Introduction
Most patients with metastatic castration-resistant
prostate cancer (mCRPC) develop bone metastases, which are
associated with an increased risk of pathologic bone fractures
(both symptomatic and found incidentally), radiation or surgery to
the bone, spinal cord compression which are called skeletal-related
events (SRE) that occur in 20–50% of mCRPC patients. Indeed, these
are the major causes of prostate cancer-specific morbidity,
worsening of quality of life and death (1).
Radium-223 dichloride (Radium-223) is a novel
targeted radioisotope that, as a bone-seeking calcium mimetic,
selectively binds mineral hydroxyapatite of newly formed bone
stroma in areas of increased metabolic activity as bone metastases
(2). It emits high-energy
alpha-particle radiation of short range (<100 mm; <10 cell
diameters) that induces double-stranded DNA breaks with cytotoxic
effects in target areas, limiting damage on surrounding normal
tissue, particularly the bone marrow (3).
Based on previous phase II trials (2,4,5), the efficacy and the safety of Radium-223
in mCRPC were demonstrated in the phase 3 Alpharadin in Symptomatic
Prostate Cancer Patients (ALSYMPCA) trial, which investigated
Radium-223 vs. placebo in patients with mCRPC with symptomatic bone
metastases and no known visceral metastases who had previously
received docetaxel or not (6). This
trial showed that Radium-223 significantly improved median overall
survival (mOS) and prolonged median time to first symptomatic
skeletal events (SSE) compared with placebo, independently to prior
exposure to docetaxel (7) and the use
of bisphosphonates (8). The end-point
SSE is similar to SRE, but includes only symptomatic pathologic
fractures in addition to radiation or surgery to the bone and
spinal cord compression. Notably, Radium-223 was associated with a
favourable toxicity profile and a higher percentage of patients who
experienced an improvement in quality of life (6,9).
The survival benefit and the better safety profile
of Radium-223 were the novel points compared to beta-emitting
radiopharmaceuticals, such as Samarium-153 and Strontium-89/90.
The results of this pivotal study led to the
regulatory approval from the American Food and Drug Administration
and European Medicines Agency of Radium-223 for the treatment of
mCRPC patients with symptomatic bone metastases. Given its
effectiveness in both docetaxel-naïve and post-docetaxel patients,
Radium-223 can be used as monotherapy both in first-line and in
subsequent lines (7).
Recent studies confirmed the efficacy and the safety
observed in the ALSYMPCA trial (10,11) but
more investigations on this novel agent in the clinical practice
setting, outside the context of clinical trials, are needed.
The aim of this retrospective study was to assess
clinical and biochemical factors related to survival and time to
first SRE (tSRE) of Radium-223 in mCRPC patients in a clinical
practice setting.
Materials and methods
Study population
From July 2015 to September 2017, we performed a
retrospective analysis of all consecutive adult mCRPC patients
treated with Radium-223 at the Policlinico Umberto I, Sapienza
University of Rome.
CRPC was defined as a serum testosterone level of
<50 ng/dl following surgical or pharmaceutical castration. All
patients had two or more symptomatic bone metastases detected by
bone scan and no known visceral metastases, except for malignant
lymphadenopathy with less than 3 cm in the short-axis diameter, an
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
score of 0–2 and adequate haematological, liver and renal
function.
Clinical data included patient characteristics [age,
ECOG PS, baseline ALP, PSA and pain score by numeric rating scale
(NRS)], tumour characteristics (Gleason score, number of bone
metastases and presence or absence of lymph node metastases) and
treatment information (median cycles and line of Radium-223, prior
use of docetaxel and concomitant use of
bisphosphonates/denosumab).
Treatment plan
All patients included in the analysis underwent
treatment with Radium-223, which consisted of six intravenous
injections of the radioisotope at a standard dose of 50 kBq/kg at
four-week intervals until disease progression or unacceptable
toxicity. The use of androgen deprivation therapy continued during
Radium-223 treatment. Concomitant treatment with Abiraterone and
Enzalutamide was not permitted. Patients were also receiving the
best standard of care, including analgesics and glucocorticoids to
control pain.
Response evaluation
Efficacy assessments included scintigraphic,
biochemical and pain response. All patients were followed
clinically by a multidisciplinary team and radiologically by bone
scan. Patients had a technetium-99 m bone scan before treatment,
after three cycles and after all six cycles of Radium-223. The best
scintigraphic result between the three assessments was considered
the scintigraphic response. The bone-specific response was assessed
as complete (CR) and partial (PR) response, stable (SD) and
progression (PD) disease according to MD Anderson criteria
(12). Overall response rate (ORR)
was defined as the sum of CR and PR and disease control rate (DCR)
as the sum of CR, PR and SD.
Biochemical and pain responses were evaluated with
the relative change of ALP, PSA and NRS values, comparing the value
at the end of the sixth cycle with the baseline value (13). A biological (ALP and PSA) response
were defined as a reduction of ≥30% from the baseline value to the
end of Radium-223 treatment. Pain response was defined as a decline
of at least one range according to the NRS.
Statistical analysis
Survival analysis was conducted on the efficacy of
Radium-223 in terms of progression-free survival (PFS) and OS. PFS
was measured from the start of Radium-223 to diagnosis of PD
evidenced by bone scan, death from any cause or last follow-up and
calculated as median PFS (mPFS). OS was measured from the start of
treatment to death from any cause or last follow-up. PFS and OS
were estimated with a 95% confidence interval. Survival curves of
PFS and OS were generated using the Kaplan-Meier method.
Differences in PFS and OS were evaluated using the log-rank test
(Mantel-Cox) for statistical significance, which was defined at the
P<0.05 level (14) using the SPSS
program-Statistical Package for the Social Sciences. Time to the
first SRE was defined as the time to first pathologic bone
fractures and spinal cord compression (evaluated clinically and
radiologically), radiation therapy and orthopaedic surgical
intervention (1).
Subgroup analyses
Subgroup analyses were performed to assess survival
outcomes based on scintigraphic response, biochemical (ALP and PSA)
response (≥50 and ≥30%), pain response, baseline ALP levels (±220
UI/l), prior docetaxel use, concomitant bisphosphonates/denosumab
and involvement of lymph node metastases. We did not assess a
subgroup analyses according to Radium-223 line because of
insufficient length of follow-up.
Toxicity evaluation
All adverse events were graded according to the
Common Terminology Criteria for Adverse Events (CTCAE) version 4.03
(15). Toxicity assessment was
performed at each cycle of treatment.
Results
Patient characteristics
Thirty-two mCRPC patients were included in the
analysis. Patient, tumour and treatment characteristics are
summarised in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Median age, years
(range) | 72 (59–86) |
| ECOG performance
status |
|
| Median
(range) | 1
(0–2) |
| 0 | 12 (38) |
| 1 | 9
(28) |
| 2 | 11 (34) |
| Gleason score at
diagnosis |
| Median
(range) | 8
(6–9) |
| ≤7 | 8
(25) |
|
8–10 | 24 (75) |
| Metastases |
| Bone
metastases | 19 (59) |
| Bone
and lymph node metastases | 13 (41) |
| Extent of
disease |
|
<6 | 1 (3) |
|
6–20 | 31 (97) |
|
>20 | 0 (0) |
| Median PSA, ng/l
(range) | 88 (0.5–3847) |
| Median ALP, U/l
(range) | 162.5
(53–1,755) |
| Baseline ALP,
U/l |
|
<220 | 20 (63) |
|
≥220 | 12 (37) |
| Pain at baseline,
NRS |
| Median
(range) | 5
(1–10) |
|
1–3 | 13 (41) |
|
4–6 | 11 (34) |
|
7–10 | 8
(25) |
| Radium-223
treatment |
| Median
cycles received, number (range) | 6
(2–6) |
| Completed 6 cycles
of Radium-223 |
|
Yes | 29 (91) |
| No | 3 (9) |
| Radium-223
treatment |
|
First-line | 6
(19) |
|
Second-line | 14 (44) |
|
Other | 12 (37) |
| Prior
chemotherapy |
|
Yes | 15 (47) |
| No | 17 (53) |
| Concomitant use of
biphosphonates/denosumab |
|
Yes | 21 (66) |
|
Biphosphonates | 15 (47) |
|
Denosumab | 6
(19) |
| No | 11 (34) |
The median age was 72 years (range 59–86 years) and
median ECOG PS was 1 (range 0–2). All patients had a histological
diagnosis of prostate cancer with a Gleason score ≥7 in 75% of
patients. Nineteen patients (59%) had only bone metastases, whereas
13 patients (41%) had also lymph node metastases. Most patients
(97%) had a number of bone metastases between 6 and 20.
Radium-223 was administered as first-line therapy in
6 patients (19%), as second-line in 14 patients (44%) and in
successive lines in 12 patients (37%). The median number of cycles
was 6 (range 2–6), and 29 patients (91%) completed all six cycles.
One patient discontinued the treatment after two cycles and 2
patients after four cycles because of death.
Fifteen patients (47%) underwent chemotherapy before
Radium-223, and 66% of patients had a concomitant use of
bisphosphonates/denosumab. Median PSA was 88 g/l (range 0.5–3847),
median ALP was 163 U/l (range 53-1755) with baseline ALP <220
UI/l in 63% of patients and median NRS score was 5 (range
1–10).
Activity evaluation
All patients were assessed for response analysis. At
scintigraphic assessment, 13 patients (41%) experienced PR and 16
patients (50%) had SD with a DCR of 91%.
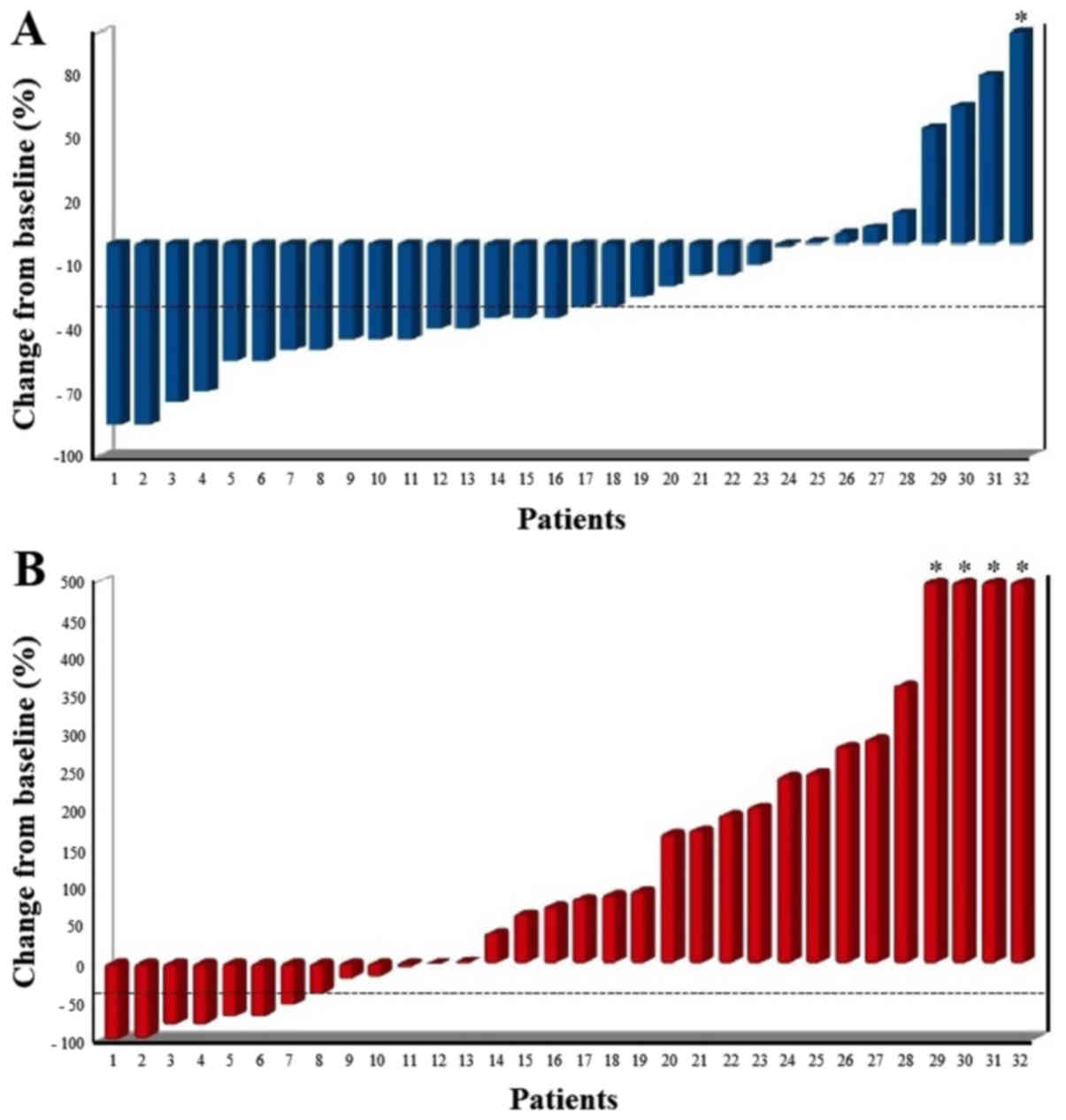
At biochemical assessment with ALP, 18 patients
(56%) showed a biochemical response (≥30% reduction) with 8
patients (25%) which showed an ALP reduction ≥50% (Fig. 1A).
At biochemical assessment with PSA, 8 patients (25%)
showed a biochemical response with 7 (22%) which showed a PSA
response ≥50% (Fig. 1B). Of the 24
patients with a PSA increase, only 2 had a scintigraphic
progression, whereas 5 experienced a scintigraphic response, which
can be evaluated as PSA flare up. Of these 5 patients, 2 patients
also experienced a pain flare up.
At pain assessment, 16 patients (50%) had pain
response and 7 patients (22%) had pain stability with a pain
control of 72%.
SREs occurred in 3 patients (9%), two vertebral
fractures and one external-beam radiation to relieve skeletal pain,
with a median tSRE of 9.5 months (range 4–18). Median PFS was 12
months (95% confidence interval (CI) 11–13 months) with a PFS rate
at 1 year of 53%, and mOS was 14 months (95% CI 10–18) with an OS
rate at 1 year of 63%.
Subgroup analyses
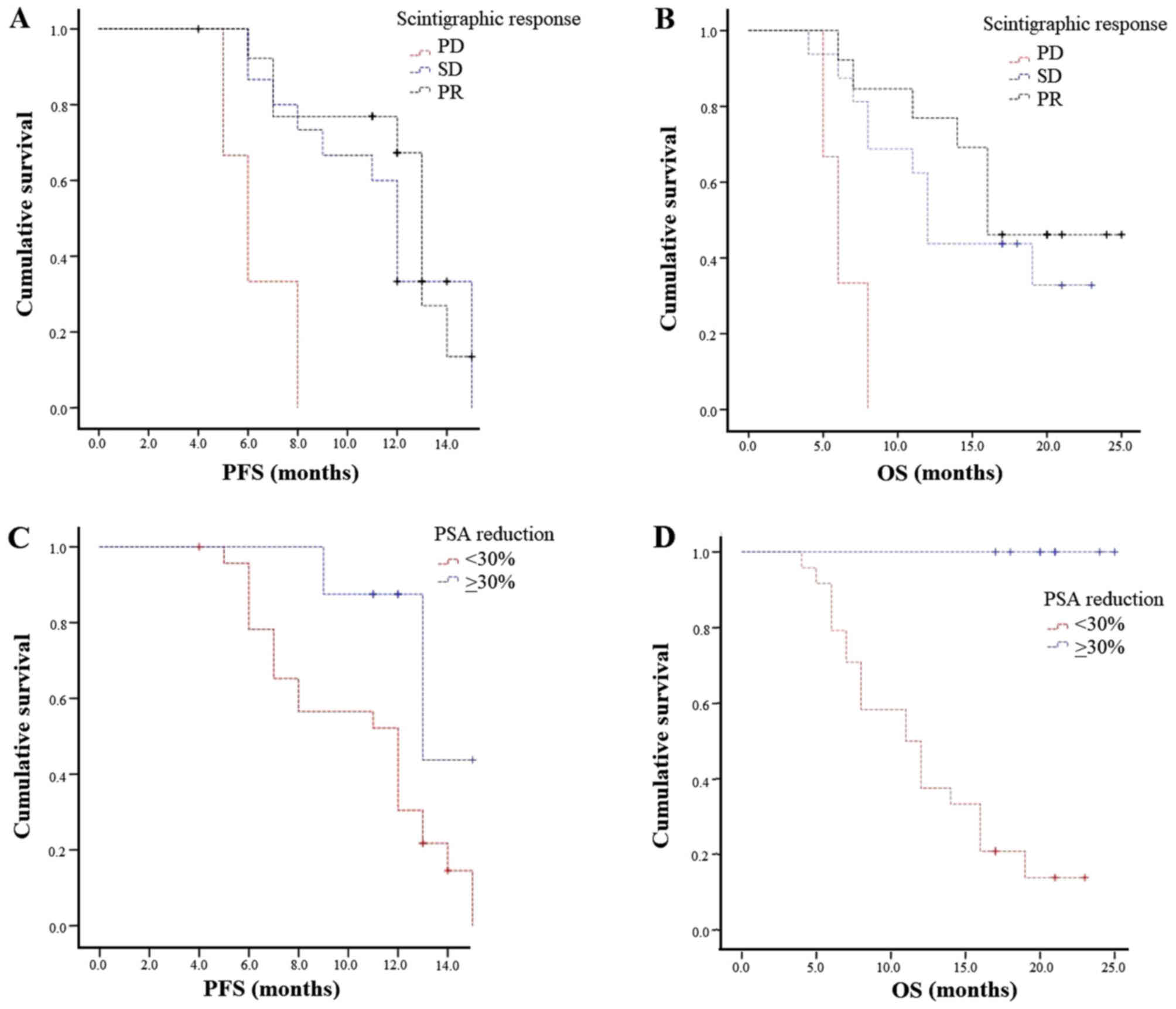
Scintigraphic response and stability were correlated
with a statistically significant benefit in terms of mPFS (13 and
12 vs. 6 months; P=0.002) and mOS (16 and 12 vs. 6 months; P=0.003;
Fig. 2A and B).
According to PSA, biochemical response (both ≥30%
and ≥50%) was associated with longer mPFS (both 13 vs. 12 months;
P=0.02 and P=0.03, respectively). Median OS was not computed
because all cases were censored (Fig. 2C
and D).
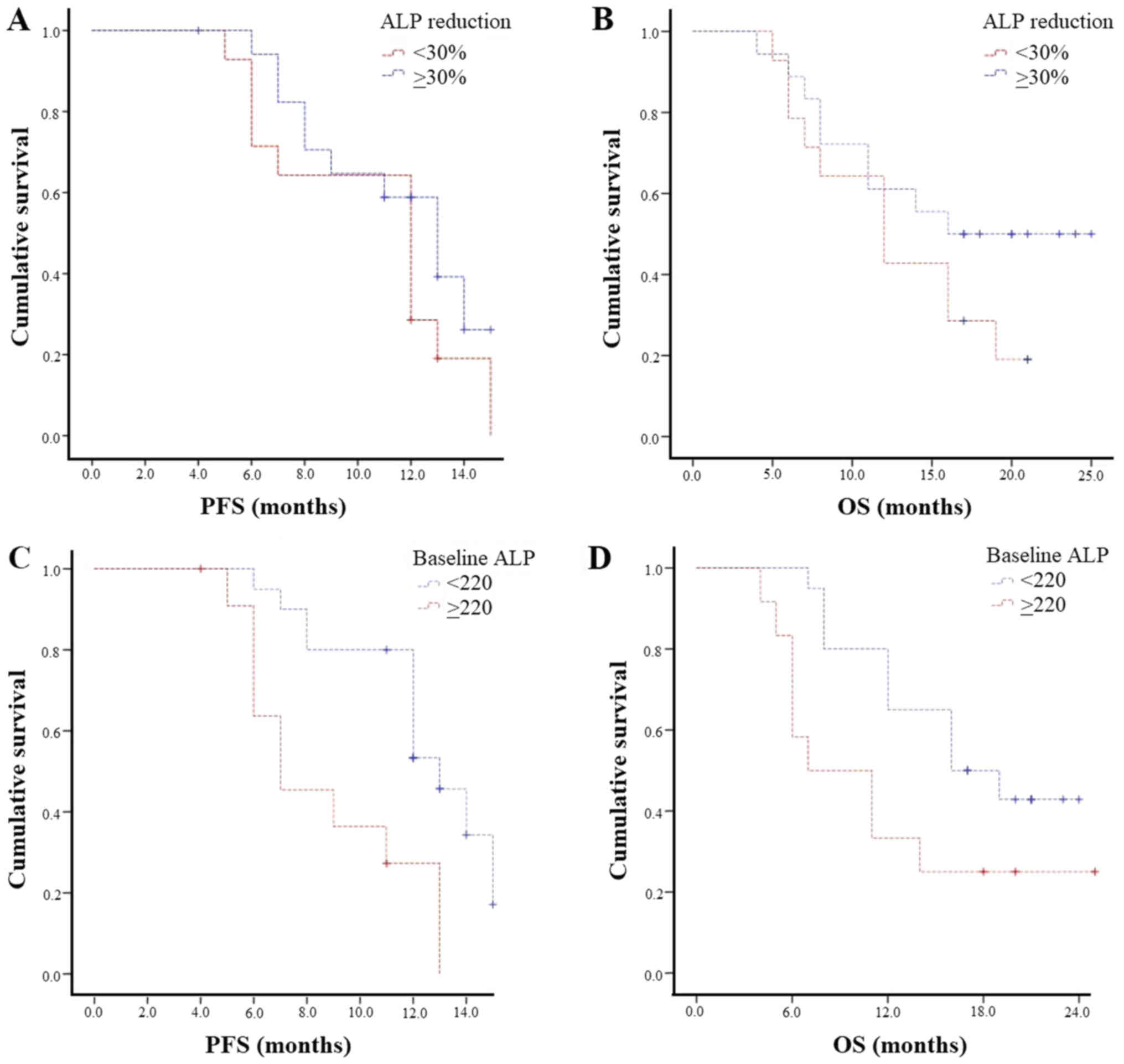
According to ALP, biochemical response (both ≥30 and
≥50%) was associated with longer mPFS (both 13 vs. 12 months; P=0.2
and P=0.7, respectively) and mOS (≥30%: 16 vs. 12 months; P=0.2 and
≥50%: 14 vs. 11 months; P=0.7; Fig. 3A
and B).
Pain response was associated with survival benefit
according to mPFS (13 vs. 12 months) and mOS (16 vs. 12 months)
without statistical significance (P=0.4 and P=0.9, respectively).
We also observed that pain stability was associated with similar
survival outcomes compared to pain response, both associated with
longer mPFS and mOS compared to pain worsening (mPFS: 13 vs. 12 vs.
9 months; P=0.6 and mOS: both 16 vs. 11 months; P=0.7).
Patients with baseline ALP <220 UI/l had a
statistically significantly longer mPFS (13 vs. 7 months, P=0.002)
and mOS (16 vs. 7 months, P=0.04) than those with baseline ALP ≥220
UI/l (Fig. 3C and D).
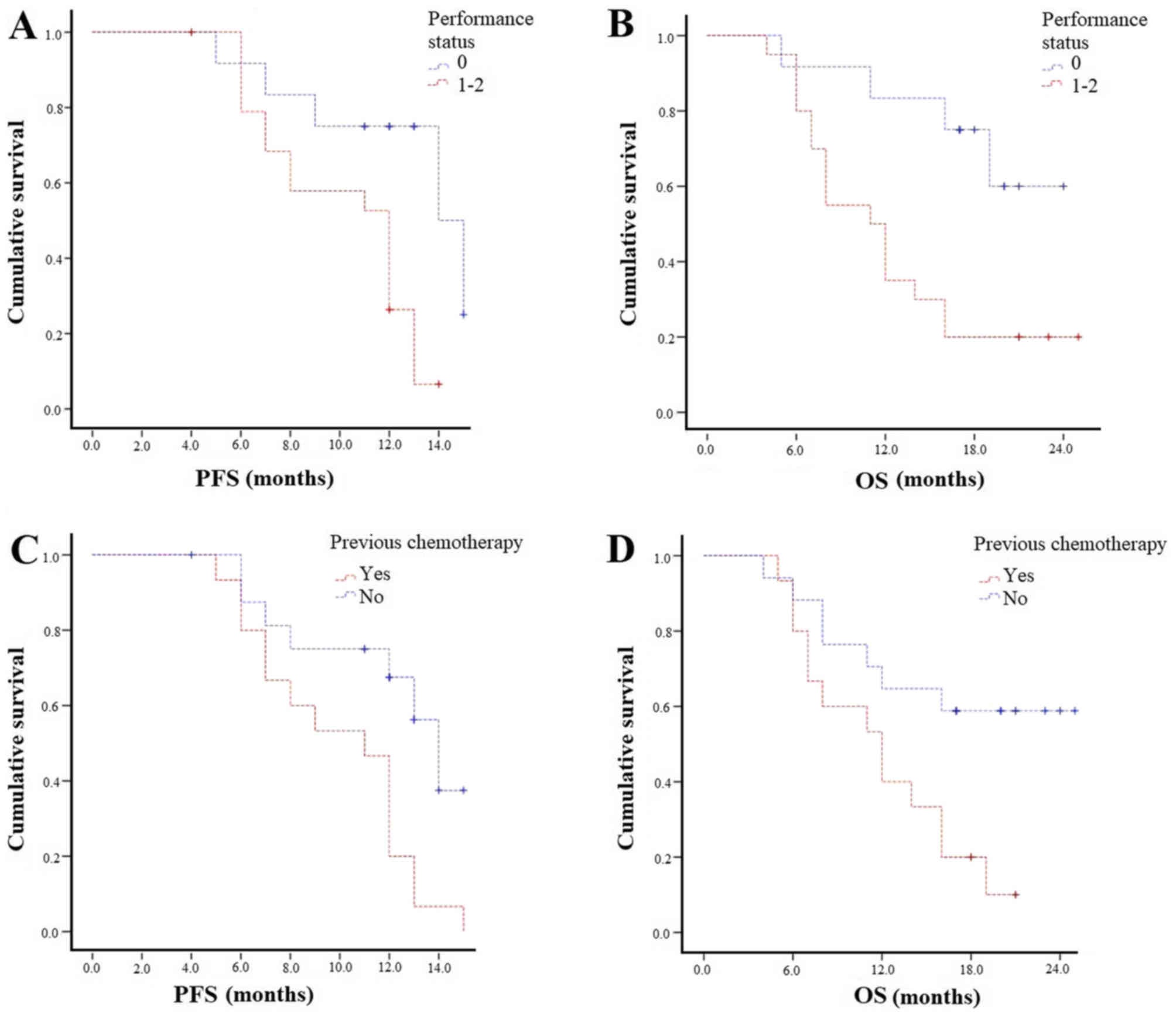
Patients with ECOG PS 0 experienced higher mPFS (14
vs. 12 months; P=0.01) and mOS (Not reached-11 months; P=0.009;
Fig. 4A and B). Those with both bone
and lymph node metastases had similar survival outcomes compared to
bone metastases patients. Patients not previously treated with
chemotherapy experienced statistically significantly longer PFS (14
vs. 11 months; P=0.007) and mOS (not reached 12 months; P=0.02;
Fig. 4C and D). The concomitant use
of bisphosphonates/denosumab did not affect survival outcomes in
terms of OS and PFS.
All the results of the subgroup analyses are
summarised in Table II.
 | Table II.Subgroup analysis. |
Table II.
Subgroup analysis.
| Variable | Patients n (%) | Median PFS,
months | P-value | Median OS,
months | P-value |
|---|
| ALP baseline
(UI/l) |
|
| 0.002 |
| 0.04 |
|
<220 | 20 (63) | 13 |
| 16 |
|
|
>220 | 12 (37) | 7 |
| 7 |
|
| ALP response |
|
| 0.2 |
| 0.2 |
|
≥30% | 18 (56) | 13 |
| 16 |
|
|
<30% | 14 (44) | 12 |
| 12 |
|
| ALP response |
|
| 0.7 |
| 0.7 |
|
≥50% | 8 (25) | 13 |
| 14 |
|
|
<50% | 24 (75) | 12 |
| 11 |
|
| PSA response |
|
| 0.02 | a | <0.0001 |
|
≥30% | 8 (25) | 13 |
|
|
|
|
<30% | 24 (75) | 12 |
|
|
|
| PSA response |
|
| 0.03 | a | 0.001 |
|
≥50% | 7 (22) | 13 |
|
|
|
|
<50% | 25 (78) | 12 |
|
|
|
| Scintigraphic
response |
|
| 0.002 |
| 0.003 |
| PR | 13 (41) | 13 |
| 16 |
|
| SD | 16 (50) | 12 |
| 12 |
|
| PD | 3 (9) | 6 |
| 6 |
|
| Pain
assessment |
|
| 0.6 |
| 0.7 |
|
Response | 16 (50) | 13 |
| 16 |
|
|
Stability | 7 (22) | 12 |
| 16 |
|
|
Worsening | 9 (28) | 9 |
| 11 |
|
| ECOG PS |
|
| 0.01 |
| 0.009 |
| 0 | 12 (37) | 14 |
| NR |
|
|
1–2 | 20 (63) | 12 |
| 11 |
|
| Previous
chemotherapy |
|
| 0.007 | NR | 0.02 |
| No | 17 (53) | 14 |
| 12 |
|
|
Yes | 15 (47) | 12 |
|
|
|
Toxicity evaluation
All 32 patients were evaluated for safety (Table III). Radium-223 was generally well
tolerated with grade 1–2 haematologic toxicities in only 9% of
patients. Grade 1–2 gastrointestinal toxicities were observed in
19% of patients and included hypertransaminasemia (3%), diarrhoea
(9%), constipation (3%) and nausea (3%); grade 1–2 creatinine
increased and oedema of limbs were both developed in 6% of
patients. Grade 3 toxicities were observed in 16% of patients and
included anaemia (13%) and thrombocytopenia (3%). No grade 4
adverse events were observed. None of the patients discontinued for
toxicity.
 | Table III.Toxicities of Radium-223 according to
the CTCAE (version 4.03). |
Table III.
Toxicities of Radium-223 according to
the CTCAE (version 4.03).
|
Chemotherapy-related toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Anaemia | 3 (9) | – | 4 (13) | – |
|
Thrombocytopenia | – | – | 1 (3) | – |
|
Hypertransaminasemia | 1 (3) | – | – | – |
| Creatinine
increased | 2 (6) | – | – | – |
| Diarrhoea | 1 (3) | 2 (6) | – | – |
| Constipation | 1 (3) | – | – | – |
| Nausea | 1 (3) | – | – | – |
| Oedema of
limbs | – | 2 (6) | – | – |
Discussion
Radium-223 was approved for the treatment of mCRPC
patients based on its OS benefit and safety profile demonstrated in
the registrational ALSYMPCA trial (6). In this phase 3 trial, Radium-223
significantly prolonged mOS of 3.6 months (14.9 vs. 11.3 months,
hazard ratio (HR)=0.70; P<0.001) compared to placebo (6). Both subgroups previously treated
(HR=0.70; P=0.002) and not previously treated with docetaxel
(HR=0.69; P=0.01) showed improved OS (7). Radium-223 prolonged median time to first
SSE compared to placebo (15.6 vs. 9.8 months, HR=0.66; P<0.001)
(6) regardless of prior docetaxel
therapy, baseline ALP level and current use of bisphosphonates
(8). It also significantly prolonged
median time to ALP increase (7.4 vs. 3.8 months; P<0.001) and a
significant number of patients experienced a total ALP response
(≥30% reduction from baseline) (47% vs. 3%; P<0.001) and an ALP
normalisation (34% vs. 1%; P<0.001) compared to placebo
(6). The PSA responses (≥30%
reduction from baseline) were less pronounced compared to ALP level
(16% vs 6%; P<0.001) (6). In this
way, ALP has emerged as the leading biomarker for Radium-223
treatment response (16). A low
incidence of grade 3–4 adverse events (56% vs. 62%) and of
study-drug discontinuation due to toxicity (16% vs. 21%) was shown,
especially in patients with a low extent of disease and no prior
docetaxel treatment (7,17).
Our study is a retrospective analysis of mCRPC
patients treated with Radium-223 in a clinical practice setting.
Our clinical experience is in line with the results of the ALSYMPCA
trial in terms of efficacy and safety. Compared to the ALSYMPCA
trial, our court of patients had similar characteristics, except
for a better performance status (ECOG PS 0: 38% vs. 27%), lower
previous chemotherapies (47% vs. 57%), lower severe pain (25% vs.
32%), lower median PSA (88 vs. 146 µg/l) and the absence of massive
bone metastases (>20 metastases: 0% vs. 32%). Most of our
patients (63%), in fact, were treated with Radium-223 in an early
setting as first- or second-line. For this reason, 91% of patients
completed all six cycles of treatment, a percentage higher than
that reported in the ALSYMPCA trial (6) and in other retrospective trials
(11,13,18–24)
(Table IV).
 | Table IV.Comparison of results between the
ALSYMPCA trial, other retrospective studies on Radium-223 and the
present study. |
Table IV.
Comparison of results between the
ALSYMPCA trial, other retrospective studies on Radium-223 and the
present study.
| Author | Type of study | No. pts. | Concomitant
abiraterone/enzalutamide | 6 cycles | ORR | DCR | ALP response | PSA response | NRS response | PFS months | OS months | tSRE months | SRE | Toxicities grade
3–4 | (Refs.) |
|---|
| ALSYMPCA trial | Phase III
placebo-controlled | 614 | No | 63% | – | – | 47% | 16% | 30% | – | 14.9 | 15.6 | 33% | 56% | (6–9) |
| Dan et
al | Retrospective | 11 | No | – | – | – | – | <22% | – | – | – | – | – | 18% | (11) |
| Modi et
al | Retrospective | 29 | Yes | 38% | 14% | 38% | <55% | 10% | – | – | – | – | – | – | (13) |
| Etchebehere et
al | Retrospective | 110 | Yes | 53% | – | – | – | – | – |
4.3 | 11.7 | 8.6 | 22% | – | (18) |
| Jadvar et
al | Retrospective | 25 | No | 24% | – | – | 44% | 20% | – | – | – | – | – | – | (19) |
| McKay et
al | Retrospective | 135 | Yes | 62% | – | – | – | 27% | – | – | 7.3 | 3 | 27% | 28% | (20) |
| Alva et
al | Retrospective | 145 | No | 51% | – | – | 48% | 16% | 51% | – | – | – | – | 13% | (21) |
| Küronya et
al | Retrospective | 41 | No | 78% | – | – | 22% | 0% | 81% | – | – | – | – | 15% | (22) |
| Wong et
al | Retrospective | 64 | Yes | 55% | – | – | 55% | 12% | – | – | 12.9 | 4.4 | – | – | (29) |
| De Luca et
al | Retrospective | 48 | No | 44% | – | – | 52% | – | 47% | – | – | – | – | – | (30) |
| Our study | Retrospective | 32 | No | 91% | 41% | 91% | 56% | 25% | 50% | 12 | 14 | 9.5 | 9% | 16% |
|
We observed a significant percentage of ALP response
(56% of patients) associated with survival benefit but without
statistical significance. However, baseline ALP <220 correlated
with longer survival outcomes in terms of mPFS and mOS with
statistical significance. These results on ALP are in line with
those of other trials (Table IV),
reflecting the potential role of ALP as a predictor of survival in
mCRPC patients treated with Radium-223 (2). On the other hand, we observed a lower
percentage of PSA response (25%), which is higher than that
reported in other trials (range 10–27%), associated with longer
mPFS with statistical significance. The primary aim of Radium-223
is to target bone metastases and therefore ALP, which is a
biomarker of osteoblast activity, should be preferred over PSA as a
biomarker of the efficacy of Radium-223 (2,4,25,26). In
fact, the increase of PSA may have resulted from the development of
lymph node or visceral metastases not influenced by Radium-223.
The best correlation observed in our study was
between scintigraphic response and survival outcomes: patients with
a scintigraphic response/stability had a longer PFS (P=0.002) and
OS (P=0.003). Bone scan response was not described in the ALSYMPCA
trial and in the majority of clinical trials on Radium-223
presented in the literature (Table
IV) although it is the main radiological exam of bone
metastases in clinical practice. According to the results observed
and the natural target of Radium-223, systematic bone scan
evaluations should be the main parameter, in association with ALP,
to evaluate the response to the radionuclide treatment.
According to bone pain, we observed pain response in
41% of patients and pain control in 75% of patients, which are
associated with better survival outcomes. Even though preliminary
studies (4) and post-hoc analyses
showed that Radium-223 treatment was associated with a better pain
relief (30% vs. 20%; P=0.01) (9), the
ALSYMPCA trial was not designed to be a pain-palliative treatment
(27).
PSA and pain relief, therefore, should not be used
as a surrogate measure of Radium-223 efficacy nor alter the
decision to administer all six radionuclide injections, the
recommended regimen for survival benefit (28).
Therefore, scintigraphic and biochemical responses
and baseline factors that reflected a less aggressive disease (such
as ALP <220, ECOG PS 0 and the absence of previous chemotherapy)
were associated with better survival with statistical
significance.
The mOS observed in our population was similar to
that reported in the ALSYMPCA trial, which was the primary
end-point of the study, confirming the benefit in survival of
Radium-223 also in clinical practice experiences. In the pivotal
trial and all the post-hoc analyses, OS was not supported by PFS
data due to the unknown direct anti-tumour effect of Radium-223,
which is reflected in the marginal effect on PSA. The choice of the
secondary end-points (ALP and tSRE) and their clinical relevance
were reflected by the pharmacodynamics of Radium-223, which is
focused on bone disease.
The safety results observed in our study were
satisfactory, with a lower percentage of grade 3–4 adverse events
(16%) compared to the ALSYMPCA trial and similar to other
retrospective analyses (Table
IV).
Some limitations are evident in this study because
it represents a relatively small and single-institution
retrospective analysis based on clinical experience. Given the
small sample size, many evaluations described did not reach
statistical significance. Despite these limitations, the
heterogeneity of our population provides a broad perspective on the
use of Radium-223 in real-life practice and provides interesting
observations on efficacy, survival outcomes and tolerability
outside prospective randomised trials.
Further analyses on the use of Radium-223 in
clinical practice settings in combination or sequence with other
agents (29,30) and on the identification of clinical
and biochemical predictors of Radium-223 benefit are needed. Having
established the efficacy of Radium-223 in metastatic CRPC, future
trials would focus on its earlier use in non-advanced and
castration sensitive metastatic prostate cancer as well as its
efficacy in patients with other primary bone-metastatic
cancers.
In conclusion, our retrospective study reported that
clinical and biochemical prognostic factors, both at baseline and
after treatment, correlated with better prognosis. It also showed
that Radium-223 is a valid treatment in terms of efficacy and
safety in mCRPC patients in the real-life clinical practice as well
as in the clinical trial setting.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AP and SER were the major contributors in writing
the manuscript, analysing and interpreting the patient data. FB,
CP, CF, VF and GAF were involved in the acquisition, analysis and
interpretation of patient data. GDV, ST and VB were involved in
analysis and interpretation of patient data, writing the manuscript
and revising it critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. Written informed consent was obtained from all
individual participants included in the study.
Patient consent to participate
Written informed consent was obtained from all
individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DePuy V, Anstrom KJ, Castel LD, Schulman
KA, Weinfurt KP and Saad F: Effects of skeletal morbidities on
longitudinal patient-reported outcomes and survival in patients
with metastatic prostate cancer. Support Care Cancer. 15:869–876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nilsson S, Franzén L, Parker C, Tyrrell C,
Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal
M, et al: Bone-targeted radium-223 in symptomatic,
hormone-refractory prostate cancer: A randomised, multicentre,
placebo-controlled phase II study. Lancet Oncol. 8:587–594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kerr C: (223)Ra targets skeletal
metastases and spares normal tissue. Lancet Oncol. 3:4532002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nilsson S, Strang P, Aksnes AK, Franzèn L,
Olivier P, Pecking A, Staffurth J, Vasanthan S, Andersson C and
Bruland ØS: A randomized, dose-response, multicenter phase II study
of radium-223 chloride for the palliation of painful bone
metastases in patients with castration-resistant prostate cancer.
Eur J Cancer. 48:678–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker CC, Pascoe S, Chodacki A,
O'Sullivan JM, Germá JR, O'Bryan-Tear CG, Haider T and Hoskin P: A
randomized, double-blind, dose-finding, multicenter, phase 2 study
of radium chloride (Ra 223) in patients with bone metastases and
castration-resistant prostate cancer. Eur Urol. 63:189–197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoskin P, Sartor O, O'Sullivan JM,
Johannessen DC, Helle SI, Logue J, Bottomley D, Nilsson S,
Vogelzang NJ, Fang F, et al: Efficacy and safety of radium-223
dichloride in patients with castration-resistant prostate cancer
and symptomatic bone metastases, with or without previous docetaxel
use: A prespecified subgroup analysis from the randomised,
double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 15:1397–1406.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sartor O, Coleman R, Nilsson S, Heinrich
D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue
J, et al: Effect of radium-223 dichloride on symptomatic skeletal
events in patients with castration-resistant prostate cancer and
bone metastases: Results from a phase 3, double-blind, randomised
trial. Lancet Oncol. 15:738–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nilsson S, Cislo P, Sartor O, Vogelzang
NJ, Coleman RE, O'Sullivan JM, Reuning-Scherer J, Shan M, Zhan L
and Parker C: Patient-reported quality-of-life analysis of
radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol.
27:868–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saad F, Carles J, Gillessen S, Heinrich D,
Gratt J, Miller K, Sten Nilsson S, O'Sullivan J, Tucci M and
Heidenreich A: Radium-223 in an international early access program
(EAP): Effects of concomitant medication on overall survival in
metastatic castration-resistant prostate cancer (mCRCP) patients
(Internet). J Clin Oncol. 33:50342015.
|
|
11
|
Dan TD, Eldredge-Hindy HB, Hoffman-Censits
J, Lin J, Kelly WK, Gomella LG, Lallas CD, Trabulsi EJ, Hurwitz MD,
Dicker AP and Den RB: Hematologic toxicity of concurrent
administration of radium-223 and next-generation antiandrogen
therapies. Am J Clin Oncol. 40:342–347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: Cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Modi D, Hwang C, Mamdani H, Kim S, Gayar
H, Vaishampayan U, Joyrich R and Heath EI: Radium-223 in heavily
pretreated metastatic castrate-resistant prostate cancer. Clin
Genitourin Cancer. 14:373–380.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
15
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE) v4.03. http://ctep.cancer.govJanuary 28–2017
|
|
16
|
Nguyen NC, Shah M, Appleman LJ, Parikh R
and Mountz JM: Radium-223 therapy for patients with metastatic
castrate-resistant prostate cancer: An update on literature with
case presentation. Int J Mol Imaging. 2016:25680312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogelzang NJ, Coleman RE, Michalski JM,
Nilsson S, O'Sullivan JM, Parker C, Widmark A, Thuresson M, Xu L,
Germino J and Sartor O: Hematologic safety of radium-223
dichloride: Baseline prognostic factors associated with
myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer.
15:42–52.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Etchebehere EC, Milton DR, Araujo JC,
Swanston NM, Macapinlac HA and Rohren EM: Factors affecting (223)Ra
therapy: Clinical experience after 532 cycles from a single
institution. Eur J Nucl Med Mol Imaging. 43:8–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jadvar H, Challa S, Quinn DI and Conti PS:
One-year postapproval clinical experience with radium-223
dichloride in patients with metastatic castrate-resistant prostate
cancer. Cancer Biother Radiopharm. 30:195–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKay RR, Jacobus S, Fiorillo M, Ledet EM,
Cotogna PM, Steinberger AE, Jacene HA, Sartor O and Taplin ME:
Radium-223 use in clinical practice and variables associated with
completion of therapy. Clin Genitourin Cancer. 15:e289–e298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alva A, Nordquist L, Daignault S, George
S, Ramos J, Albany C, Isharwal S, McDonald M, Campbell G,
Danchaivijitr P, et al: Clinical correlates of benefit from
radium-223 therapy in metastatic castration resistant prostate
cancer. Prostate. 77:479–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Küronya Z, Sinkovics I, Ágoston P, Bíró K,
Bodrogi I, Böde I, Dank M, Gyergyay F, Vajdics T, Kolonics Z, et
al: A retrospective analysis of the first 41 mCRPC patients with
bone pain treated with radium-223 at the national institute of
oncology in hungary. Pathol Oncol Res. 23:777–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha TL, Wu TT, Vogelzang NJ, Huang CY,
Huang SP, Lin C, Ou YC, Pang ST, Shen DH, Wu WJ and Chang WY:
Optimal usage of radium-223 in metastatic castration-resistant
prostate cancer. J Formos Med Assoc. 116:825–836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hague C and Logue JP: Clinical experience
with radium-223 in the treatment of patients with advanced
castrate-resistant prostate cancer and symptomatic bone metastases.
Ther Adv Urol. 8:175–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomblyn M, Nilsson S, Vogelzang N, Sartor
AO, Cislo P, Van Gool R, Aksnes AK and Parker C: 714 Pain and
quality of life (QoL) analyses from the phase 3 randomized ALSYMPCA
study with radium-223 dichloride (Ra-223) in castration-resistant
prostate cancer (CRPC) patients with bone metastases. J Urol.
189:e2932013. View Article : Google Scholar
|
|
26
|
Nilsson S: Radionuclide therapies in
prostate cancer: Integrating radium-223 in the treatment of
patients with metastatic castration-resistant prostate cancer. Curr
Oncol Rep. 18:142016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nevedomskaya E, Baumgart SJ and Haendler
B: Recent advances in prostate cancer treatment and drug discovery.
Int J Mol Sci. 19(pii): E13592018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saad F, Carles J, Gillessen S, Heidenreich
A, Heinrich D, Gratt J, Lévy J, Miller K, Nilsson S, Petrenciuc O,
et al: Radium-223 and concomitant therapies in patients with
metastatic castration-resistant prostate cancer: An international,
early access, open-label, single-arm phase 3b trial. Lancet Oncol.
17:1306–1316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong WW, Anderson EM, Mohammadi H, Daniels
TB, Schild SE, Keole SR, Choo CR, Tzou KS, Bryce AH, Ho TH, et al:
Factors associated with survival following radium-223 treatment for
metastatic castration-resistant prostate cancer. Clin Genitourin
Cancer. 15:e969–e975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Luca R, Costa RP, Tripoli V, Murabito A
and Cicero G: The clinical efficacy of radium-223 for bone
metastasis in patients with castration-resistant prostate cancer:
An italian clinical experience. Oncology. 94:161–166. 2018.
View Article : Google Scholar : PubMed/NCBI
|