
comparable with the results of the phase IV SAiL trial conducted
between 2006 and 2008 in Europe. SAiL reported 14.6 months (95% CI,
13.8–15.3) OS, that was shorter than the reported OS in AVALANCHE.
The PFS in AVALANCHE was 7.162±0.282 months (CI95%:
6.609–7.715). SAiL trial reported TTP of 7,8 months (95% CI,
7.5–8.1) but not PFS. The SAiL study let the choice of platinum
doublet chemotherapy regimen to the investigator's decision
similarly to our study. However, non-platinum doublets and
single-agent chemotherapy regimens were also allowed in SAiL study
unlike in AVALANCHE. Other differences included that SAiL enrolled
a selected patient population that was generally healthier and
younger (29).
ORR outcomes in AVALANCHE were also comparable with
the ORR results of the above-mentioned studies. The 46.7% ORR was
higher than the 34.6%, 37.8% and the 34.9% of the AVAiL 7.5 mg/kg,
AVAiL 15 mg/kg and the E4599 trials, respectively. The SAiL and
ARIES trials showed higher ORR. SAiL reported 3% CR and 48% PR
(29) which is also comparable to the
2.3% CR and 44.4% PR rate of AVALANCHE.
Sandler et al (24) reported that women had significantly
lower OS in the E4599 trial. They, however, also stated that this
difference could be the result of imbalances of treatment regiments
or baseline prognostic factors between the two groups (24). The AVAiL studies (26) and our AVALANCHE trial, on the other
hand, found comparable results between women and men. Women had
longer PFS and OS than men in the AVALANCHE, however, only OS was
on the boundary of significance (P=0.071). Although, OS was
reported higher in both AVAiL studies and the AVALANCHE trial, this
survival advantage of women can also be accounted for by the
generally longer survival of women with lung cancer that has been
previously reported in statistical reports (1,30).
As for the patients' age, nearly 10% of the patient
population with evaluable data were over 70 years of age and no
significant difference was found between the two groups regarding
PFS. Surprisingly, however, OS was reported to be longer in
patients over 70 years of age, although this difference was not
significant. Contrary to our findings, the E4599 study found that
patients older than 65 years of age had a significantly higher HR
for death and suggested that these patients might not benefit from
BEV treatment (24). The AVAiL
studies reported similar HRs for OS in both groups. One concern in
previous studies was that the risk of bleeding could be higher in
older patients, however neither the E4599, nor the AVAiL studies
nor the SAiL study back up this hypothesis (31).
We observed higher PFS and OS in patients with an
ECOG status of 0 at enrolment, although only OS showed a
significant difference. This result is not surprising in light of
the fact that ECOG performance status is an important prognostic
factor in lung cancer (32–35). Of note, the E4599 and the AVAiL
studies did not find a significant difference in the HR for OS
between the ECOG 0 and the ECOG 1 group (24,26).
Johnson et al (22) assumed that central tumour location
might cause pulmonary haemorrhage more often thus decreasing the
OS. However, this was not supported by subsequent data. Neither
SAiL, nor ARIES showed significantly more pulmonary bleeding with
centrally located tumours (36,37). Based
on a retrospective analysis of the E4599 study data, Sandler et
al (38) suggested that pulmonary
haemorrhage was connected to cavitation of NSCLC instead of central
location. Further studies did not support this assumption. Our data
do not reinforce any of these suggestions. Neither the PFS, nor the
OS was significantly longer with central tumours, and cavitated
tumours were not assessed separately.
Although previous chemo- or radiotherapy did not
influence PFS or OS, we found significantly longer PFS and OS in
the patient group that underwent surgery before enrolment in this
study. There is no available data to back up this finding. The most
probable reason behind it is that the number of cancer sites is
lower in these patients. Further assessment would be needed to draw
further conclusions.
Platinum based chemotherapy has been shown in
multiple studies to result in a small but significant survival
benefit when compared to supportive care (39,40). The
most commonly used platinum derivatives are cisplatin and
carboplatin. Neither of the above mentioned two drugs were
associated with higher PFS, OS or lower toxicity when compared to
each other (41–46). Interestingly, patients treated with
cisplatin were found to have a longer OS (16.953±1.775 months) than
those receiving carboplatin (OS: 12.977±1.692 months). The
statistical difference was on the boundary of significance
(P=0.06). Santana-Davila et al (42) showed that oncologists more often
administered cisplatin to relatively younger patients with less
comorbidities. This could be a reason for the longer OS. However,
it has also been shown that morbidity is higher in patients
receiving cisplatin and they experience a higher need for health
care (42).
Our patients receiving BEV maintenance therapy
showed significantly higher PFS and OS, which correlates with
previous results published by Reck et al (25). In addition, Dranitsaris et al
(47) found that BEV maintenance
therapy contributed to a significant OS benefit. In the Phase IIIB
AvaALL study, BEV was administered even after disease progression.
A significantly higher PFS of 10.1 months was achieved in this
experimental arm compared to the control arm where only supportive
care was used after disease progression (48). There are several trials debating
whether BEV or BEV with pemetrexed is more effective for
maintenance therapy. AVAPERL and POINTBREAK, two phase III trials
designed to evaluate BEV maintenance therapy with or without
pemetrexed, showed significantly longer PFS, however the difference
regarding OS was not significant in either of them (49,50).
Our rate of reported adverse events falls behind
that of expected based on previous trials. Lynch et al
(28) reported that in the ARIES
trial 19.7% of patients experienced one or more protocol-specified
adverse event, which is somewhat lower than the 20.8% of patients
reported in AVALANCHE. However, when looking at the serious adverse
events, the 10.9% reported in ARIES is appreciably higher than the
0.5% reported in AVALANCHE. Notably, the study protocols can vary
in the qualification of serious adverse events. Crinò et al
(29) reported a rate of 38% for
serious adverse events, although only 13% was deemed related to BEV
by the investigators. There is a special interest in similar
studies regarding pulmonary bleeding, one of the most common
serious adverse event following BEV therapy. AVAiL 7.5 mg/kg, AVAiL
15 mg/kg, E4599, ARIES and SAiL reported 4, 5, 4.7, 4.1 and 9.5%
for the prevalence of any grade pulmonary haemorrhage,
respectively. In contrary to this, pulmonary haemorrhage only
occurred in 2 patients (0.7%) in AVALANCHE.
In summary, patients in Hungary commonly receive BEV
for advanced NSCLC in combination with a range of
chemotherapeutics. Despite the less strictly selected patient
population and treatment regimens survival outcomes and treatment
response rates are comparable with those of the previous large RCT
(randomised clinical trials). In our study, both PFS and OS were
significantly longer and ORR significantly higher in patients who
received BEV maintenance therapy. The adjuvant/neoadjuvant
chemotherapy or radiotherapy received prior to enrolment, the
localization of the primary tumour, the presence of metastases or
the age of the patient had no influence on the efficacy of BEV
treatment. On the other hand, previous surgery and cisplatin
chemotherapy were associated with better outcomes. We also found
low rates of adverse events and acceptable safety profile.
The study design did not allow the comparison of PFS
and OS assessed in the study, with placebo or any active
comparator, and the comparative assessment of the significance of
the prognostic factors studied, either. Due to the high censoring
rate, the median OS could not be determined after the closure of
the study; therefore, a retrospective data collection was
required.
The Avalanche study, like most OCSs had limitations
such as reporting errors, missing data, potential biases regarding
data entry and confoundment. In this study, reporting centres were
asked to enrol all eligible patients to reduce selection bias,
however, unintended selection bias cannot be excluded. All known
strong confounders were collected and analysed to reduce
confounding bias. Clinical reporting errors were reduced by
systematic data reviews occurring every 3 months.
A further limitation of the current study was that
in 12/40 planned sites, due to their lower patient turnover, we did
not identify eligible patients within the recruiting period. Thus,
representing the real life setting, not all centres enrolled
patients and there were also smaller centres where fewer patients
were recruited.
Acknowledgements
The authors would like to thank the AVALANCHE study
investigators who collected the present data reported. The abstract
was presented at the 2014 Annual Meeting of American Society of
Clinical Oncology May 30-June 3 2014 in Chicago, IL and published
as an abstract in J Clin Oncol 32 (15 suppl): 2014.
Funding
The present study was sponsored by F Hoffmann-La
Roche AG (Basel, Switzerland).
Availability of data and materials
The datasets generated and analysed during the
present study are not publicly available due to patient
confidentiality but are available from the corresponding author on
reasonable request and with permission of F Hoffmann-La Roche
AG.
Authors' contributions
ET analysed and interpreted the data and contributed
to the study design. ÁKG analysed and interpreted the collected
data and wrote the manuscript. EJ, ZS, GL, PD, ZV, LH and EC
enrolled the patients to the present study and collected the data.
VS enrolled the patients and designed the study.
Ethics approval and consent to
participate
The present study was approved by the Hungarian
Ethics Committee and Health Authority. All patients provided
written informed consent.
Patient consent for publication
All patients provided written informed consent for
the publication of any associated data.
Competing interests
The BEV used in the present study was obtained from
Genentech/Roche (South San Francisco, CA, USA). The present study
was also sponsored by F Hoffmann-La Roche. The funding body
contributed to data collection and analysis; however, the sponsor
did not influence the content of the report and did not contribute
to the writing of this manuscript. The authors declare that they
have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AE
|
adverse event
|
|
ALK
|
anaplastic lymphoma kinase
|
|
BEV
|
bevacizumab
|
|
CI
|
confidence interval
|
|
CR
|
complete response
|
|
CRF
|
case report form
|
|
ECOG
|
Eastern Cooperative Oncology group
|
|
EGFR
|
epidermal growth factor receptor
|
|
EMA
|
European medicines agency
|
|
NA
|
not applicable
|
|
NSCLC
|
non-small cell lung cancer
|
|
nsNSCLC
|
non-squamous non-small cell lung
cancer
|
|
OCS
|
observational cohort study
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PFS
|
progression-free survival
|
|
PR
|
partial response
|
|
RCT
|
randomized clinical trial
|
|
SAE
|
serious adverse event
|
|
SD
|
stable disease
|
|
SD
|
standard deviation
|
|
TNM
|
Internationally accepted
classification of malignant tumours
|
|
TTP
|
time-to-progression
|
|
VEGF
|
vascular endothelial growth factor
|
|
WHO
|
World Health Organization
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer Society. Cancer Facts
& Figures 2015 (Atlanta). American Cancer Society. 2015.
|
|
4
|
Kris MG, Johnson BE, Kwiatkowski DJ,
Iafrate AJ, Wistuba II, Aronson SL, Engelman JA, Shyr Y, Khuri FR,
Rudin CM, et al: Identification of driver mutations in tumor
specimens from 1,000 patients with lung adenocarcinoma: The NCI's
lung cancer mutation consortium (LCMC). J Clin Oncol. 29 (18
Suppl):787S2011. View Article : Google Scholar
|
|
5
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutationpositive non-small-cell lung cancer (EURTAC):
A multicentre, open-label, randomised phase 3 trial. Lancet Oncol.
13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gentzler RD, Yentz SE and Patel JD:
Bevacizumab in advanced NSCLC: Chemotherapy partners and duration
of use. Curr Treat Options Oncol. 14:595–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattern J, Koomägi R and Volm M:
Association of vascular endothelial growth factor expression with
intratumoral microvessel density and tumour cell proliferation in
human epidermoid lung carcinoma. Br J Cancer. 73:931–934. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Manseau EJ, Senger DR and Dvorak HF: Expression of vascular
permeability factor (vascular endothelial growth factor) and its
receptors in adenocarcinomas of the gastrointestinal tract. Cancer
Res. 53:4727–4735. 1993.PubMed/NCBI
|
|
9
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Guidi AJ, Dvorak HF, Senger DR, Connolly JL and Schnitt SJ:
Expression of vascular permeability factor (vascular endothelial
growth factor) and its receptors in breast cancer. Hum Pathol.
26:86–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seto T, Higashiyama M, Funai H, Imamura F,
Uematsu K, Seki N, Eguchi K, Yamanaka T and Ichinose Y: Prognostic
value of expression of vascular endothelial growth factor and its
flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung
Cancer. 53:91–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factorinduced angiogenesis suppresses tumour growth in vivo.
Nature. 36:841–844. 1993. View Article : Google Scholar
|
|
13
|
Kabbinavar FF, Wong JT, Ayala RE, Wintroub
AB, Kim KJ and Ferrara N: The effect of antibody to vascular
endothelial growth factor and cisplatin on the growth of lung
tumors in nude mice. Proc Am Assoc Cancer Res. 36:4881995.
|
|
14
|
Armanini M, Gillett N, Phillips HS and
Ferrara N: Importance of VEGF for breast cancer angiogenesis in
vivo: Implications from intravital microscopy of combination
treatments with an anti-VEGF neutralizing monoclonal antibody and
doxorubicin. Anticancer Res. 19:4203–4214. 1999.PubMed/NCBI
|
|
15
|
Qu CY, Zheng Y, Zhou M, Zhang Y, Shen F,
Cao J and Xu LM: Value of bevacizumab in treatment of colorectal
cancer: A meta-analysis. World J Gastroenterol. 21:5072–5080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshida H, Yabuno A and Fujiwara K:
Critical appraisal of bevacizumab in the treatment of ovarian
cancer. Drug Des Devel Ther. 9:2351–2358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller K, Armanini M, Gillett N, Phillips
HS and Ferrara N: Paclitaxel plus bevacizumab versus paclitaxel
alone for metastatic breast cancer. N Engl J Med. 357:2666–2676.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Liu Q, Robidoux A, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher
L, et al: Neoadjuvant plus adjuvant bevacizumab in early breast
cancer [NSABP B-40 (NRG Oncology)]: Secondary outcomes of a phase
3, randomised controlled trial. Lancet Oncol. 16:1037–1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turnbull JD, Cobert J, Jaffe T, Harrison
MR, George DJ and Armstrong AJ: Activity of single-agent
bevacizumab in patients with metastatic renal cell carcinoma
previously treated with vascular endothelial growth factor tyrosine
kinase inhibitors. Clin Genitourin Cancer. 11:45–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–3111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Novotny WF, Holmgren E, Griffing S, et al:
Identification of squamous cell histology and central, cavitary
tumors as possible risk factors for pulmonary hemorrhage (PH) in
patients with advanced NSCLC receiving bevacizumab (BV). Proc Am
Soc Clin Oncol. 20:A13182001.
|
|
24
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et
al: Overall survival with cisplatin-gemcitabine and bevacizumab or
placebo as first-line therapy for nonsquamous non-small-cell lung
cancer: Results from a randomised phase III trial (AVAiL). Ann
Oncol. 21:1804–1809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saad ED and Katz A: Progression-free
survival and time to progression as primary end points in advanced
breast cancer: Often used, sometimes loosely defined. Ann Oncol.
20:460–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lynch TJ Jr, Spigel DR, Brahmer J,
Fischbach N, Garst J, Jahanzeb M, Kumar P, Vidaver RM, Wozniak AJ,
Fish S, et al: Safety and effectiveness of bevacizumab-containing
treatment for non-small-cell lung cancer: Final results of the
ARIES observational cohort study. J Thorac Oncol. 9:1332–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crinò L, Dansin E, Garrido P, Griesinger
F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL
and Zhou C: Safety and efficacy of first-line bevacizumab-based
therapy in advanced non-squamous non-small-cell lung cancer (SAiL,
MO19390): A phase 4 study. Lancet Oncol. 11:733–740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quaresma M, Coleman MP and Rachet B:
40-year trends in an index of survival for all cancers combined and
survival adjusted for age and sex for each cancer in England and
Wales, 1971–2011: A population-based study. Lancet. 385:1206–1218.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lauro S, Onesti CE, Righini R and
Marchetti P: The use of bevacizumab in non-small cell lung cancer:
An update. Anticancer Res. 34:1537–1545. 2014.PubMed/NCBI
|
|
32
|
Simmons CP, Koinis F, Fallon MT, Fearon
KC, Bowden J, Solheim TS, Gronberg BH, McMillan DC, Gioulbasanis I
and Laird BJ: Prognosis in advanced lung cancer-A prospective study
examining key clinicopathological factors. Lung Cancer. 88:304–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Kock I, Mirhosseini M, Lau F, Thai V,
Downing M, Quan H, Lesperance M and Yang J: Conversion of karnofsky
performance status (KPS) and eastern cooperative oncology group
performance status (ECOG) to palliative performance scale (PPS) and
the interchangeability of PPS and KPS in prognostic tools. J
Palliat Care. 29:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sengupta A, Banerjee SN, Biswas NM, Jash
D, Saha K, Maji A, Bandyopadhyaya A and Agarwal S: The incidence of
hyponatraemia and its effect on the ECOG performance status among
lung cancer patients. J Clin Diagn Res. 7:1678–1682.
2013.PubMed/NCBI
|
|
35
|
Young J, Badgery-Parker T, Dobbins T,
Jorgensen M, Gibbs P, Faragher I, Jones I and Currow D: Comparison
of ECOG/WHO performance status and ASA score as a measure of
functional status. J Pain Symptom Manage. 49:258–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Griesinger F, B.A, Eberhardt W, Garrido P,
Isla D, Ko Y, Kohlhaufl M, Schneider C, Thatcher T and Palakis N:
Safety of first line bevacizumab-based therapy in the SAiL
(MO19390) trial: Central trumor location and hypertension in
patients with advanced non-small cell lung cancer. Ann Oncol. 21
Suppl 8:(viii): 1442010.
|
|
37
|
Kumar P, Fishbach NA, Brahmer JR, Spigel
DR, Beatty S, Teng S, Flick ED, Sing A and Lynch TJ: Baseline
radiographic characteristics and severe pulmonary hemorrhage in
bevacizumab-treated non-small cell lung cancer patients: Results
from ARIES, an observational cohort study. J Clin Oncol. 28
Suppl:(15s): A76192010. View Article : Google Scholar
|
|
38
|
Sandler AB, Schiller JH, Gray R, Dimery I,
Brahmer J, Samant M, Wang LI and Johnson DH: Retrospective
evaluation of the clinical and radiographic risk factors associated
with severe pulmonary hemorrhage in first-line advanced,
unresectable non-small-cell lung cancer treated with Carboplatin
and Paclitaxel plus bevacizumab. J Clin Oncol. 27:1405–1412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chemotherapy in non-small cell lung
cancer: A meta-analysis using updated data on individual patients
from 52 randomised clinical trials. Non-small Cell Lung Cancer
Collaborative Group. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marino P, Pampallona S, Preatoni A,
Cantoni A and Invernizzi F: Chemotherapy vs supportive care in
advanced non-small-cell lung cancer. Results of a meta-analysis of
the literature. Chest. 106:861–865. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ezer N, Smith CB, Galsky MD, Mhango G, Gu
F, Gomez J, Strauss GM and Wisnivesky J: Cisplatin vs.
carboplatin-based chemoradiotherapy in patients >65 years of age
with stage III non-small cell lung cancer. Radiother Oncol.
112:272–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Santana-Davila R, Szabo A, Arce-Lara C,
Williams CD, Kelley MJ and Whittle J: Cisplatin versus
carboplatin-based regimens for the treatment of patients with
metastatic lung cancer. An analysis of Veterans Health
Administration data. J Thorac Oncol. 9:702–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smit E, Moro-Sibilot D, Carpeño Jde C,
Lesniewski-Kmak K, Aerts J, Villatoro R, Kraaij K, Nacerddine K,
Dyachkova Y, Smith KT, et al: Cisplatin and carboplatin-based
chemotherapy in the first-line treatment of non-small cell lung
cancer: Analysis from the European FRAME study. Lung Cancer.
92:35–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Castria TB, da Silva EM, Gois AF and
Riera R: Cisplatin versus carboplatin in combination with
third-generation drugs for advanced non-small cell lung cancer.
Cochrane Database Syst Rev. 8:CD0092562013.
|
|
45
|
Sun X and Zheng Y: Cisplatin or
Carboplatin for advanced non-small-cell lung cancer? J Thorac
Oncol. 9:e702014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tiseo M, Ardizzoni A and Boni L:
First-line chemotherapy treatment of advanced non-small-cell lung
cancer: Does cisplatin versus carboplatin make a difference? J
Thorac Oncol. 9:e822014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dranitsaris G, Beegle N, Ravelo A,
Kalberer T, Yu E and Thomas S: Evaluating the impact of bevacizumab
maintenance therapy on overall survival in advanced non-small-cell
lung cancer. Clin Lung Cancer. 14:120–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gridelli C, Bennouna J, de Castro J,
Dingemans AM, Griesinger F, Grossi F, Rossi A, Thatcher N, Wong EK
and Langer C: Randomized phase IIIb trial evaluating the
continuation of bevacizumab beyond disease progression in patients
with advanced non-squamous non-small-cell lung cancer after
first-line treatment with bevacizumab plus platinum-based
chemotherapy: Treatment rationale and protocol dynamics of the
AvaALL (MO22097) trial. Clin Lung Cancer. 12:407–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Patel JD, Socinski MA, Garon EB, Reynolds
CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA,
et al: PointBreak: A randomized phase III study of pemetrexed plus
carboplatin and bevacizumab followed by maintenance pemetrexed and
bevacizumab versus paclitaxel plus carboplatin and bevacizumab
followed by maintenance bevacizumab in patients with stage IIIB or
IV nonsquamous non-small-cell lung cancer. J Clin Oncol.
31:4349–4357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barlesi F, Scherpereel A, Gorbunova V,
Gervais R, Vikström A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M,
et al: Maintenance bevacizumab-pemetrexed after first-line
cisplatin-pemetrexed-bevacizumab for advanced nonsquamous
nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL
(MO22089) randomized phase III trial. Ann Oncol. 25:1044–1052.
2014. View Article : Google Scholar : PubMed/NCBI
|