Introduction
The presence of BRCA mutations increases the
risk of breast (~80%) and ovarian cancer (~40%). The incidence of
BRCA mutations in breast and ovarian cancer are <1–7% for
BRCA1 and 1–3% for BRCA2 independently from family
history or age at diagnosis. In literature, a family history of
breast or ovarian cancer, young age at diagnosis, male breast
cancer or multiple tumors (bilateral breast cancer or breast and
ovarian cancer in the same patient) occur more often in BRCA
mutation carriers. The median time of diagnosis of breast cancer in
patients with germline BRCA mutation is lower (in age under
50 years) than for patients with sporadic cancer (1). External factors which can modify
BRCA associated breast cancer risk are hormonal and
reproductive factors such as pregnancy, history of breast feeding
and oral contraceptives (2,3).
It has previously been demonstrated that tumors in
patients with BRCA1 mutation frequently exhibit negative
steroid receptor status, with expression of p53 protein. Mutations
in TP53 gene also seem to be increased in tumors with
BRCA1 mutation. A previous study indicated that familial
breast cancers with BRCA1 mutation are different from
BRCA2 tumors and sporadic cancers (4).
The triple negative breast cancer (TNBC) phenotype
is the most commonly observed molecular subtype in patients with
BRCA1 mutation. The presence of triple negative diseases in
BRCA1 mutation carriers is higher than in sporadic breast
cancer patients and is 11–20% (5).
Recent data show that survival rate of BRCA carriers who
were administrated systemic treatment (chemotherapy) was similar to
non-carriers (6,7). Various studies both clinical and
preclinical, showed that BRCA is an important factor
affecting chemotherapy response and treatment toxicity in breast
cancer patients (8). In Poland, three
founder mutations in BRCA1 (i.e., 5382insC, C61G, 4153delA)
are under investigation (9).
In the present study, we compare hereditary and
sporadic breast cancer according to clinicopathological factors and
overall survival (OS) time.
Materials and methods
In a study conducted in the years 2007–2016 in the
Maria Skłodowska Curie Memorial Cancer Center and Institute of
Oncology (COI; Gliwice, Poland), we analyzed prognostic factors and
survival in 60 patients with breast cancer with confirmed
BRCA1 mutations. A control group was selected from breast
cancer patients without the BRCA mutation (n=386). The
patients in both groups were treated according to the same
protocol. All patients had signed a written informed consent
allowing their biological material to be used in clinical
research.
All patients were females diagnosed, treated and
followed up at the COI in Gliwice. Patients underwent clinical
follow-up examinations every three months in the first two years,
every six months afterwards until the fifth year after diagnosis
and every year subsequently. Inclusion criteria were: Breast cancer
confirmed by microscopic examination, performance status ZUBROD
0–1, age above 18, the correct value of renal and liver function
and normal values of bone marrow. The data of age at onset,
menopausal status, surgical procedure, disease stage according to
TNM classification, histology, estrogen and progesterone receptor
(PR) status, HER2 status and contralateral breast cancer were
gathered from hospital records and pathology reports. The analysis
of patient medical records was performed according to national law
regulation.
All patients had genetic tests and consultation in
Genetic Outpatient Clinic. Mutation profile was assessed by
RFLP-PCR technique. We evaluated the three most common mutations in
the Polish population, including 5382insC, C61G and 4153delA. All
patients were tested for the presence of BRCA1 and
BRCA2 mutations. Mutation analysis was conducted by a
multiplex allele-specific polymerase chain reaction assay.
Statistical analysis was carried out using
STATISTICA 7 software (StatSoft, Inc., Tulsa, OK, USA). The
frequency of side effects was monitored. The qualitative features
were presented as the percentage of their occurrence and evaluated
with Fisher's test and χ2 test with Yates correction.
P<0.05 was considered to indicate a statistically significant
difference. Prognostic factors of OS were estimated by Cox
proportional hazards model. The probability of survival was
estimated using the Kaplan-Meier method.
Results
Patient characteristics
For the total group of 446 cases, the median age at
diagnosis was 51.8 years (range, 23.7–78.3 years). In BRCA
mutation carriers (n=60) and non-carriers (n=386) the median age
was 43.5 years (range, 23.7–74.4 years) and 53.1 years (range,
25.6–78.3 years), respectively. BRCA carriers were
significantly younger (P<0.0001) than non-carriers. A total of
263 women (59.0%) were in premenopausal period (80% carriers and
56% non-carriers) (P=0.0004). The majority of patients had early
stage breast cancer. Distant metastases were observed only in 7
(1.6%) of women (1 case in BRCA mutation carriers and 6 in
non-carriers). Lymph node metastases (N+) was detected more
frequently in non-carriers (45.9%; vs. 18.3%, P=0.0001).
Conversely, locally advanced breast cancer (T3-T4) was reported
frequently in BRCA mutation carriers (38.3% vs. 19.4%,
P=0.002). Lobular invasive carcinoma was reported more often in
patients without BRCA mutation than in BRCA carriers
(12.2% vs. 5%). As expected, patients with BRCA mutation had
more frequent estrogen receptor (ER; 66.7% vs. 35.5%, P=0.0001) and
PR (71.7% vs. 41.7%, P=0.0002) negative receptor status, higher
histological grade (G3; 50% vs. 29.5%, P=0.002), negative HER2
receptor status (98.3% vs. 56.2%, P=0.0001) and TNBC (61.7% vs.
15.0%, P=0.0001). There was also an observed predisposition to the
development of secondary cancers in mutation carriers (35% vs.
9.6%, P=0.0001). Clinicopathological patient characteristics are
presented in Table I.
 | Table I.Clinicopathological patient's
characteristics according to BRCA1 mutation carriers. |
Table I.
Clinicopathological patient's
characteristics according to BRCA1 mutation carriers.
|
|
|
| BRCA1
carriers | BRCA1 non
carriers |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | n | Percentage of total
n (%) | n | % of n | n | % of n | P-value |
|---|
| Total cases | 446 | 100 | 60 | 100 | 386 | 100 | – |
| Age (range, 24–78
years; median 52 years) |
|
≤65 | 386 | 86.5 | 55 | 91.7 | 331 | 85.8 | 0.308 |
|
>65 | 60 | 13.5 | 5 | 8.3 | 55 | 14.2 |
|
| Age (years) |
|
≤40 | 67 | 15.0 | 22 | 36.7 | 45 | 11.7 | 0.0001 |
|
>40 | 379 | 85.0 | 38 | 63.3 | 341 | 88.3 |
|
| Menopausal
status |
|
Postmenopausal | 183 | 41.0 | 12 | 20.0 | 171 | 44.3 | 0.0004 |
|
Premenopausal | 263 | 59.0 | 48 | 80.0 | 215 | 55.7 |
|
| Clinical
staging |
| I | 90 | 20.2 | 8 | 13.3 | 82 | 21.2 | 0.030 |
|
IIA | 136 | 30.5 | 23 | 38.3 | 113 | 29.3 |
|
|
IIB | 128 | 28.7 | 23 | 38.3 | 105 | 27.2 |
|
|
IIIA | 69 | 15.5 | 2 | 3.3 | 67 | 17.4 |
|
|
IIIB | 11 | 2.5 | 3 | 5.0 | 8 | 2.1 |
|
|
IIIC | 5 | 1.1 | 0 | 0.0 | 5 | 1.3 |
|
| IV | 7 | 1.6 | 1 | 1.7 | 6 | 1.6 |
|
| T |
| T1 | 131 | 29.4 | 10 | 16.7 | 121 | 31.3 | 0.0001 |
| T2 | 217 | 48.7 | 27 | 45.0 | 190 | 49.2 |
|
| T3 | 77 | 17.3 | 14 | 23.3 | 63 | 16.3 |
|
| T4 | 21 | 4.7 | 9 | 15.0 | 12 | 3.1 |
|
| Clinical staging
nodes |
| N0 | 258 | 57.8 | 49 | 81.7 | 209 | 54.1 | 0.001 |
| N1 | 133 | 29.8 | 8 | 13.3 | 125 | 32.4 |
|
| N2 | 47 | 10.5 | 3 | 5.0 | 44 | 11.4 |
|
| N3 | 8 | 1.8 | 0 | 0.0 | 8 | 2.1 |
|
| G |
| G1 | 27 | 6.1 | 1 | 1.7 | 26 | 6.7 | 0.002 |
| G2 | 111 | 24.9 | 6 | 10.0 | 105 | 27.2 |
|
| G3 | 144 | 32.3 | 30 | 50.0 | 114 | 29.5 |
|
|
Missing | 164 | 36.8 | 23 | 38.3 | 141 | 36.5 |
|
| Tumor type |
| Ductal
invasive | 363 | 81.4 | 56 | 93.3 | 307 | 79.5 | 0.035 |
| Lobular
invasive | 50 | 11.2 | 3 | 5.0 | 47 | 12.2 |
|
|
Other | 33 | 7.4 | 1 | 1.7 | 32 | 8.3 |
|
| ER |
|
Negative | 177 | 39.7 | 40 | 66.7 | 137 | 35.5 | 0.0001 |
|
Positive | 269 | 60.3 | 20 | 33.3 | 249 | 64.5 |
|
| PR |
|
Negative | 204 | 45.7 | 43 | 71.7 | 161 | 41.7 | 0.0002 |
|
Positive | 242 | 54.3 | 17 | 28.3 | 225 | 58.3 |
|
| Steroid
receptor |
|
Negative | 161 | 36.1 | 37 | 61.7 | 124 | 32.1 | 0.0002 |
|
Positive | 285 | 63.9 | 23 | 38.3 | 262 | 67.9 |
|
| HER2
overexpression |
|
Negative | 276 | 61.9 | 59 | 98.3 | 217 | 56.2 | 0.0001 |
|
Positive | 170 | 38.1 | 1 | 1.7 | 169 | 43.8 |
|
| Triple
negative |
| No | 351 | 78.7 | 23 | 38.3 | 328 | 85.0 | 0.0001 |
|
Yes | 95 | 21.3 | 37 | 61.7 | 58 | 15.0 |
|
In the subgroup analysis, there were no significant
differences between younger (≤40 years) and older (> 40 years)
BRCA mutation carriers according to clinicopathological
factors. Among younger patients (≤40 years) there was an observed
increased occurrence of TNBC (68% vs. 58%; P=0.583), tumors with
negative ER status (ER-) (77% vs. 60%; P=0.258) and with negative
PR status (PR-) (77% vs. 68%; P=0.560) and without HER2
overexpression (100% vs. 97.4%, P=1.00) (Table II). In BRCA non-carriers,
younger patients (≤40 years) in comparison to older exhibited an
increased rate of diagnosis of TNBC (20.0% vs. 14.4%, P=0.373),
tumors with ER- status (42.2% vs. 34.6%, P=0.324) and HER2
overexpression (48.9% vs. 43.1%, P=0.524). There were no
differences observed in negative PR status (PR-) (42.2% vs.
41.6%).
 | Table II.Patient's characteristics according
to age. |
Table II.
Patient's characteristics according
to age.
|
|
|
| Age ≤40 years | Age >40
years |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | Total n | Percentage of total
n (%) | n | % of n | n | % of n | P-value |
|---|
| BRCA1
carriers | 60 | 100 | 22 | 100 | 38 | 100 | – |
| T |
| T1 | 10 | 16.7 | 4 | 18.2 | 6 | 15.8 | 0.635 |
| T2 | 27 | 45.0 | 8 | 36.4 | 19 | 50.0 |
|
|
T3-T4 | 23 | 38.3 | 10 | 45.5 | 13 | 34.2 |
|
| Clinical staging
nodes |
| N0 | 49 | 81.7 | 20 | 90.9 | 29 | 76.3 | 0.0001 |
| N+ | 11 | 18.3 | 2 | 9.1 | 9 | 23.7 |
|
| G |
|
G1-G2 | 7 | 11.7 | 1 | 4.5 | 6 | 15.8 | 0.261 |
| G3 | 30 | 50.0 | 10 | 45.5 | 20 | 52.6 |
|
|
Missing | 23 | 38.3 | 11 | 50.0 | 12 | 31.6 |
|
| ER |
|
Negative | 40 | 66.7 | 17 | 77.3 | 23 | 60.5 | 0.258 |
|
Positive | 20 | 33.3 | 5 | 22.7 | 15 | 39.5 |
|
| PR |
|
Negative | 43 | 71.7 | 17 | 77.3 | 26 | 68.4 | 0.560 |
|
Positive | 17 | 28.3 | 5 | 22.7 | 12 | 31.6 |
|
| HER2
overexpression |
|
Negative | 59 | 98.3 | 22 | 100.0 | 37 | 97.4 | 1.00 |
|
Positive | 1 | 1.7 | 0 | 0.0 | 1 | 2.6 |
|
| Triple
negative |
| No | 23 | 38.3 | 7 | 31.8 | 16 | 42.1 | 0.583 |
|
Yes | 37 | 61.7 | 15 | 68.2 | 22 | 57.9 |
|
Treatment strategies
Treatment strategies are presented in Table III. The surgical treatment was
performed in 402 (90.1%) patients, including mastectomy for 292
(65.5%) and breast conserving treatment (BCT) for 110 (24.7%). BCT
was conducted more often in non-carriers in comparison to carriers
(28.2% vs. 21.6%, P=0.401). Radiotherapy was administered to 66.7%
of mutation carriers and 67.1% non-carriers (P=1.00). The total
radiotherapy dose administered was 50 Gy in 25 fractions. If
indicated, a boost was delivered. All patients underwent
chemotherapy. A total of 97.3% (434) patients received
anthracycline based chemotherapy (AC, FAC) at The Clinical and
Experimental Oncology Department. Chemotherapy regiments with
taxanes (paclitaxel) were used in 13% of patients. Patients with
steroid positive receptor breast cancer were treated with
anti-estrogen therapy: 61.1% of non-carriers and 30.0% of
BRCA mutation carriers (P<0.0001). The lower frequency of
HT in carriers was due to the high frequency of ER (−) in that
group. Trastuzumab was used in women with HER2 positive breast
cancer confirmed by immunohistochemistry examination or by the FISH
method (gene amplification) (1.7% BRCA carriers and 41.2%
non-carriers, P<0.0001).
 | Table III.Treatment strategy according to
BRCA1 mutation. |
Table III.
Treatment strategy according to
BRCA1 mutation.
|
|
|
| BRCA1
carriers | BRCA1 non
carriers |
|
|---|
|
|
|
|
|
|
|
|---|
| Treatment | Total n | Percentage of total
n (%) | n | % of n | n | % of n | P-value |
|---|
| Total cases | 446 | 100 | 60 | 100 | 386 | 100 | – |
| Chemotherapy
regimen |
| AC
FAC | 376 | 84.3 | 44 | 73.3 | 332 | 86.0 | 0.005 |
| AC +
taxanes | 58 | 13.0 | 11 | 18.3 | 47 | 12.2 |
|
|
CMF | 12 | 2.7 | 5 | 8.3 | 7 | 1.8 |
|
| Trastuzumab
therapy |
|
Yes | 160 | 35.9 | 1 | 1.7 | 159 | 41.2 | 0.0001 |
| No | 286 | 64.1 | 59 | 98.3 | 227 | 58.8 |
|
| Hormonotherapy |
|
Yes | 254 | 57.0 | 18 | 30.0 | 236 | 61.1 | 0.0001 |
| No | 192 | 43.0 | 42 | 70.0 | 150 | 38.9 |
|
| Local
treatment |
|
Mastectomy | 292 | 65.5 | 40 | 66.7 | 252 | 65.3 | 0.224 |
| Breast
conservation surgery | 110 | 24.7 | 11 | 18.3 | 99 | 25.6 |
|
| Without
surgery | 44 | 9.9 | 9 | 15.0 | 35 | 9.1 |
|
| Radiotherapy |
|
Yes | 299 | 67.0 | 40 | 66.7 | 259 | 67.1 | 1.00 |
| No | 147 | 33.0 | 20 | 33.3 | 127 | 32.9 |
|
Survival analysis in BRCA (−) negative
patients
Patients with positive nodes (N +) exhibited a
significantly worse OS than those without node involvement (5-year
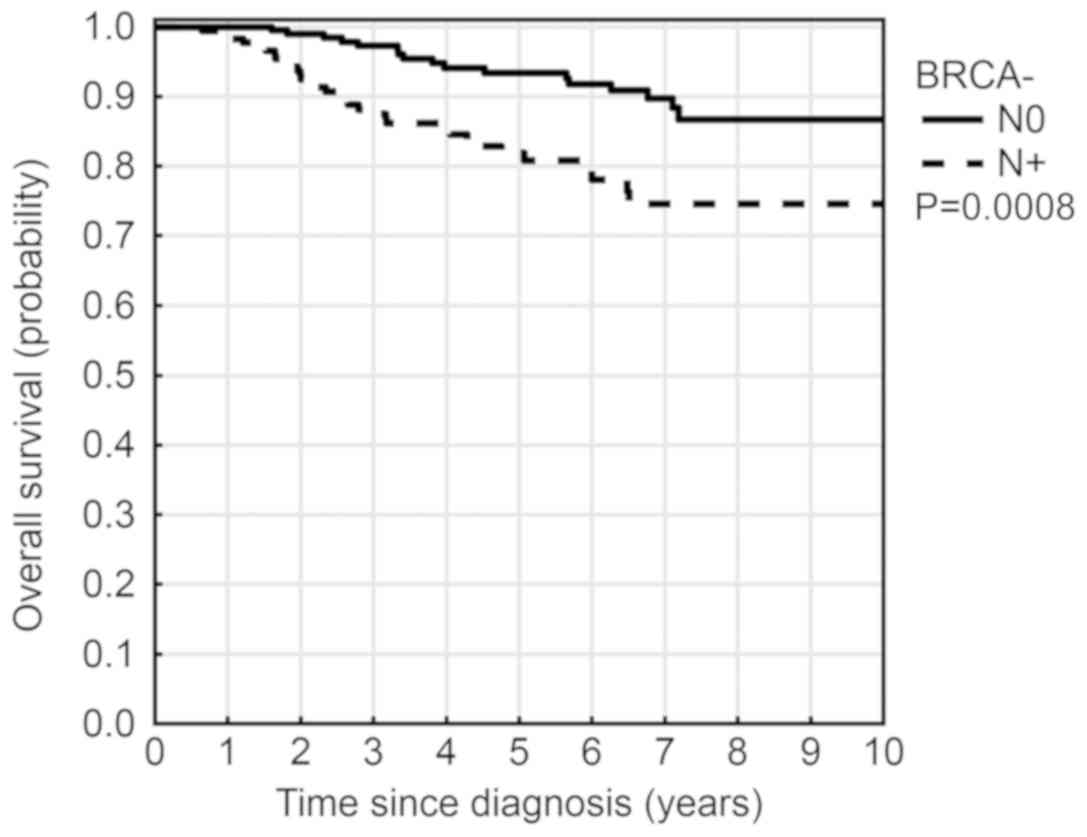
survival rate 82% vs. 93%, P=0.0008) (Fig. 1). Risk of mortality was 2.7 fold
higher for patients with lymph node metastases. The 5 year OS rate
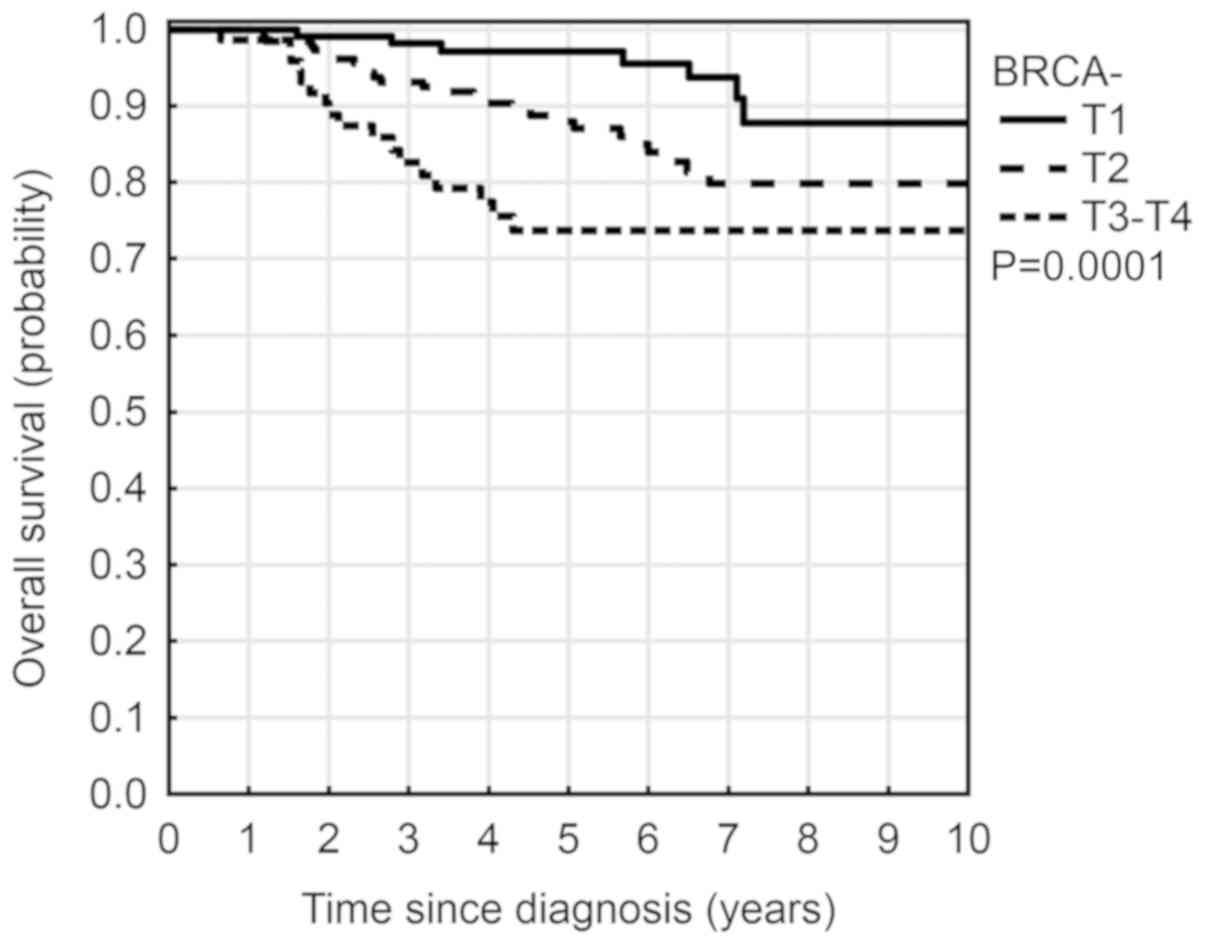
depending on the depth (T) was 97% for T1, 88% for T2 and 74% for
the T3-T4 (Fig. 2). The risk of
mortality depended on the stage of the disease and was higher at
the advanced T3-T4 stages, HR=4.7; (P=0.0006). Patients with
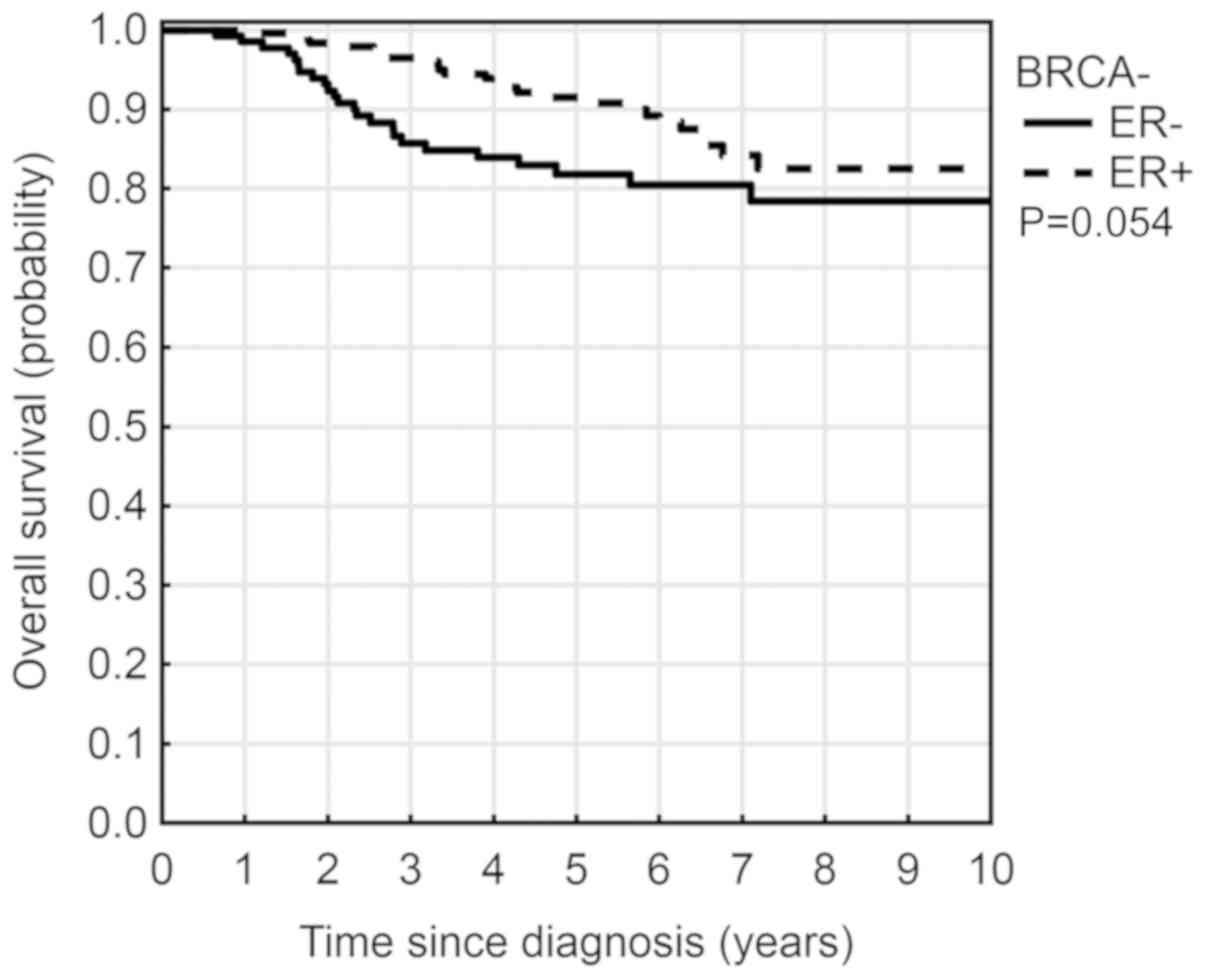
positive ER status (ER+) had a longer OS rate (5-year OS 91% vs.
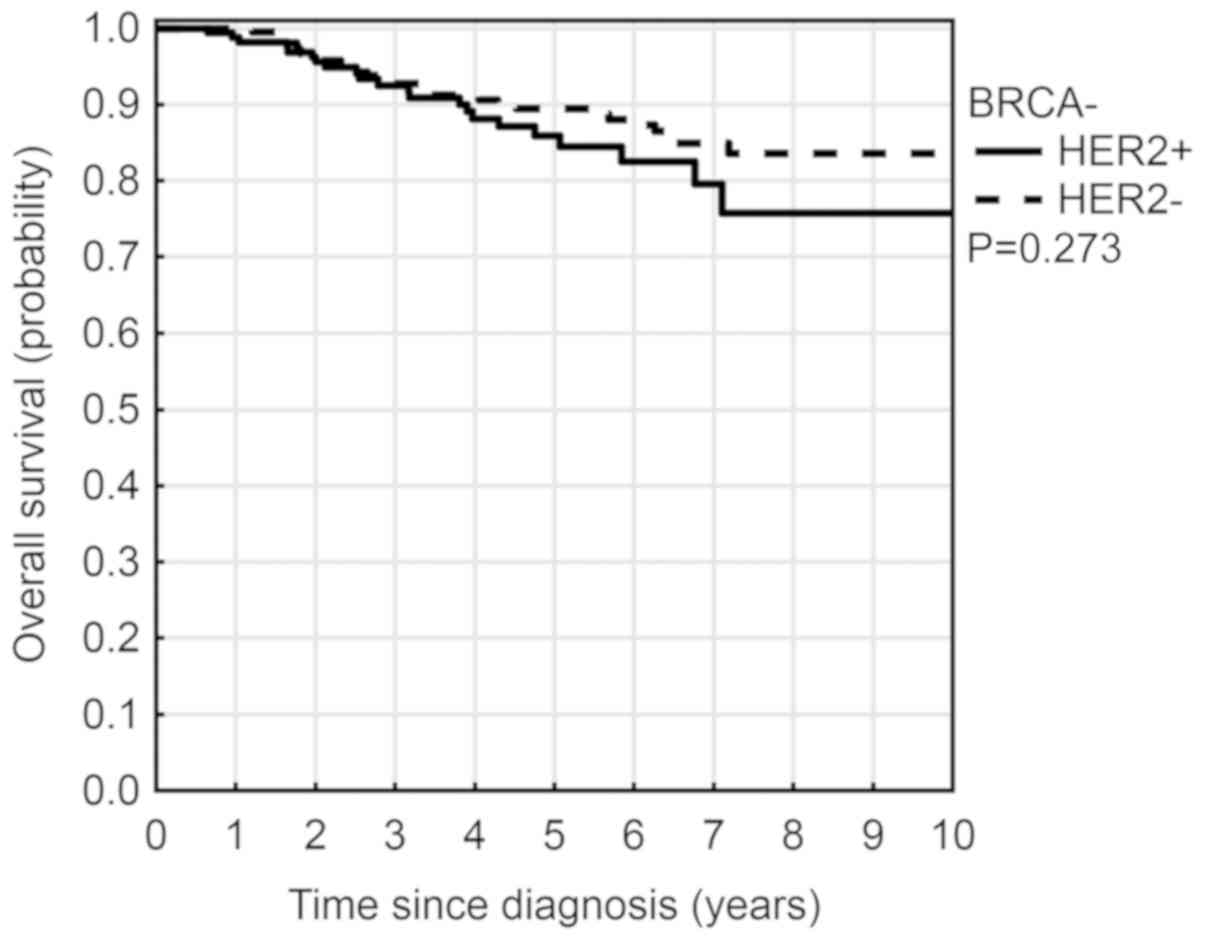
82%, P=0.054) however this was not significant (Fig. 3). Patients with tumor HER2
overexpression had a lower OS rate (5-year OS 86% vs. 89%, P=0.273)
(Fig. 4), which was also not
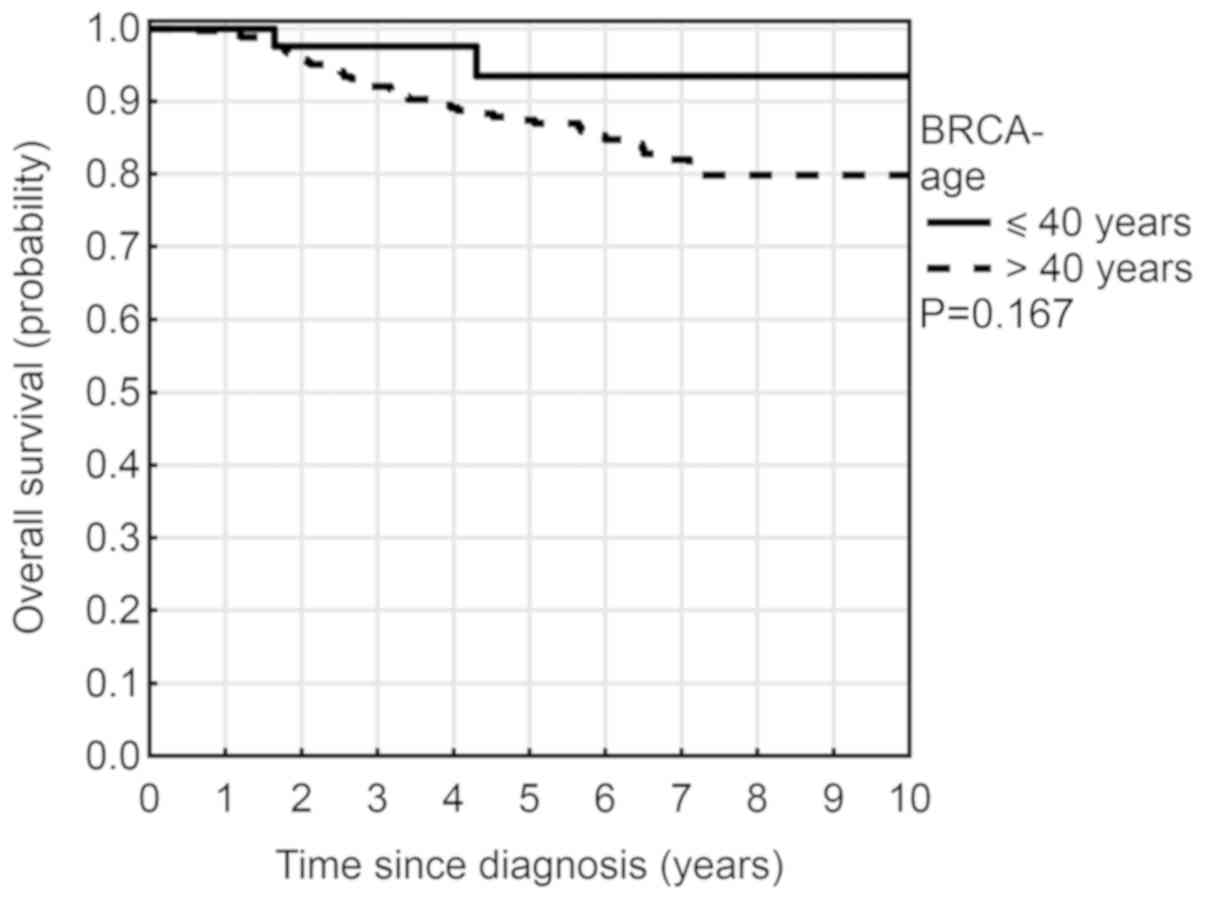
significant. Younger patients (≤40 years) had an increased OS rate
(5-year OS 93% vs. 87%; P=0.167) (Fig.
5) however this was again not significant. They also had a
lower risk of mortality (HR=0.36; P=0.167) compared with older
patients. In uni- and multivariate analyses, increased tumor size,
lymph node metastasis and higher tumor grade were all associated
with increased risk of mortality (Table
IV). Similarly, steroid receptor status (ER negative)
insignificantly increased risk of mortality.
 | Table IV.5-year survival rates, and uni- and
multivariate hazard ratios for mortalities in BRCA1
non-carriers and carriers. |
Table IV.
5-year survival rates, and uni- and
multivariate hazard ratios for mortalities in BRCA1
non-carriers and carriers.
| A, BRCA1
non-carriers |
|---|
|
|---|
|
|
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|---|
| Factor | Total n | 5-year survival
rate (%) | Test log rank
P-value | HR | P-value | HR | 95% CI | P-value |
|---|
| Total cases | 386 | 88.1 | – | – | – | – | – | – |
| Age (years) |
|
≤40 | 45 | 93.5 |
| 0.36 | 0.161 | 0.37 | 0.09–1.53 | 0.169 |
|
>40 | 341 | 87.4 | 0.167 | 1.0 |
| 1.0 |
|
|
| T Stage |
| T1 | 121 | 97.1 |
| 1.0 |
| 1.0 |
|
|
| T2 | 190 | 87.9 | 0.0001 | 2.59 | 0.026 | 2.26 | 0.98–5.22 | 0.057 |
|
T3-T4 | 75 | 73.7 |
| 4.71 | 0.0006 | 3.32 | 1.34–8.20 | 0.009 |
| Clinical staging
nodes |
| N0 | 209 | 93.4 |
| 1.0 |
| 1.0 |
|
|
| N+ | 177 | 81.9 | 0.0008 | 2.67 | 0.001 | 2.40 | 1.30–4.42 | 0.005 |
| G |
|
G1-G2 | 131 | 94.8 |
| 1.0 |
| 1.0 |
|
|
| G3 | 114 | 84.0 | 0.0039 | 3.71 | 0.004 | 2.93 | 1.19–7.19 | 0.019 |
|
Missing | 141 | 85.3 |
| 3.04 | 0.009 | 2.95 | 1.27–6.86 | 0.012 |
| ER status |
|
Negative | 137 | 81.8 |
| 1.0 |
| 1.0 |
|
|
|
Positive | 249 | 91.5 | 0.054 | 0.58 | 0.057 | 0.54 | 0.28–1.04 | 0.064 |
| Triple
negative |
| No | 328 | 88.5 |
| 1.0 |
| 1.0 |
|
|
|
Yes | 58 | 85.2 | 0.745 | 1.12 | 0.754 | 0.69 | 0.30–1.59 | 0.382 |
|
| B, BRCA1
carriers |
|
|
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
|
| Factor | N | 5-year survival
rate | P-value | HR | Test log rank
P-value | HR | 95% CI | P-value |
|
| Total cases | 60 | 77.3 | – | – | – | – | – | – |
| Age (years) |
|
≤40 | 22 | 81.8 | 0.310 | 0.59 | 0.326 | 0.44 | 0.12–1.60 | 0.213 |
|
>40 | 38 | 75.0 |
| 1.0 |
| 1.0 |
|
|
| T Stage |
| T1 | 10 | 90.0 |
| 1.0 |
| 1.0 |
|
|
| T2 | 27 | 84.5 | 0.243 | 2.91 | 0.318 | 2.71 | 0.31–23.4 | 0.365 |
|
T3-T4 | 23 | 63.5 |
| 5.07 | 0.125 | 5.39 | 0.64–45.1 | 0.120 |
| Clinical staging
nodes |
| N0 | 49 | 82.9 | 0.034 | 1.0 |
| 1.0 |
|
|
| N+ | 11 | 51.9 |
| 3.00 | 0.031 | 3.29 | 1.08–9.99 | 0.036 |
| G |
|
G1-G2 | 7 | 83.3 |
| 1.0 |
| 1.0 |
|
|
| G3 | 30 | 75.3 | 0.798 | 1.98 | 0.516 | 1.61 | 0.19–13.72 | 0.663 |
|
Missing | 23 | 77.8 |
| 1.77 | 0.596 | 1.37 | 0.15–12.14 | 0.779 |
| ER status |
|
Negative | 40 | 74.4 | 0.417 | 1.0 |
| 1.0 |
|
|
|
Positive | 20 | 83.3 |
| 0.63 | 0.419 | 0.14 | 0.02–0.99 | 0.049 |
| Triple
negative |
| No | 23 | 81.3 |
| 1.0 |
| 1.0 |
|
|
|
Yes | 37 | 75.1 | 0.884 | 1.08 | 0.883 | 0.20 | 0.03–1.17 | 0.073 |
Survival analysis in BRCA (+) mutation
carriers
The 5-year OS rate was 77.3% [95% confidence
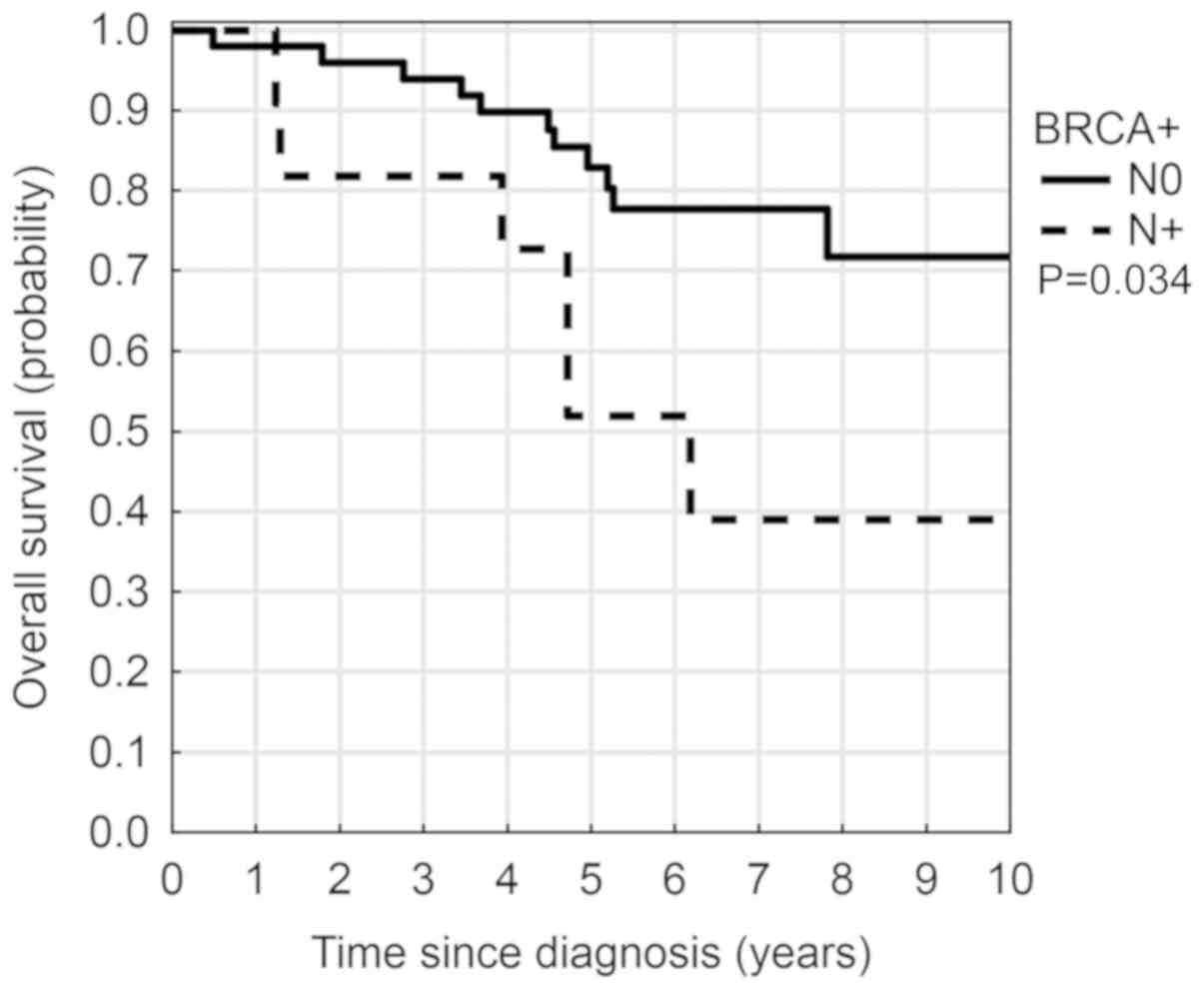
interval (CI), 66.4–88.2%]. Patients with lymph node metastases (N
+) had a significantly lower 5-year OS compared with patients
without lymph node involvement (52% vs. 83%, P=0.03) and 3.0 fold
higher risk of death (Fig. 6). 5-year
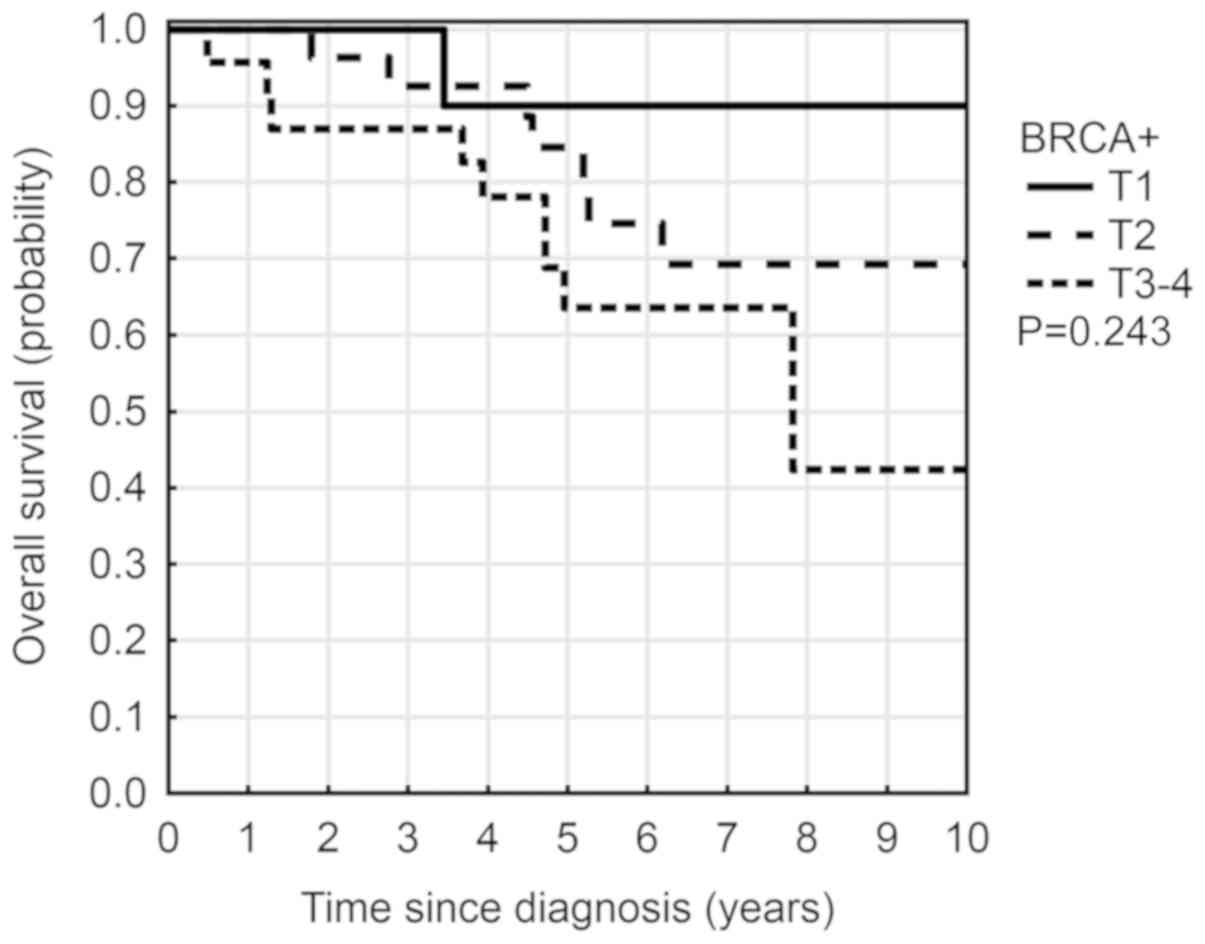
OS was associated with tumor size (T) and was 90% for T1, 84% for
T2 and 63% for T3-T4. The risk of mortality depended on stage of
disease and was the greatest at the advanced T3-T4 stages, HR=5.07;
(95% CI, 0.64–40.33 P=0.125) (Fig.
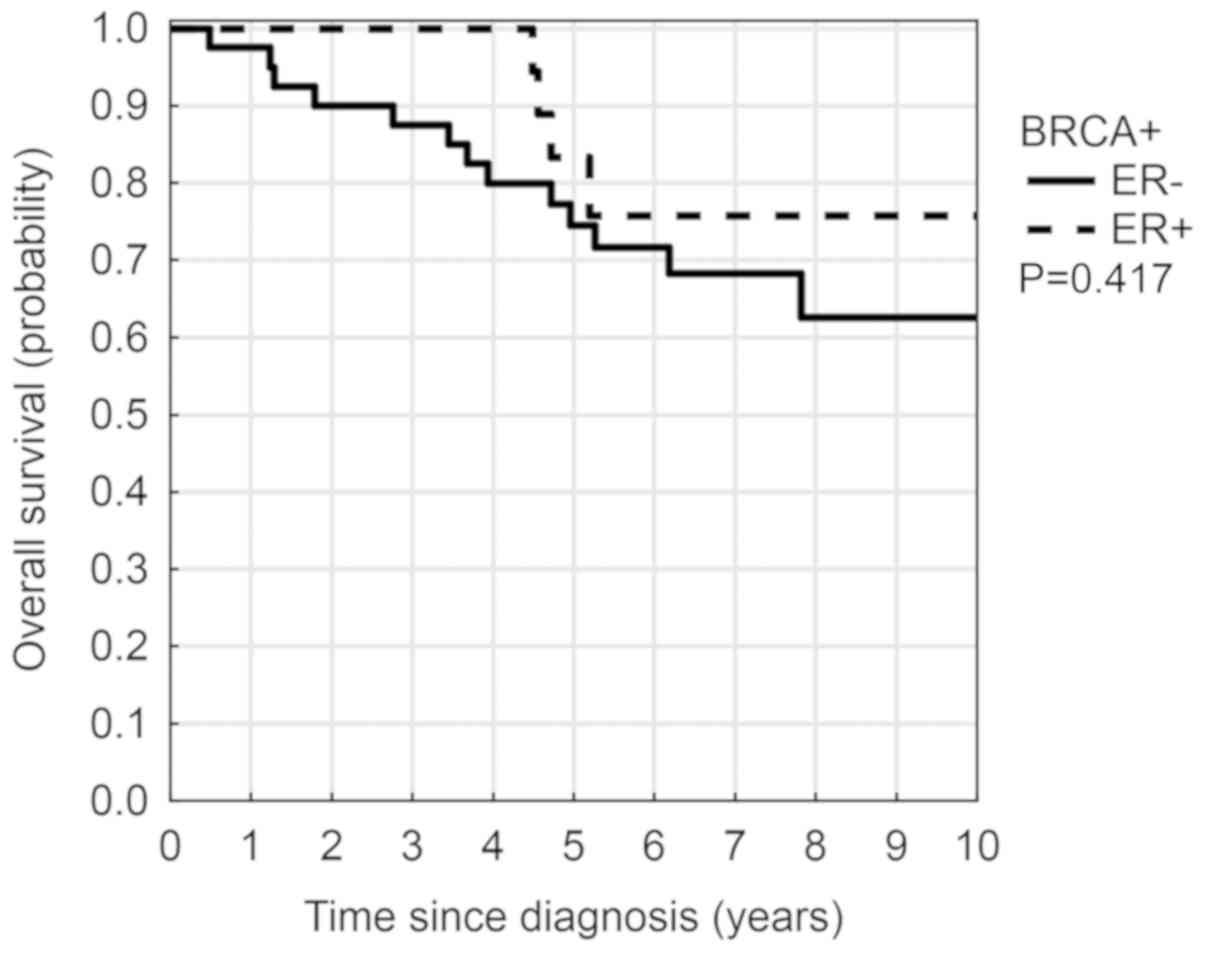
7). Patients who had tumors with ER+ status had an
insignificantly higher 5-year OS (83% vs. 74%, P=0.417) (Fig. 8). Younger patients (≤40 years)
exhibited an insignificantly higher OS (82% vs. 75%; P=0.310)
(Table IV). In univariate analysis,
lymph node metastasis was a significant prognostic factor. In
multivariate analysis, lymph node metastases (HR=3.29, P=0.036) and
ER- status (HR=7.14, P=0.049) were identified as negative
prognostic factors in BRCA mutation carriers. Conversely,
TNBC was a favorable prognostic factor in this group (HR=0.20,
P=0.073) (Table IV).
BRCA mutation carriers had a significantly
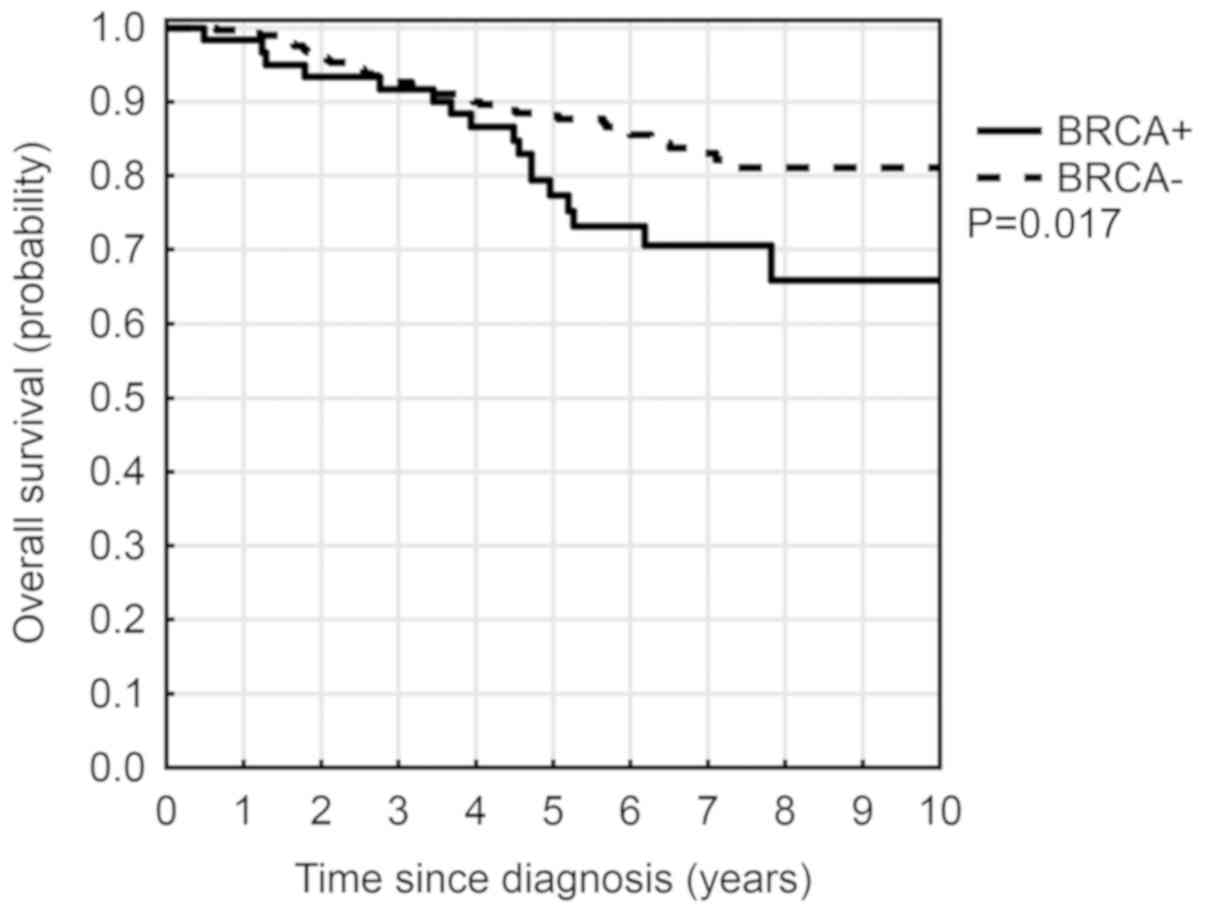
worse survival rate compared with non-carriers (P=0.017) (Fig. 9). The ten-year OS rate was 78.0% for
all analyzed groups: 65.9% for BRCA mutation carriers and
81.1% for non-carriers. The 5-year (OS) rate was 86.2% for all
analyzed groups: 77.3% for BRCA mutation carriers and 88.1%
for non-carriers. In univariate analyses, BRCA mutation
carriers had a significantly higher risk of mortality in comparison
to non-carriers (HR=1.87, 95% CI, 1.08–3.25) (Table V). After adjusting for other
prognostic factors, there was a significant difference in survival
between carriers and non-carriers (HR=2.28, P=0.019). Higher tumor
grade (T3-4) (HR=3.64), lymph node metastases (N+) (HR=2.45) and G3
(HR=2.84) were significant factors for a worse OS. ER+ status was
associated with a better OS (HR=0.49, P=0.022). Younger age (≤40
years) (HR=0.48, P=0.081) was a favorable factor, but was not
significant. Detailed results for multivariate analysis are shown
in Table V.
 | Table V.Multivariate analysis for overall
survival. |
Table V.
Multivariate analysis for overall
survival.
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| BRCA mutation
carriers vs. non carriers (univariable) | 1.87 | 1.08–3.25 | 0.026 |
| BRCA mutation
carriers vs. non carriers (adjusted) | 2.28 | 1.15–4.55 | 0.019 |
| Adjusted for: |
| Age
(years) | 0.48 | 0.21–1.10 | 0.081 |
| T2 vs.
T1 | 2.33 | 1.07–5.08 | 0.033 |
| T3-4
vs. T1 | 3.64 | 1.61–8.20 | 0.002 |
| N+ vs.
N0 | 2.45 | 1.45–4.14 | 0.001 |
| G3 vs.
G1-2 | 2.84 | 1.26–6.42 | 0.012 |
| G
missing vs. G1-2 | 2.82 | 1.29–6.16 | 0.009 |
| ER
positive vs. ER negative | 0.49 | 0.27–0.90 | 0.022 |
| TNBC
vs. others | 0.61 | 0.29–1.28 | 0.192 |
Discussion
In this retrospective study, we reported the
negative factors for OS in breast cancer patients with BRCA
mutation which were: Infiltration of armpit lymph nodes (P=0.034),
increased size of primary tumor (T3-T4, P=0.243), age >40 years
(P=0.310) and negative steroid receptor status (P=0.417). In case
of non-carriers, negative factors for OS were also: Lymph node
metastasis (N+) (P=0.0008), increased tumor size (T3-T4)
(P=0.0001), negative steroid receptor status (P=0.054) and HER2
overexpression, however this was not significant (P=0.273).
In a previous study involving a group of patients
with stage I breast cancer, BRCA mutation carriers, the
ten-year survival rate was 89.9%. Huzarski et al (9) reported that the ten-year OS among breast
cancer patients with BRCA1 mutation is similar to OS in
women without a BRCA1 mutation. Similarly, survival outcomes
of BRCA1 mutation carriers were similar to those of sporadic
breast cancer patients in a study conducted by Goodwin et al
(10). Worse survival outcomes in
BRCA2 mutation carriers were observed in univariable
analysis (more adverse tumor characteristics). However, similar
outcomes of BRCA2 mutation carriers and sporadic disease
were identified in multivariable analyses (10). In previous reports, breast cancer
BRCA mutation carriers exhibited a worse prognosis compared
with breast cancer patients of the same age that did not have the
BRCA mutation (11,12). In our study, the ten-year OS rate was
65.9% for BRCA mutation carriers and 81.1% for non-carriers,
irrespective of disease stage. Lee et al (4) showed that the presence of BRCA1
mutation decreases short-term and long-term OS rate, and short-term
progression-free survival rate (PFSR). Conversely, there was no
reported association between BRCA2 mutation and short-term
or long-term survival rate. This suggests that carcinogenic
pathways for BRCA1 and BRCA2 are different (13). Baretta et al (14) revealed that patients with BRCA1
mutation have worse OS in comparison to BRCA-sporadic
patients (HR 1.30; 95% CI, 1.11–1.52). Similarly, worse breast
cancer-specific survival was reported in BRCA1 mutation
carriers among patients with stage I–III breast cancer (HR, 1.45;
95% CI, 1.01–2.07) (14). The
meta-analyses conducted by Van der Broek et al (15) did not support worse survival in breast
cancer for patients with BRCA1/2 mutation in the adjuvant
treatment. They only improved a 10% worse unadjusted
recurrence-free survival for BRCA1 mutation carriers
(15). In the present study,
BRCA mutation carriers had a significantly worse survival
rate compared with non-carriers (P=0.017). However, patients with
the BRCA mutation had an increased rate of TNBC diagnosis in
comparison to those with sporadic breast cancer (61.7% vs. 15.0%,
P=0.0001).
Clinicopathological factors affecting OS were also
analyzed in various studies. The survival rate for BRCA
positive women without lymph node infiltration and tumor size <1
cm was not increased, compared with patients with tumor size
between 1 and 2 cm (10). In the
present study, the risk of mortality depended on the stage of the
disease and was higher at the advanced T3-T4 stages in BRCA
mutation non-carriers and in patients with the BRCA
mutation. Huzarski et al (9)
reported that oophorectomy significantly improved survival among
women with a BRCA1 mutation. BRCA1 mutation carriers
who received chemotherapy had better survival in comparison to
women treated without chemotherapy (9). In the Goodwin et al (10) study, the survival of BRCA1
mutation carriers treated with chemotherapy was similar to that of
BRCA 1 non-carriers. However, in case of treatment without
chemotherapy, the survival of BRCA1 mutation carriers was
worse (HR=1.97; 95% CI, 0.65–5.94) (10). In our study, all patients received
chemotherapy; 97.3% of patients received chemotherapy regimens with
anthracycline.
Foulkes et al (11) confirmed that BRCA1 mutation
carrier status was associated with clinicopathological factors of
breast cancer associated with worse prognosis, including young age
at diagnosis, high nuclear grade, negative steroid receptor status
(ER-), and the presence of somatic TP53 mutations. In the
group of patients with negative steroid receptor status (ER-)
tumors, higher nuclear grade 3 and tumor size <20 mm the
BRCA1 positive status was associated with a significantly
worse prognosis (11). Previous
studies have confirmed these results (7,16,17). Osin and Lakhani reported that
BRCA1-associated tumors are more likely to be steroid
receptor negative, and more frequently express p53 protein.
Mutations in the TP53 gene also appear to be increased in
tumors with BRCA1 mutation (18). The presence of steroid receptor status
(ER) in tumors with BRCA1 mutation was significantly lower
(8 vs. 26%) in comparison with a grade-matched control group. In
contrast, the presence of ER in tumors with BRCA2 mutation
appears to be similar to that in sporadic breast cancers (13,19). In
some studies, there was no difference between mutation carriers and
non-carriers according to HER2/neu overexpression or amplification
(17,20). Crook et al (20) showed that tumors with BRCA
mutation were more often p53 positive in comparison to sporadic
breast cancers (77% BRCA1, 45% BRCA2, 35% sporadic).
The presence of mutations in the TP53 gene have also been
reported to be increased in BRCA1 tumors (18). In our analysis, negative prognostic
factors for both groups (BRCA mutation carriers and
non-carriers) were lymph node metastases, negative steroid receptor
status and larger tumor size.
BRCA mutation carriers were characterized by
younger age, negative steroid receptor status, tumors without HER2
overexpression and larger tumor size (T3-T4). The ten-year survival
rate among breast cancer patients with the BRCA1 mutation
was significantly worse than in patients without a BRCA1
mutation. Negative factors for OS in breast cancer patients who
were carriers of BRCA mutations included infiltration of
armpit lymph nodes, negative steroid receptor status and increased
size of the primary tumor.
Acknowledgements
The authors would like to thank Dr Karolina Tęcza,
Dr Jolanta Pamuła Piłat and Magdalena Mazur from the Center for
Translational Research and Molecular Biology of Cancer for their
assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JH analyzed and interpreted the patient data and was
a major contributor in writing the manuscript. ZK performed
statistical analysis, and analyzed and interpreted the patient
data. EG made substantial contributions to conception and design,
or acquisition of data, or analysis and interpretation of data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent
allowing for their biological material to be used in clinical
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balmaña J, Díez O, Rubio LT and Cardoso F:
ESMO Guidelines Working Group: BRCA in breast cancer: ESMO clinical
practice guidelines. Ann Oncol. 22 Suppl 6:vi31–vi34. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrieu N, Goldgar DE, Easton DF, Rookus
M, Brohet R, Antoniou AC, Peock S, Evans G, Eccles D, Douglas F, et
al: Pregnancies, breast-feeding, and breast cancer risk in the
International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer
Inst. 98:535–544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ritte R, Tikk K, Lukanova A, Tjønneland A,
Olsen A, Overvad K, Dossus L, Fournier A, Clavel-Chapelon F, Grote
V, et al: Reproductive factors and risk of hormone receptor
positive and negative breast cancer: A cohort study. BMC Cancer.
13:5842013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee EH, Park SK, Park B, Kim SW, Lee MH,
Ahn SH, Son BH, Yoo KY and Kang D: KOHBRA Research Group; Korean
Breast Cancer Society: Effect of BRCA1/2 mutation on short-term and
long-term breast cancer survival: A systematic review and
meta-analysis. Breast Cancer Res Treat. 122:11–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peshkin BN, Alabek ML and Isaacs C:
BRCA1/2 mutations and triple negative breast cancer. Breast Dis.
32:25–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bayraktar S, Gutierrez-Barrera AM, Liu D,
Tasbas T, Akar U, Litton JK, Lin E, Albarracin CT, Meric-Bernstam
F, Gonzalez-Angulo AM, et al: Outcome of triple-negative breast
cancer in patients with or without deleterious BRCA mutations.
Breast Cancer Res Treat. 130:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rennert G, Bisland-Naggan S,
Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS and Narod SA:
Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2
mutations. N Engl J Med. 357:115–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Górski B, Byrski T, Huzarski T, Jakubowska
A, Menkiszak J, Gronwald J, Pluzańska A, Bebenek M,
Fischer-Maliszewska L, Grzybowska E, et al: Founder mutations in
the BRCA1 gene in Polish families with breast-ovarian cancer. Am J
Hum Genet. 66:1963–1968. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huzarski T, Byrski T, Gronwald J, Górski
B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka
M, et al: Ten-year survival in patients with BRCA1-negative and
BRCA1-positive breast cancer. J Clin Oncol. 31:3191–3196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodwin PJ, Phillips KA, West DW, Ennis M,
Hopper JL, John EM, O'Malley FP, Milne RL, Andrulis IL, Friedlander
ML, et al: Breast cancer prognosis in BRCA1 and BRCA2 mutation
carriers: An International Prospective Breast Cancer Family
Registry population-based cohort study. J Clin Oncol. 30:19–26.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Foulkes WD, Chappuis PO, Wong N, Brunet
JS, Vesprini D, Rozen F, Yuan ZQ, Pollak MN, Kuperstein G, Narod SA
and Bégin LR: Primary node negative breast cancer in BRCA1 mutation
carriers has a poor outcome. Ann Oncol. 11:307–313. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stoppa-Lyonnet D, Ansquer Y, Dreyfus H,
Gautier C, Gauthier-Villars M, Bourstyn E, Clough KB, Magdelénat H,
Pouillart P, Vincent-Salomon A, et al: Familial invasive breast
cancers: Worse outcome related to BRCA1 mutations. J Clin Oncol.
18:4053–4059. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osin P, Gusterson BA, Philp E, Waller J,
Bartek J, Peto J and Crook T: Predicted anti-oestrogen resistance
in BRCA-associated familial breast cancers. Eur J Cancer.
34:1683–1686. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baretta Z, Mocellin S, Goldin E, Olopade
OI and Huo D: Effect of BRCA germline mutations on breast cancer
prognosis: A systematic review and meta-analysis. Medicine
(Baltimore). 95:e49752016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van der Broek AJ, Schmidt MK, van't Veer
LJ, Tollenaar RA and van Leeuwen FE: Worse breast cancer prognosis
of BRCA1/BRCA2 mutation carriers: What's the evidence? A systematic
review with meta-analysis. PLoS One. 10:e01201892015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robson M, Rajan P, Rosen PP, Gilewski T,
Hirschaut Y, Pressman P, Haas B, Norton L and Offit K:
BRCA-associated breast cancer: Absence of a characteristic
immunophenotype. Cancer Res. 58:1839–1842. 1998.PubMed/NCBI
|
|
17
|
Eisinger F, Stoppa-Lyonnet D, Longy M,
Kerangueven F, Noguchi T, Bailly C, Vincent-Salomon A, Jacquemier
J, Birnbaum D and Sobol H: Germ line mutation at BRCA1 affects the
histoprognostic grade in hereditary breast cancer. Cancer Res.
56:471–474. 1996.PubMed/NCBI
|
|
18
|
Osin PP and Lakhani SR: The pathology of
familial breast cancer: Immunohistochemistry and molecular
analysis. Breast Cancer Res. 1:36–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armes JE, Trute L, White D, Southey MC,
Hammet F, Tesoriero A, Hutchins AM, Dite GS, McCredie MR, Giles GG,
et al: Distinct molecular pathogeneses of early-onset breast
cancers in BRCA1 and BRCA2 mutation carriers: A population-based
study. Cancer Res. 59:2011–2017. 1999.PubMed/NCBI
|
|
20
|
Crook T, Brooks LA, Crossland S, Osin P,
Barker KT, Waller J, Philp E, Smith PD, Yulug I, Peto J, et al: p53
mutation with frequent novel condons but not a mutator phenotype in
BRCA1-and BRCA2-associated breast tumors. Oncogene. 17:1681–1689.
1998. View Article : Google Scholar : PubMed/NCBI
|