Introduction
MicroRNAs (miRNAs) are a class of ~22-bp non-coding
RNAs that are expressed in many organisms (1,2). Mature
miRNAs arise from one arm of endogenous hairpin transcripts, the
primary miRNAs (pri-miRNAs), by sequential processing in the
nucleus and cytoplasm. Mature miRNAs regulate the expression of
target genes post-transcriptionally. In particular, miRNAs inhibit
translation through imperfect base-pairing interactions with the
3′-UTRs of their respective target genes or degrade their target
mRNAs through perfect or near-perfect base paring (3,4). An
individual miRNA is capable of regulating dozens of distinct mRNAs.
More than 650 human miRNAs are believed to modulate about one-third
of the mRNA species encoded in the genome.
MicroRNAs are associated with the development and
progression of various types of cancers, including breast cancer
(BC), brain cancer, chronic lymphocytic leukemia, colorectal
neoplasia, hepatocellular carcinoma, and lung cancer (5–8). BC is one
of the most common cancers affecting adult females, and studies
have shown that BC is also associated with the deregulation of
several miRNAs. Previous studies reported that various miRNAs, such
as miR-139-5p (9), miR-17-5p
(10), and miR-219a-5p (11), function as tumor suppressors to
regulate BC development and progression. In the present study, our
findings revealed that miR-146a-5p is downregulated in BC cells.
Interleukin-1 receptor-associated kinase 1 (IRAK1) was predicted as
a direct target of miR-146a-5p. Elevated expression of IRAK1 in BC
tissues promotes the growth, migration, and invasion of BC
cells.
Therefore, we aimed to explore the biological
function of miR-146a-5p in BC and to verify the potential
mechanisms involved. Results revealed that strong miR-146a-5p
expression in BC patients significantly repressed cell
proliferation, migration, and invasion. Furthermore, IRAK1 as a
direct target of miR-146a-5p can reverse the tumor suppressive
effects of miR-146a-5p.
Materials and methods
Patient and tissue samples
A total of 20 paired BC tissues were collected from
patients who underwent surgical treatment between October 2015 and
December 2017 at the Women's Hospital, Zhejiang University School
of Medicine (Zhejiang, China). Tissue samples were snap frozen in
liquid nitrogen immediately after surgical resection and stored at
−80°C. Sample collection was performed after obtaining written
informed consent from all patients. The present study was approved
by the Institutional Review Board of Women's Hospital, Zhejiang
University School of Medicine. Patients who received radiotherapy
and/or immunotherapy before or after surgical treatment were not
included in the present study. The study protocol conformed to the
ethical guidelines of the 1975 Declaration of Helsinki and was
approved by the Women's Hospital, Zhejiang University School of
Medicine. All patients enrolled in the present study provided
written informed consents.
Cell lines and culture
BC cell lines, including the noncancerous breast
cell line MCF10A and the BC cell lines MDA-MB-453 and MCF7 were
purchased from the Chinese Academy of Sciences Cell Bank (Shanghai,
China). All cells were cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen; Thermo Fisher Scientific, Inc.) and
grown in humidified 5% CO2 at 37°C. miR-146a-5p mimics
and controls were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Transfection was performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as previously
described.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BC and noncancerous
breast cells using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. For
microRNA analysis, RT-qPCR was performed using the TaqMan MicroRNA
Reverse Transcription Kit, TaqMan Universal PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the target-specific
primers. For mRNA analysis, RT-qPCR was performed using the TaqMan
High-Capacity cDNA Reverse Transcription Kit, TaqMan Fast PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
the corresponding primers. GAPDH was used as an internal control
for normalizing IRAK1 expression levels. RT-qPCR was performed in
triplicate on a RealPlex4 real-time PCR detection system from
Eppendorf (Hamburg, Germany). The thermocycling conditions for
RT-qPCR were as follows: Initial denaturation at 95°C for 5 min,
followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C
for 10 sec. The relative expression levels were calculated using
the 2−∆∆Cq method (12).
Immunohistochemistry (IHC)
Tissue specimens were fixed in 10% neutral buffered
formalin, dehydrated, and embedded in paraffin. Afterwards,
sections were deparaffinized and rehydrated. Sections were treated
with hydrogen peroxide for 10 min to block endogenous peroxide
activity. After antigen retrieval using a microwave, the sections
were treated with 1% bovine serum albumin to block non-specific
binding. Sections were then incubated with rabbit anti-IRAK1
(Abcam, Cambridge, MA, USA) in a humidified chamber overnight at
4°C. After washing thrice with phosphate-buffered saline (PBS) for
5 min each wash, tissue sections were treated with biotinylated
anti-rabbit secondary antibody (Abcam), followed by further
incubation with streptavidin-horseradish peroxidase complex. After
rinsing, sections were treated with diaminobenzidine (DAB; Abcam)
as chromogen, and counterstained with hematoxylin. Samples
incubated with PBS instead of the primary antibody served as
negative controls.
Cell proliferation assay
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT) (Amresco, LLC, Solon, OH, USA) assays were performed
to evaluate cell proliferation. Briefly, 5×103 cells
were separately seeded in 96-well microtiter plates (Corning
Incorporated, Corning, NY, USA) and transfected with miR-146a-5p,
control or miR-146a-5p mimics + IRAK1 plasmid, and then routinely
cultured for 3 days. Medium supplemented with 0.5 mg/ml MTT was
added to each well. The cells were incubated at 37°C for 4 h, after
which 100 µl of dimethyl sulfoxide (DMSO) was added to each well to
dissolve the formazan. Absorption at the wavelength of 490 nm was
measured using a SpectraMax M5 microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Cell migration and invasion assay
Cell migration was evaluated by performing a wound
healing assay. Briefly, transfected cells were cultured in 6-well
plates (5×104 cells/well). Upon reaching 90–95%
confluence, the cell monolayer was scratched using a sterile
plastic micropipette tip, after which cells were cultured under
standard conditions for 24 h. Following several washes, wound
recovery was observed and photographed using an X71 inverted
microscope (Olympus, Tokyo, Japan).
The Transwell invasion assay was performed to
determine cell invasion. First, 1×105 transfected cells
were seeded into the upper chamber of Matrigel-coated inserts
containing serum-free medium. Medium containing 10% FBS was added
to the lower chamber as chemoattractant. The cells were allowed to
invade the chamber for 48 h at 37°C with 5% CO2. Cells
that invaded the lower surface of the filter were fixed in 70%
ethanol for 30 min and stained with 0.1% crystal violet for 10 min.
The number of cells that migrated to the lower side was counted in
five randomly selected fields under an X71 inverted microscope
(Olympus).
Luciferase activity assay
The 3′-UTR of wild-type IRAK1 was amplified from a
human cDNA library. Mutations in the miR-146a-5p binding site were
introduced by site-directed mutagenesis using a fast mutation kit
(NEB, Beverly, MA, USA). The PCR fragment was cloned into the
psiCHECK-2 vector downstream of the firefly luciferase coding
region within XhoI and NotI (Takara Bio, Inc., Otsu,
Japan). The psiCHECK-2-control was used as internal control.
Western blot analysis
Total protein lysates were resolved by 10% SDS-PAGE
and transferred onto polyvinyl difluoride membranes (EMD Millipore,
Bedford, MA, USA). After blocking in Tris-buffered saline
containing 0.1% Tween-20 (TBS-T) with 5% non-fat dry milk for 30
min, membranes were washed four times in TBS-T and incubated with
primary antibodies overnight at 4°C. All primary antibodies were
obtained from Abcam and used at the following dilutions: IRAK1
(1/500), Twist (1/500), MMP-2 (1/500), MMP-9 (1/500), and
anti-GAPDH (1/1,000). After thorough washing, membranes were
incubated with horseradish peroxidase-linked goat polyclonal anti
rabbit IgG secondary antibodies at a dilution of 1:2,000 for 1 h at
room temperature. Immunoreactivity was detected by enhanced
chemiluminescence (ECL Kit; Pierce; Thermo Fisher Scientific, Inc.)
and exposure to radiography film. GAPDH served as the loading
control.
Statistical analysis
Data were presented as mean ± standard deviation
(SD). Statistical analysis was performed using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). Two-tailed Student's t-test was
performed to compare the differences between two groups. One-way
analysis of variance followed by Dunnett's multiple comparison was
employed to compare the differences among three independent
treatment groups. Correlation between miR-146a-5p and IRAK1
expression levels in BC tissues was determined by conducting
Pearson's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-146a-5p expression in human BC
tissues and cell lines
In the initial effort to determine the biological
role of miR-146a-5p in BC, we examined miR-146a-5p expression
levels in 20 BC patient tissue samples and normal tissues by
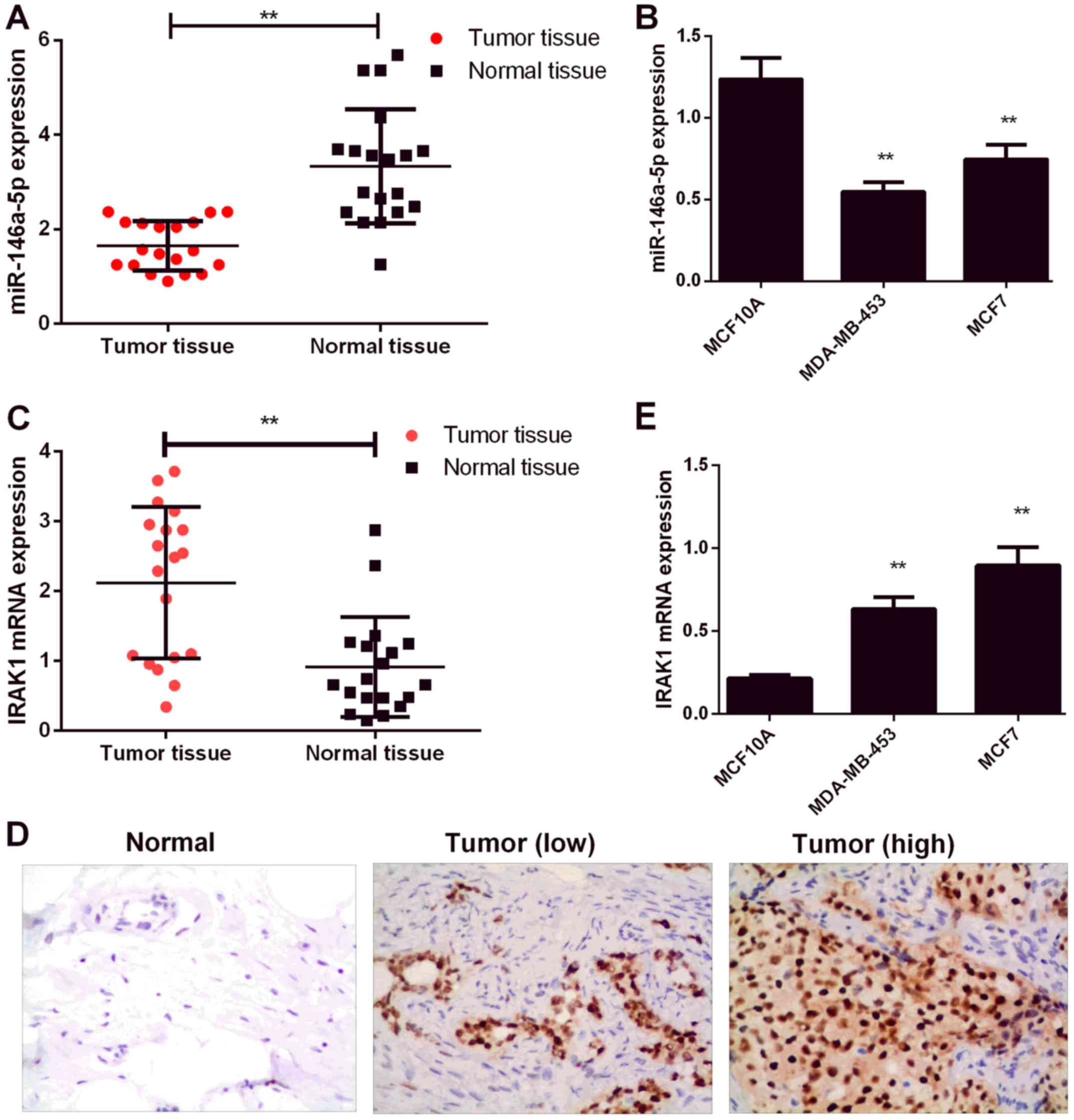
RT-PCR. As shown in Fig. 1A,
miR-146a-5p expression was significantly downregulated in BC
tissues relative to that in normal tissues (P<0.01). Consistent
with the above results, BC cell lines (MDA-MB-453 and MCF7) showed
miR-146a-5p downregulation relative to those of normal human breast
tissue MCF10A cells (Fig. 1B). IRAK1
expression is known to be significantly upregulated in BC (13). In addition, IRAK1 expression levels
were determined by RT-PCR and IHC. As shown in Fig. 1C and D, IRAK1 levels were
significantly upregulated in the BC tissues (P<0.01). IRAK1
levels were also upregulated in BC cell lines (MDA-MB-453 and MCF7)
relative to those in MCF10A cells (P<0.01, Fig. 1E).
miR-146a-5p overexpression inhibits
the proliferation of BC cells
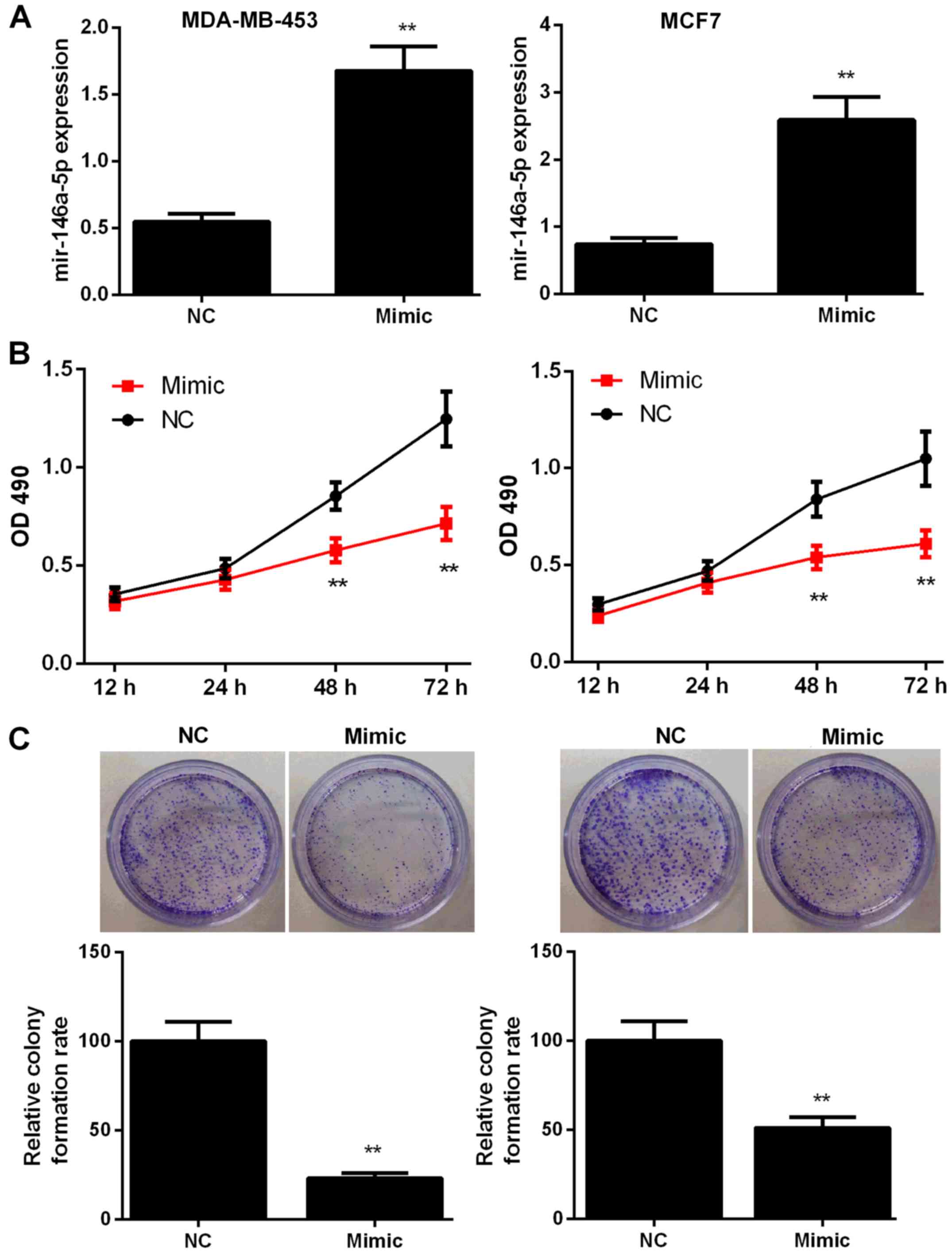
To explore the potential role of miR-146a-5p in BC,
MDA-MB-453 and MCF7 cells were transfected with miR-control or
miR-146a-5p mimic. The transfection efficiency was assessed by
RT-qPCR (Fig. 2A). The effects of
miR-146a-5p mimic on cell proliferation and colony formation were
then evaluated. As shown in Fig. 2B,
BC cells treated with the miR-146a-5p mimic showed significant
inhibition of proliferation at 48 and 72 h compared with those in
the NC groups (P<0.01). In addition, colony formation rate in
the mimic-treated group was significantly lower at 48 h compared
with that in the NC group (P<0.01, Fig. 2C).
miR-146a-5p represses BC cell
migration and invasion
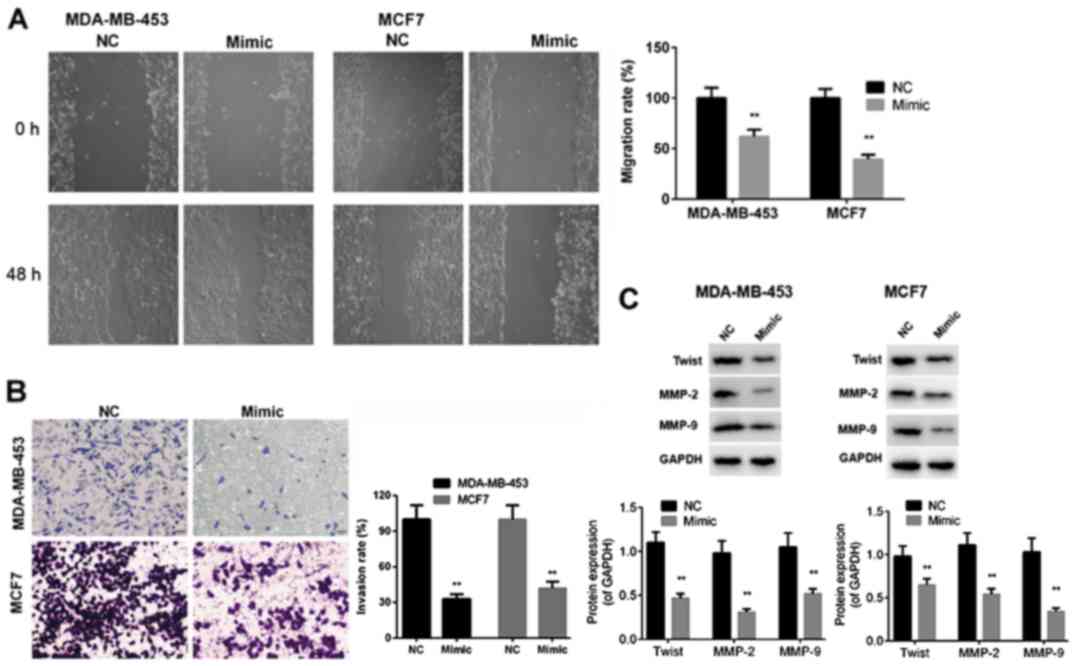
To determine whether miR-146a-5p influences the
mobility of BC cells, we performed wound healing and Transwell
assays to evaluate migration and invasion capabilities of
MDA-MB-453 and MCF7 cells after transfection with miR-control or
miR-146a-5p mimic. The results indicated that the migration and
invasion capabilities of MDA-MB-453 and MCF7 cells were markedly
reduced by transfection with miR-146a-5p mimic compared with those
of the miR-control cells (P<0.01, Fig.
3A and B). In addition, protein expression levels of Twist,
MMP-2, and MMP-9 were significantly downregulated in MDA-MB-453 and
MCF7 cells after transfection miR-146a-5p mimic compared to those
in cells transfected with miR-control (P<0.01, Fig. 3C). The above-mentioned findings
demonstrated that miR-146a-5p exerted inhibitory effects on the
migration and invasion capabilities of BC cells.
IRAK1 is a direct target of
miR-146a-5p
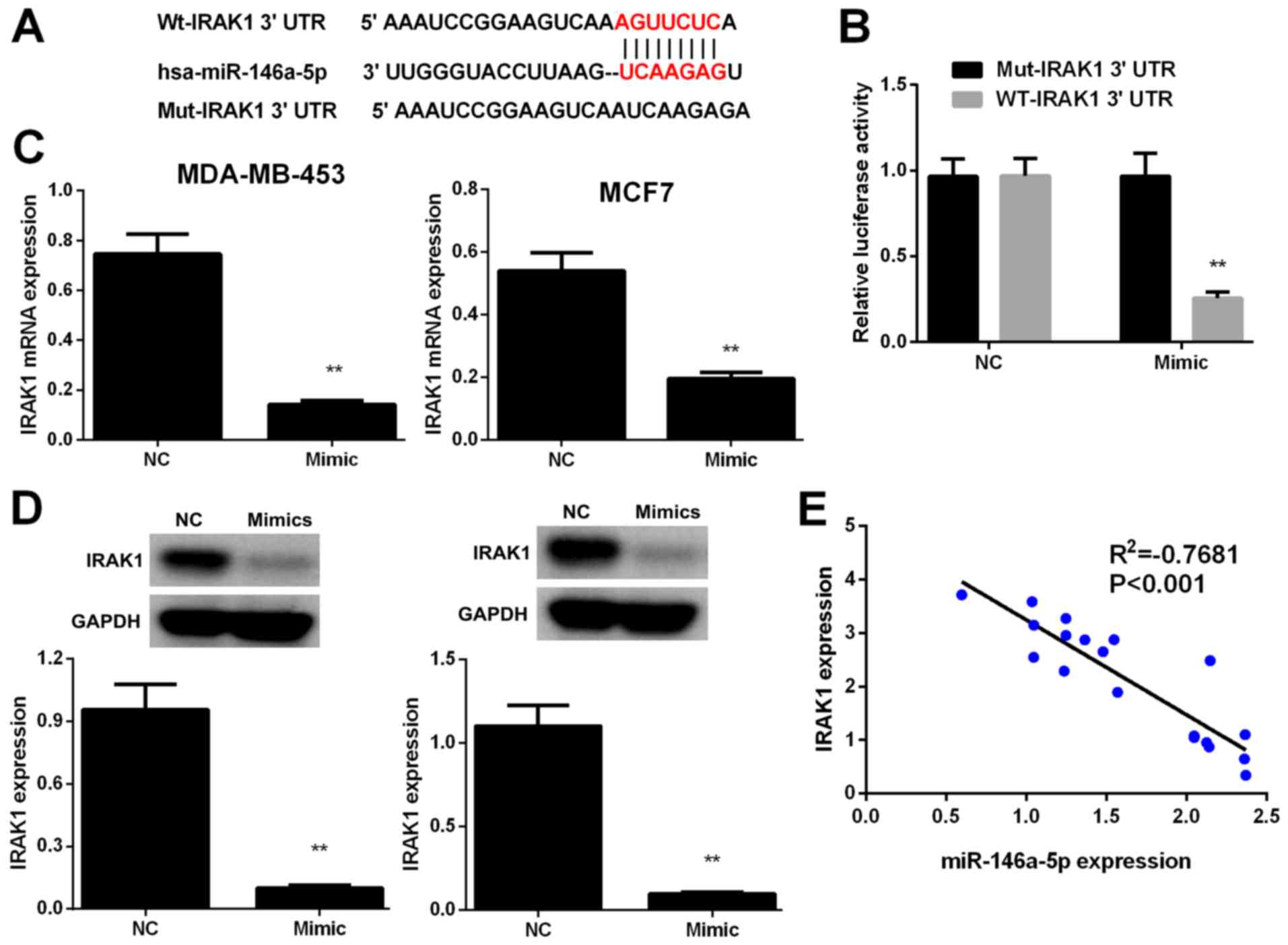
To verify the potential mechanism by which
miR-146a-5p suppresses BC cell proliferation, migration, and
invasion, we used TargetScan and miRanda algorithms to identify
putative miR-146a-5p targets. IRAK1, which is frequently reported
to be involved in the occurrence, metastasis, and progression of
human BC, was selected as a candidate miR-146a-5p target (Fig. 4A). Luciferase reporter assays were
performed to confirm whether IRAK1 is regulated via direct binding
of miR-146a-5p to its 3′-UTR. IRAK1 3′-UTR fragments harboring
miR-146a-5p target sequences and its corresponding mutant fragments
were subcloned into firefly luciferase vectors. Results
demonstrated that luciferase activity was markedly lower in the
cells co-transfected with miR-146a-5p mimic and wild-type IRAK1
3′-UTR compared to those in the NC cells, whereas co-transfection
with miR-146a-5p mimic and the mutant IRAK1 3′-UTR failed to
repress luciferase activity (Fig.
4B).
IRAK1 expression levels in MDA-MB-453 and MCF7 cells
were evaluated by RT-PCR and western blot analysis. Results showed
that IRAK1 was downregulated in BC cells transfected with
miR-146a-5p mimics relative to that in the NC cells (Fig. 4C and D). In addition, results of
Pearson's correlation analysis revealed that miR-146a-5p expression
was inversely correlated with IRAK1 expression (P<0.01, Fig. 4E). The above results indicated that
miR-146a-5p directly binds to the IRAK1 3′-UTR to suppress its
expression.
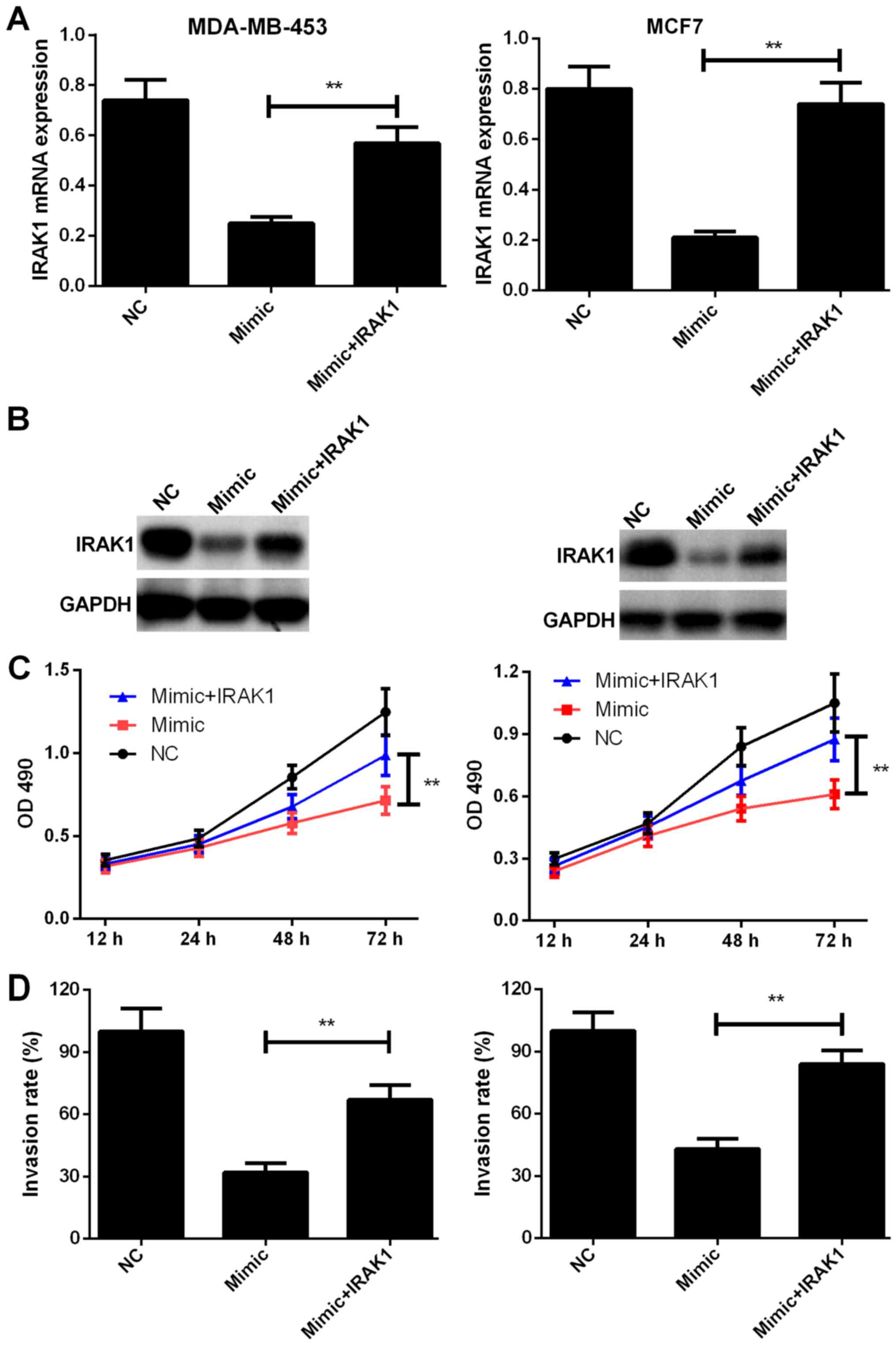
IRAK1 overexpression alleviates the inhibitory
effects of miR-146a-5p on the proliferation, migration, and
invasion capabilities of BC cells. We further evaluated the
functional correlation between miR-146a-5p and IRAK1. IRAK1
expression was rescued in MDA-MB-453 and MCF7 cells transfected
with the miR-146a-5p mimic. The transfection efficiencies were
determined by RT-PCR and western blotting (P<0.01, Fig. 5A and B). As shown in Fig. 5C and D, IRAK1 overexpression in BC
cells transfected with miR-146a-5p mimic group significantly
enhanced cell proliferation and promoted the invasion capabilities
(P<0.01), which suggested that restoring IRAK1 expression
alleviates miR-146a-5p-induced suppression of proliferation and
invasion of MDA-MB-453 and MCF7 cells. The above-mentioned results
indicated that miR-146a-5p exerts repressive effects on the
proliferation and invasion behavior of BC cells by targeting
IRAK1.
Discussion
Increasing evidence has demonstrated that abnormal
miRNA expression patterns play crucial roles in BC occurrence and
metastasis. A better understanding of the biological functions of
miRNAs is important for the development of effective therapeutic
strategies for BC treatment and for identifying novel diagnostic
markers for BC. Multiple studies have shown that miR-146a-5p
downregulation is involved in tumorigenesis and metastasis of
diverse human cancers. Xu et al (14) reported that miR-146a plays a critical
role in the apoptosis of androgen-independent prostate cancer cells
apoptosis by regulating ROCK/Caspase-3 signaling. Yuwen et
al (15) suggested that the serum
exosomal miR-146a-5p can be used as a novel putative biomarker for
predicting the efficacy of cisplatin for the treatment of lung
cancer and for real-time monitoring of drug resistance. The
findings of Min et al (16)
revealed that miR-146a-5p influences cell proliferation and
apoptosis in a cellular context-dependent manner and selectively
disarms the TRAF6-mediated branch of the TGF-β signaling in oral
squamous cell carcinoma cell lines by sparing Smad4 involvement.
Nevertheless, the biological role of miR-146a-5p in BC remains
unknown.
In the present study, we first examined the
expression of miR-146a-5p and interleukin-1 receptor-associated
kinase 1 (IRAK1). Our results revealed that miR-146a-5p was
markedly downregulated and IRAK1 was significantly upregulated in
BC tissues and cell lines. We further conducted functional studies
to elucidate the role of miR-146a-5p in BC. Results revealed that
miR-146a-5p overexpression inhibited BC cell proliferation, colony
formation, migration, and invasion. The above findings suggested
the potential role of miR-216a as a tumor suppressor in BC.
To verify the potential molecular mechanisms by
which miR-146a-5p exerts antitumor effects in BC, we performed
bioinformatics analysis using TargetScan and miRanda algorithms.
The analysis predicted IRAK1 as a direct target of miR-146a-5p.
Subsequently, results of dual luciferase reporter assays supported
the above findings and indicated that IRAK1 is a direct target of
miR-146a-5p. IRAK1 is a downstream factor in the toll-like receptor
(TLR) signaling, and IRAK1 expression is known to be significantly
upregulated in many human cancers, including lung cancer (17), hepatocellular carcinoma (18), endometrial carcinoma (19), papillary thyroid carcinoma (20), and BC (21). Abnormal IRAK1 expression is known to
be closely correlated with tumorigenesis and metastasis. A previous
study demonstrated that IRAK1 is overexpressed in BC and was found
to promote aggressive cell growth, metastasis, and development of
resistance to paclitaxel treatment (21). Si et al (22) found that miR-146a-5p regulates
proliferation and metastasis of triple-negative BC (TNBC) cells by
regulating SOX5. First, our analysis identified IRAK1 as a novel
miR-146a-5p target. Results of RT-PCR and western blot analysis
revealed that IRAK1 expression was considerably downregulated in BC
cells transfected with miR-146a-5p mimics when compared to the
controls. In addition, IRAK1 expression was found to be markedly
upregulated in BC tissues and inversely correlated with miR-146a-5p
expression. In addition, we rescued IRAK1 expression in BC cells
transfected with miR-146a-5p mimic. Restoring IRAK1 expression was
demonstrated to alleviate the inhibitory effects of miR-146a-5p on
the proliferation, migration, and invasion of BC cells. The
above-mentioned results indicated that miR-146a-5p acts as a tumor
suppressor partially by directly targeting IRAK1.
In summary, miR-146a-5p is downregulated in BC
tissues and cell lines and plays a tumor suppressor role by
directly targeting IRAK1. Our current findings provided new
insights into the molecular mechanism underlying BC occurrence and
progression. Therefore, miR-146a-5p can be considered as a novel
therapeutic target for the treatment of BC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JPL and LFD designed and performed the experiments.
FFC contributed to data analysis. YFF enrolled the patients and
measured the RNA levels in the clinical samples. JPL conceived and
initiated the present study, and wrote the manuscript. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Women's
Hospital, Zhejiang University School of Medicine (Zhejiang, China).
All patients enrolled in the present study provided written
informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang Z and Xi Y: MicroRNAs mediate
therapeutic and preventive effects of natural agents in breast
cancer. Chin J Nat Med. 14:881–887. 2016.PubMed/NCBI
|
|
2
|
Chernyi VS, Tarasova PV, Kozlov VV, Saik
OV, Kushlinskii NE and Gulyaeva LF: Search of MicroRNAs regulating
the receptor status of breast cancer in silico and experimental
confirmation of their expression in tumors. Bull Exp Biol Med.
163:655–659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lü L, Mao X, Shi P, He B, Xu K, Zhang S
and Wang J: MicroRNAs in the prognosis of triple-negative breast
cancer: A systematic review and meta-analysis. Medicine.
96:e70852017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sempere LF, Keto J and Fabbri M: Exosomal
MicroRNAs in breast cancer towards diagnostic and therapeutic
applications. Cancers. 9(pii): E712017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Wang H, Song H, Xu H, Zhao B, Wu C,
Hu J, Wu T, Xie D, Zhao J, et al: The microRNAs miR-200b-3p and
miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and
motility of breast cancer cells. Oncotarget. 8:85276–85289.
2017.PubMed/NCBI
|
|
6
|
Mjelle R, Sellæg K, Sætrom P, Thommesen L,
Sjursen W and Hofsli E: Identification of metastasis-associated
microRNAs in serum from rectal cancer patients. Oncotarget.
8:90077–90089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Awasthi R, Rathbone MJ, Hansbro PM, Bebawy
M and Dua K: Therapeutic prospects of microRNAs in cancer treatment
through nanotechnology. Drug Deliv Transl Res. 8:97–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YN, Chen ZH and Chen WC: Novel
circulating microRNAs expression profile in colon cancer: A pilot
study. Eur J Med Res. 22:512017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pajic M, Froio D, Daly S, Doculara L,
Millar E, Graham PH, Drury A, Steinmann A, de Bock CE,
Boulghourjian A, et al: miR-139-5p modulates radiotherapy
resistance in breast cancer by repressing multiple gene networks of
DNA repair and ROS defense. Cancer Res. 78:501–515. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Lai Y, Ma J, Liu Y, Bi J, Zhang L,
Chen L, Yao C, Lv W, Chang G, et al: miR-17-5p suppresses cell
proliferation and invasion by targeting ETV1 in triple-negative
breast cancer. BMC Cancer. 17:7452017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang C, Yuan Y, Song T, Wang H, Huang L,
Luo X, He H, Huo L, Zhou H, Wang N, et al: miR-219a-5p inhibits
breast cancer cell migration and epithelial-mesenchymal transition
by targeting myocardin-related transcription factor A. Acta Biochim
Biophys Sin. 49:1112–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wee ZN, Yatim SM, Kohlabauer VK, Feng M,
Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al: Corrigendum:
IRAK1 is a therapeutic target that drives breast cancer metastasis
and resistance to paclitaxel. Nat Commun. 6:100542015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong
N, Chen S, Liu N, Zhu W and Chen M: Hsa-miR-146a-5p modulates
androgen-independent prostate cancer cells apoptosis by targeting
ROCK1. Prostate. 75:1896–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuwen DL, Sheng BB, Liu J, Wenyu W and Shu
YQ: MiR-146a-5p level in serum exosomes predicts therapeutic effect
of cisplatin in non-small cell lung cancer. Eur Rev Med Pharmacol
Sci. 21:2650–2658. 2017.PubMed/NCBI
|
|
16
|
Min SK, Jung SY, Kang HK, Park SA, Lee JH,
Kim MJ and Min BM: Functional diversity of miR-146a-5p and TRAF6 in
normal and oral cancer cells. Int J Oncol. 51:1541–1552. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Dang Y, Li P, Rong M and Chen G:
Expression of IRAK1 in lung cancer tissues and its
clinicopathological significance: A microarray study. Int J Clin
Exp Pathol. 7:8096–8104. 2014.PubMed/NCBI
|
|
18
|
Ye ZH, Gao L, Wen DY, He Y, Pang YY and
Chen G: Diagnostic and prognostic roles of IRAK1 in hepatocellular
carcinoma tissues: An analysis of immunohistochemistry and
RNA-sequencing data from the cancer genome atlas. Onco Targets
Ther. 10:1711–1723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Wang Y, Duan X, Wang Y and Zhang
Z: Interleukin-1 receptor-associated kinase 1 correlates with
metastasis and invasion in endometrial carcinoma. J Cell Biochem.
119:2545–2555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou CK, Chi SY, Huang CH, Chou FF, Huang
CC, Liu RT and Kang HY: IRAK1, a target of miR-146b, reduces
cell aggressiveness of human papillary thyroid carcinoma. J Clin
Endocrinol Metab. 101:4357–4366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wee ZN, Yatim SM, Kohlbauer VK, Feng M,
Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al: IRAK1 is a
therapeutic target that drives breast cancer metastasis and
resistance to paclitaxel. Nat Commun. 6:87462015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Si C, Yu Q and Yao Y: Effect of
miR-146a-5p on proliferation and metastasis of triple-negative
breast cancer via regulation of SOX5. Exp Ther Med. 15:4515–4521.
2018.PubMed/NCBI
|