Introduction
Fat mass and obesity-associated (FTO) protein was
first identified as a protein associated with obesity (1,2). Its
overexpression is associated with obesity occurrence, whereas its
underlying mechanisms remain unclear. As a member of AlkB-like
DNA/RNA demethylase family, FTO contains a universal amino-terminal
AlkB-like domain and a carboxy-terminal domain (3,4). In
contrast to other family members, an extra loop in FTO, which
competes with the unmethylated strand of the DNA duplex for binding
to FTO, engages higher specificity to single-stranded RNA or DNA
substrates (4). Importantly, a
catalytic activity of FTO towards RNA has been previously
identified (5). FTO contains a Fe(II)
binding domain and substrate recognition domain, which catalyzes
the removal of the methyl group on N6-adenosine in mRNA
(5). As a prevalent internal
modification of mRNA, N6-adenosine methylation
(m6A) serves important roles in mRNA metabolism,
including localization and stability. Oxidative demethylation by
FTO represses the recognition of mRNA by YTH domain family protein
for mRNA decay (6). The
aforementioned observations indicate an important uncharacterized
regulatory mechanism for gene expression.
By using a genetically engineered mouse model, the
physiological functions of FTO have been extensively studied.
Germline loss of FTO leads to perinatal lethality, growth
retardation and reduced body weight, indicating that FTO is
required for mammalian development (2,7). However,
the underlying roles of FTO in other pathological processes,
including the occurrence and development of cancer, remain
unclear.
Treatment of pancreatic cancer remains inefficient,
due to its high resistance to traditional therapeutic strategies,
including chemotherapy, radiotherapy and surgical resection
(8–10). Therefore, future studies to provide
additional insight for the molecular and cellular mechanisms for
the development of novel treatment strategies are required.
In the present study, the function of FTO in
pancreatic cancer cell homeostasis was characterized to establish
the association between them. Whether FTO participates in
pancreatic cancer cell homeostasis through regulation of
m6A modification was also studied. It was revealed that
FTO was highly expressed in pancreatic cancer cells, and FTO
knockdown resulted in compromised pancreatic cell proliferation and
reduced DNA synthesis. Accordingly, mRNA methylation was
accumulated. In conclusion, a previously unknown function of FTO in
pancreatic cancer was uncovered, shedding light on a novel
molecular target for pancreatic cancer treatment.
Materials and methods
Tissues sample collection
Fresh frozen tumor and normal tissue samples were
obtained from surgical specimens of 2 patients at Jiangyin People's
Hospital (Wuxi, China) between July 2014 and May 2015. One patient
was a 52 year-old male and the other was a 43 year-old female. The
present study had been reviewed and approved by the Institutional
Review Board of Jiangyin People's Hospital, School of Medicine
(Southeast Medical University College, Wuxi, China). Informed
written consent was obtained from all patients, in accordance to
the Declaration of Helsinki and its amendments.
Cell culture and transfection
Human pancreatic ductal cell (HPDE) and pancreatic
cancer cell lines SW1990, PANC-1 and BXPC-3 were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) at 37°C in a humidified atmosphere containing 5%
CO2. For empty pcDNA 3.1 plasmid (cat. no. V79020;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) or
c-Myc-overexpressing pcDNA 3.1 plasmid (cat. no. V79020; Thermo
Fisher Scientific, Inc.) transfection, 2×105 PANC-1
cells were seeded into 6-well plates overnight prior to
transfection. A total of 2.5 µg expression plasmids or empty
vectors were transfected into cells using Lipofectamine™ 2000 (cat.
no. 11668027; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After 48 h, the transfected cells were
harvested for further analysis.
RNA interference
FTO RNA interference (RNAi)
(5′-GCACAAGCATGGCTGCTTA-3′), scramble control
(5′-GCAACGACGGTCGTACTTA-3′) and pSilencer 3.1-H1 neo plasmid were
purchased from Thermo Fisher Scientific, Inc. PANC1, SW1990 and
BXPC-3 cells (~5×105) were seeded in 35-mm plates and
cultured overnight at 37°C. Cells were subsequently transfected
with the mixture of RNAi (50 nM) and Lipofectamine® 2000
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacture's protocol.
MTT assay
Following FTO RNAi transfection for 12 h, PANC1,
SW1990 and BXPC-3 cells were plated into 96-well plates for further
culture at 37°C and samples were taken at day 2, 4 and 8. A total
of 20 µl of MTT solution (5 mg/ml), dissolved in DMEM, was
subsequently added into the cells and incubated for 2 h at 37°C.
Precipitates were subsequently dissolved by dimethylsulfoxide for
20 min at room temperature. Absorbance was recorded at a wavelength
of 540 nm using a microplate reader elx 800 (BioTek Instruments,
Inc., Winooski, VT, USA).
Bromodeoxyuridine (BrdU) assay
For BrdU incorporation assay, PANC-1, SW1990 and
BXPC-3 cells were plated in 8-well chamber slides at density of
3×104/ml. Following 48 h of transfection, cells were
incubated with 10 µM BrdU for an additional 2 h. Cells were then
fixed with 2% formaldehyde for 30 min at room temperature and
permeabilized with 0.2% Triton X-100, followed by incubation with
anti-BrdU primary antibody (1:1,000; cat. no. ab152095; Abcam,
Cambridge, UK) overnight at 4°C. Cells were subsequently incubated
with PE-Cy7-labeled mouse anti-rabbit secondary antibody (1:400;
cat. no. sc-516721; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) for 1 h at room temperature. Images were acquired using a
fluorescent microscope (magnification, ×400; Leica Microsystems,
Inc., Buffalo Grove, IL, USA) and analyzed with ImageJ software
(v1.8.0; National Institutes of Health, Bethesda, Maryland,
USA).
TUNEL assay
For TUNEL assay, cells at a density of
3×104/ml were fixed with 2% formaldehyde for 30 min at
room temperature and the TUNEL assay was performed according to the
manufacturer's protocol (Click-iT® TUNEL Alexa
Fluor® 488 Imaging assay; cat. no., C10245; Thermo
Fisher Scientific, Inc.). Ten fields of view at ×400 magnification
were observed using a fluorescent microscope (Leica Microsystems,
Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RNA from PANC-1, SW1990 and BXPC-3 cells was
isolated by TRIzol (Thermo Fisher Scientific, Inc.) and
subsequently used for cDNA synthesis with High-Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. RT-qPCR was performed
using StepOne/StepOnePlus™ Real-time PCR System with SYBR Green PCR
Master mix (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The sequence of the FTO primers used in
RT-qPCR were the following: Forward, 5′-ACTTGGCTCCCTTATCTGACC-3′
and reverse, 5′-TGTGCAGTGTGAGAAAGGCTT-3′. An optimal reaction was
obtained with the following thermocycling conditions: Initial
denaturation at 95°C for 10 min; 45 cycles of denaturation at 95°C
for 15 sec, annealing at 60°C for 1 min and elongation at 72°C for
1 min; and a final extension at 72°C for 10 min. β-actin mRNA was
used as a control and the level of FTO mRNA expression was
normalized to β-actin mRNA using the 2−ΔΔCq method
(11). The sequence of the β-actin
primers were as follows: Forward, 5′-CTCCATCATGAAGTGTGACGTT-3′ and
reverse, 5′-ATCTCCTTCTGCATCCTGTCAG-3′. For amanitin treatment, a
final concentration of 2.5 mg/l amanitin (cat. no., A2263;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to the
culture medium and incubated at 37°C for 30, 60 and 120 min.
RNA immunoprecipitation
A total of two 10-cm plates of PANC-1 cells
(selected as representative cells) were crosslinked by 1%
paraformaldehyde for 10 min at room temperature. Cells at a density
of ~2×107/ml were subsequently neutralized with 2 mg/ml
glycine and lyzed in cell lysis buffer (150 mM KCl, 10 mM HEPES pH
7.6, 2 mM EDTA, 0.5% NP-40, 0.5 mM DTT, RNase inhibitor) on ice for
30 min. Cell lysate were subsequently sonicated (20 KHz; 5s
sonication, 5s pause, 12 cycles) at 4°C and precleaned by
Dynabeads® Protein G (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. FTO antibody (1:50; cat.
no. ab92821; Abcam) and control IgG (1:50; catalog no. ab188776;
Abcam) were incubated with the sonicated lysates overnight at 4°C.
Dynabeads® Protein G was subsequently incubated with the
lysate for an additional 2 h at 4°C. Immunoprecipitated complex
were subsequently washed with standard Chromatin
immunoprecipitation (cat. no., 17-295, Merck KGaA, Darmstadt,
Germany), according to the manufacturer's protocols. TRIzol was
subsequently added into the supernatant to extract
immunoprecipitated RNA.
Western blot analysis
Cells were harvested in lysis buffer (100 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40 and protein inhibitor
cocktail) and centrifuged at 1,000 × g for 3 min at 4°C to collect
the supernatants. Samples were separated with 12%
Mini-PROTEAN® TGX™ Precast Gels (cat. no., 4561093;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and 50 µg total
protein determined with BCA assay (cat. no. 23235; Thermo Fisher
Scientific, Inc.) was loaded into each lane. The gel was
subsequently transferred to Immobilon®-P polyvinylidene
difluoride Membrane (cat. no., IPVH00010; EMD Millipore, Billerica,
MA, USA). The membrane was subsequently blocked with 5%
Blotting-Grade Blocker (cat. no., 1706404; Bio-Rad Laboratories,
Inc.) dissolved in Tris-buffered saline containing Tween-20 (PBST)
at room temperature for 2 h. The blots were incubated with
antibodies against FTO (cat. no., sc-271713; Santa Cruz
Biotechnology, Inc.), GAPDH (cat. no., sc-47724; Santa Cruz
Biotechnology, Inc.) or anti-FLAG-M2 (cat. no., F1804;
Sigma-Aldrich; Merck KGaA) diluted at 1:1,000 with blocking buffer
overnight at 4°C, followed by washing with PBST and horseradish
peroxidase (HRP)-conjugated anti-mouse secondary antibody (cat.
no., sc-2005; Santa Cruz Biotechnology, Inc.) diluted at 1:5,000
with blocking buffer for 30 min at room temperature. Following
washing with PBST, protein bands were visualized by enhanced
chemiluminescence (cat. no., 7003; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and analyzed with ImageJ software (v1.8.0;
National Institutes of Health).
Dot blot
mRNA was isolated with Magnetic mRNA Isolation kit
(New England BioLabs, Inc., Ipswich, MA, USA), according to the
manufacturer's protocols. 200 ng total mRNA was spotted onto a
nitrocellulose membrane (Thermo Fisher Scientific, Inc.), which was
subsequently crosslinked by ultraviolet exposure. The membrane was
subsequently incubated with 5% non-fat milk and blocked for 30 min
at room temperature. Anti-m6A antibody (cat. no. 202
111; 1:1,000; Synaptic Systems, Goettingen, Germany) was incubated
with the membrane overnight at 4°C. HRP-conjugated rabbit secondary
antibody (cat. no. sc-2357; 1:10,000; Santa Cruz Biotechnology) was
incubated with the membrane at room temperature for 2 h.
Subsequently, the membrane was used for further chemiluminescence
analysis.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Comparisons
between 2 groups were analyzed using an unpaired Student's t-test.
Data are presented as the mean ± standard error of mean.
Comparisons between 3 or more were analyzed by one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference
Results
Overexpression of FTO in pancreatic
cancer cells
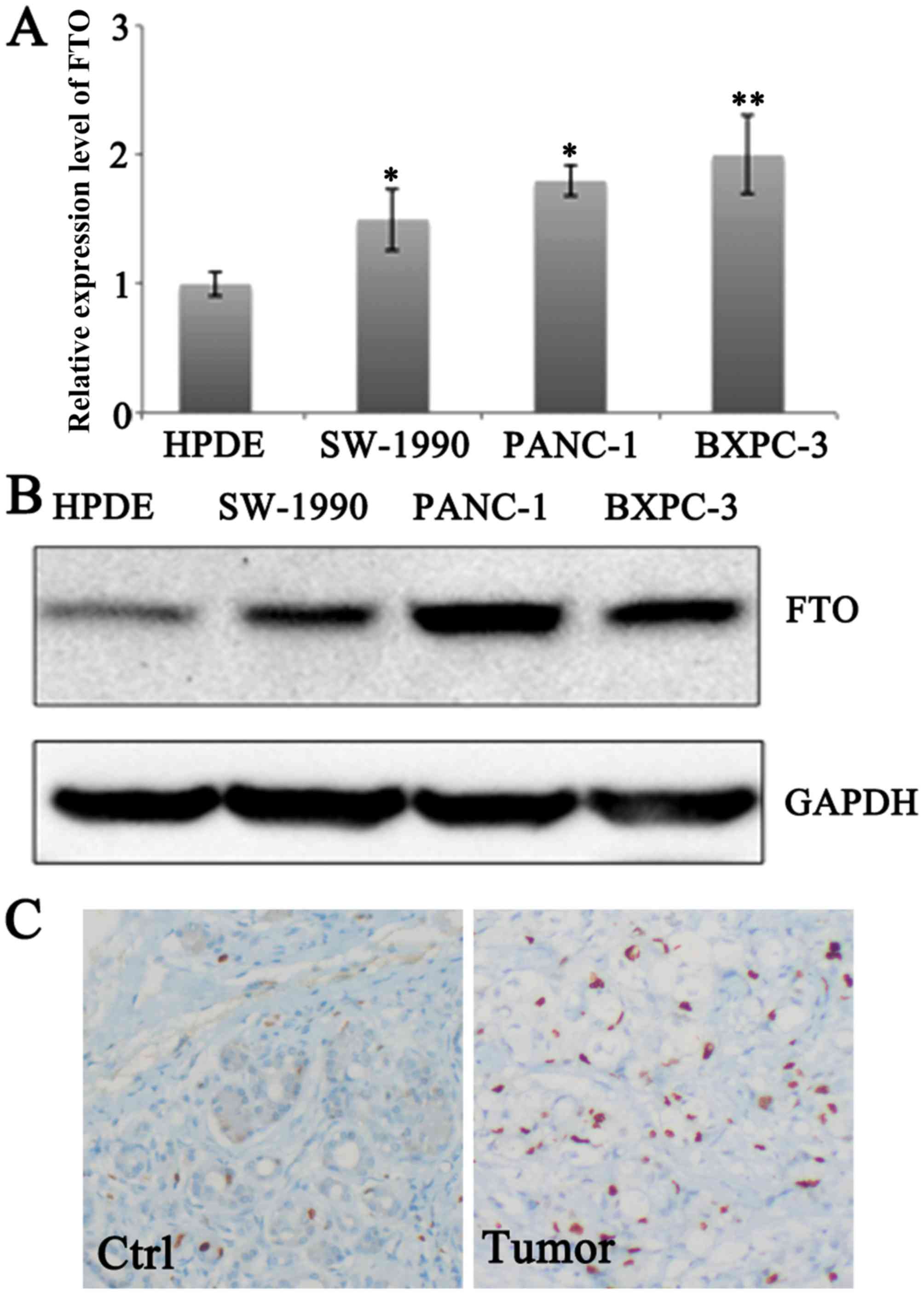
To determine the expression profile of FTO in
pancreatic cancer cells, the expression levels in normal pancreatic
epithelial cells HPDE and pancreatic cancer cell lines SW1990,
PANC-1 and BXPC-3 were examined. Higher mRNA expression levels of
FTO were observed in SW1990, PANC-1 and BXPC-3 cells compared with
expression level of HPDE (Fig. 1A).
Accordingly, the protein expression level of FTO was upregulated in
SW1990, PANC-1 and BXPC-3 compared with that in HPDE cells
(Fig. 1B). Increased FTO expression
was also observed in pancreatic tumors (Fig. 1C).
FTO knockdown compromises pancreatic
cancer cell proliferation
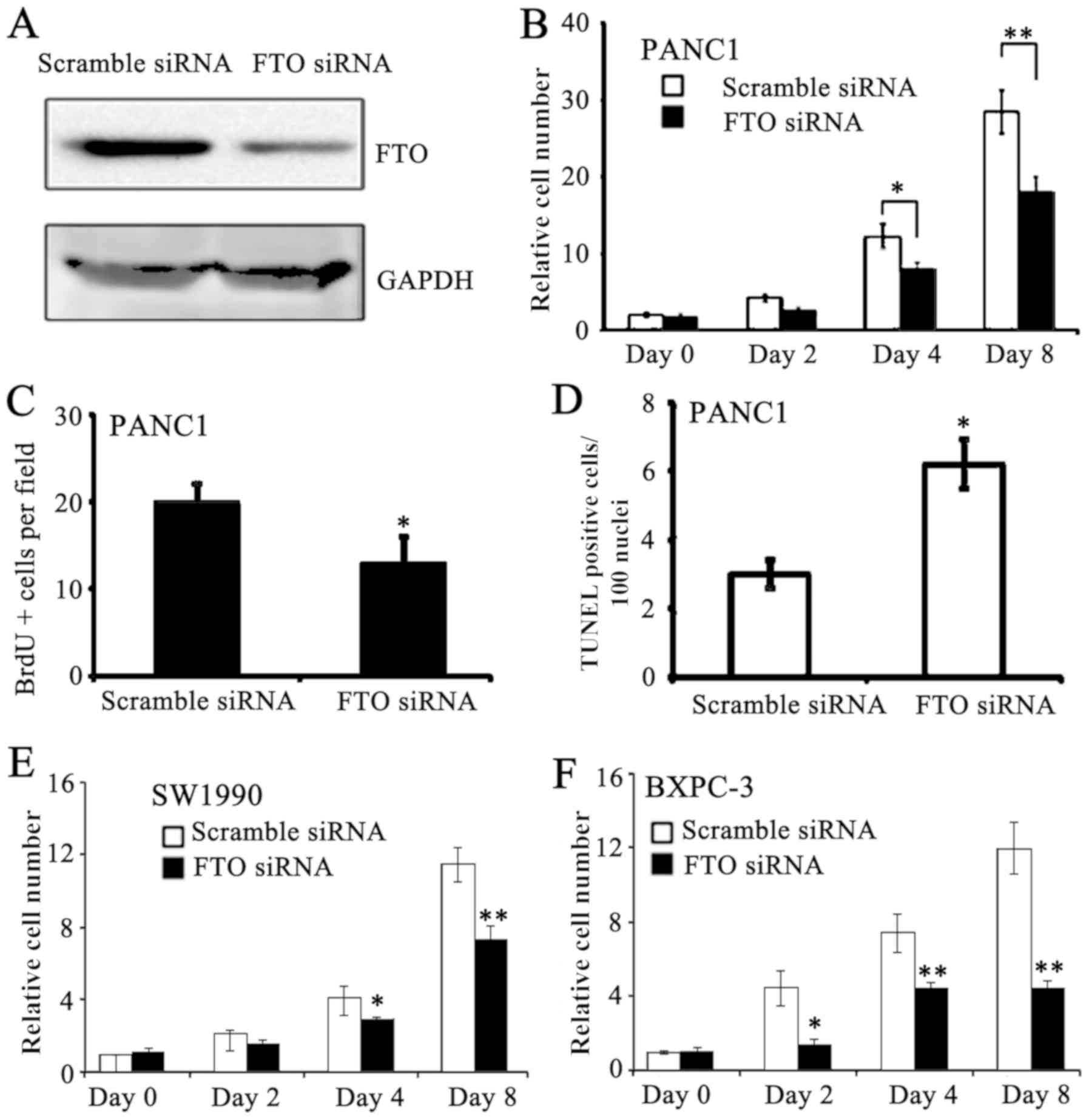
To examine the roles of FTO in pancreatic cancer
cells, RNAi was used to knockdown the expression levels of FTO in
PANC-1 cells. FTO protein expression was successfully knockdown in
PANC-1 cell lines (Fig. 2A). FTO
siRNA-treated PANC-1 cells exhibited reduced proliferation compared
with scramble siRNA-treated PANC-1 cells at days 4 and 8 (Fig. 2B). Accordingly, DNA synthesis was
reduced in FTO siRNA-treated PANC-1 cells (Fig. 2C). Furthermore, apoptosis was
increased in FTO knockdown PANC-1 cells (Fig. 2D). To verify the results from the
PANC-1 cells, proliferation in SW1990 and BXPC-3 in response to
treatment using FTO siRNA was examined. FTO knockdown lead to
similar cytotoxic effects on the proliferation of SW1990 and BXPC-3
cells for 8 days (Fig. 2E and F).
These data indicate that FTO may regulate proliferation in
pancreatic cancer cells by modulating cell cycle progression.
FTO regulates mRNA m6A
levels
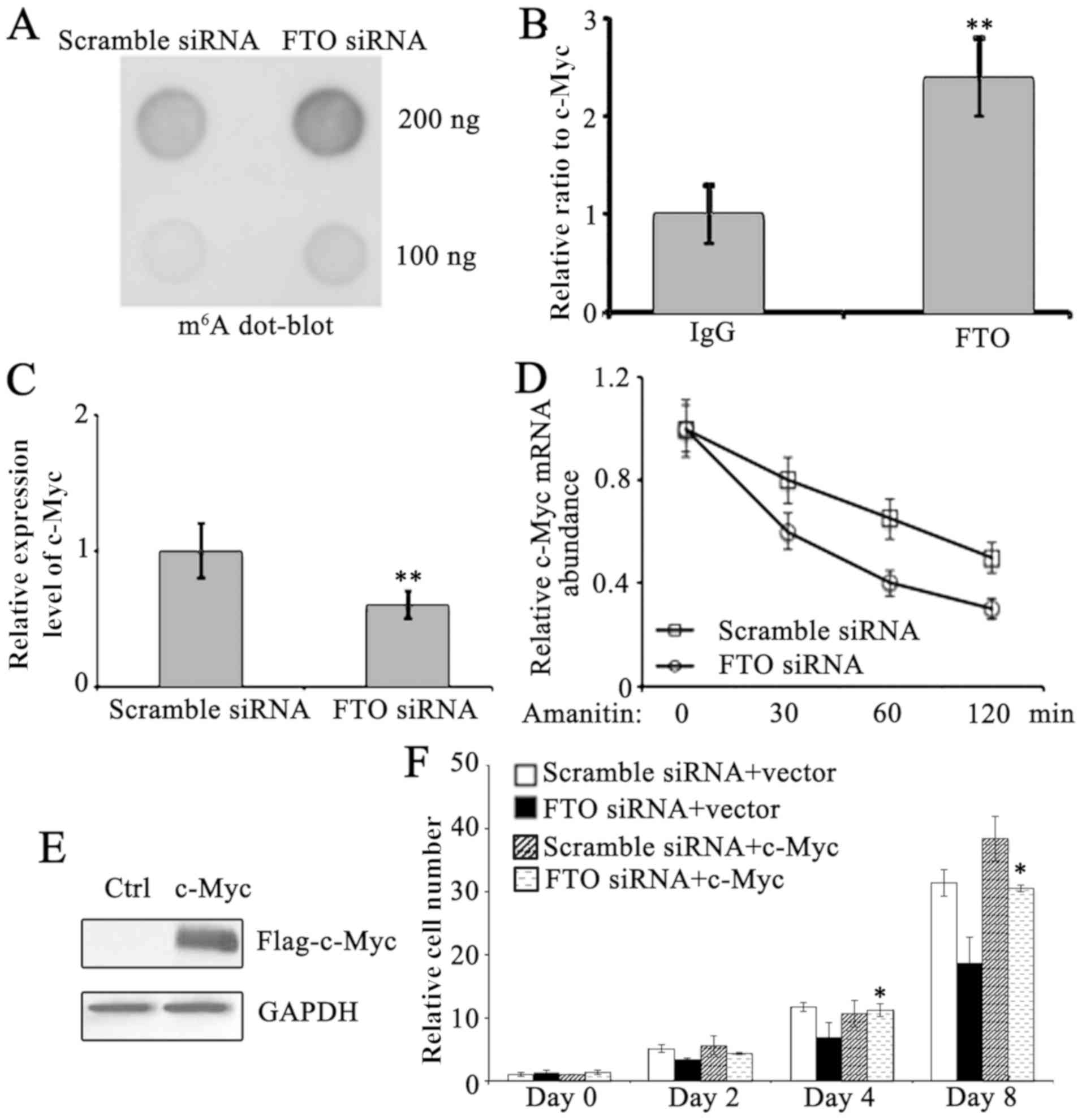
To examine the underlying mechanisms of FTO in the
proliferation and cell cycle of pancreatic cancer cells, the
m6A levels in PANC-1 cells were determined. FTO
knockdown resulted in increased m6A expression levels
compared with scramble siRNA-treated PANC-1 cells (Fig. 3A), indicating FTO serves important
roles in the regulation of m6A expression levels in
PANC-1 cells. Since c-Myc is a critical mediator in regulating cell
entry into S phase of cell cycle (12), further investigation on whether FTO
may interact with c-Myc expression and regulate its metabolism in
PANC-1 cells was carried out. By using RNA immunoprecipitation,
which enriches FTO-interacting RNA, with FTO antibody in PANC-1
cells, it was observed that FTO interacts with c-Myc expression
(Fig. 3B). c-Myc expression was
reduced following FTO knockdown (Fig.
3C). To test the stability of c-Myc expression, amanitin, a
specific inhibitor of RNA polymerase II, was used to inhibit mRNA
de novo synthesis in PANC-1 cells. The degradation of
existent c-Myc expression was subsequently monitored. As expected,
enhanced loss of c-Myc expression was detected in FTO knockdown
PANC-1 cells, indicating its stability was compromised in FTO
deficient PANC-1 cells (Fig. 3D).
These data strongly suggest that c-Myc is a primary target for
FTO-mediated cell cycle regulation in pancreatic cancer cells. To
verify this, c-Myc was overexpressed in FTO knockdown PANC-1 cells
(Fig. 3E). To verify its significance
in FTO-regulated proliferation, c-Myc was overexpressed in FTO
knockdown cells and it was indicated that c-Myc significantly
restored proliferation (Fig. 3F).
Therefore, c-Myc is suggested to serve a major role in FTO-mediated
proliferation.
Discussion
mRNA carries genetic information between the DNA and
the protein, and the underlying mechanisms for mRNA generation and
decay have been extensively studied. Similar to DNA, mRNA can also
be modified (13–15). One of its most documented internal
modifications is in its m6A sites (16,17). Until
recently, the functions of this modification remained unclear.
m6A has been reported to decrease mRNA stability and
lead to its degradation. Once m6A is demethylated, mRNA
is stabilized and relocated into the cytosol for protein
translation (6). These observations
indicate a novel mechanism for regulation of gene expression.
However, its physiological and pathological significance remain
unclear. In the present study, the existence of the aforementioned
mechanisms in pancreatic cancer cells was examined. It was
indicated that FTO, a primary demethylase in vivo, was
overexpressed in pancreatic cancer cells compared with normal
pancreatic epithelial cells, implying that it is involved in
pancreatic cancer development and progression. The knockdown of FTO
resulted in a compromised proliferation in pancreatic cancer cells,
as well as an increased in apoptosis. These observations
demonstrated FTO is required for the proliferation of pancreatic
cancer cells. In addition, regulating m6A modification
in mRNA may affect pancreatic cancer homeostasis.
The regulation of mRNA stability is critical for
gene expression in cancer cells (18). In the past decade, a central role of
microRNA in mRNA decay has been established (19,20). In
the present study, it was revealed that m6A
demethylation is an important missing mechanism for the regulation
of mRNA stability in pancreatic cancer cells. In addition, it was
demonstrated that the stability of c-Myc mRNA was compromised upon
FTO knockdown, which subsequently repressed DNA synthesis. These
data indicated an endogenous target for FTO to regulate pancreatic
cancer cell proliferation.
A number of limitations existed in the present
study. Due to a lack of knowledge of the accurate m6A
sites on c-Myc mRNA, an in vitro demethylation assay,
combining FTO recombinant protein and methylated c-Myc, was not
possible in the present study. Therefore, further investigation is
required, as it would assist to identify the direct effects of FTO
on c-Myc. In addition, c-Myc was only determined as a primary
target of FTO, while other cell cycle-associated proteins have yet
to be identified. Further studies will focus on other genes
regulated by FTO in pancreatic cancer cells. Given the roles of FTO
in energy metabolism, it is possible that metabolism-associated
mRNAs were controlled by FTO for pancreatic cancer homeostasis.
Therefore, further investigation is required.
In conclusion, it was indicated that mRNA stability
regulated by FTO serves important roles in pancreatic cancer cells,
suggesting an underlying mechanism for the development and
progression of pancreatic cancer. The data of the present study
suggest a novel possible therapeutic target for the treatment of
pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and XT designed the experiment. XT and SL
performed the experiments with cancer cell lines. DC and ZZ
performed histological experiments with tissue samples. All authors
contributed in the writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study had been reviewed and approved by the
Institutional Review Board of Jiangyin People's Hospital, School of
Medicine, Southeast University (Wuxi, China). Informed written
consent was obtained from all patients, in accordance to the
Declaration of Helsinki and its amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frayling TM, Timpson NJ, Weedon MN,
Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H,
Rayner NW, et al: A common variant in the FTO gene is associated
with body mass index and predisposes to childhood and adult
obesity. Science. 316:889–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fischer J, Koch L, Emmerling C, Vierkotten
J, Peters T, Brüning JC and Rüther U: Inactivation of the Fto gene
protects from obesity. Nature. 458:894–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerken T, Girard CA, Tung YC, Webby CJ,
Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill
LA, et al: The obesity-associated FTO gene encodes a
2-oxoglutarate-dependent nucleic acid demethylase. Science.
318:1469–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han Z, Niu T, Chang J, Lei X, Zhao M, Wang
Q, Cheng W, Wang J, Feng Y and Chai J: Crystal structure of the FTO
protein reveals basis for its substrate specificity. Nature.
464:1205–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N6-methyladenosine-dependent regulation of messenger RNA stability.
Nature. 505:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao X, Shin YH, Li M, Wang F, Tong Q and
Zhang P: The fat mass and obesity associated gene FTO functions in
the brain to regulate postnatal growth in mice. PLoS One.
5:e140052010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayraktar S and Rocha-Lima CM: Advanced or
metastatic pancreatic cancer: Molecular targeted therapies. Mt
Sinai J Med. 77:606–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loos M, Kleeff J, Friess H and Buchler MW:
Surgical treatment of pancreatic cancer. Ann NY Acad Sci.
1138:169–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Dominissini D, Rechavi G and He C:
Gene expression regulation mediated through reversible
m6A RNA methylation. Nat Rev Genet. 15:293–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He C: Grand challenge commentary: RNA
epigenetics? Nat Chem Biol. 6:863–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei CM, Gershowitz A and Moss B:
Methylated nucleotides block 5′terminus of HeLa cell messenger RNA.
Cell. 4:379–386. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dominissini D, Moshitch-Moshkovitz S,
Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K,
Jacob-Hirsch J, Amariglio N, Kupiec M, et al: Topology of the human
and mouse m6A RNA methylomes revealed by m6A-seq. Nature.
485:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meyer KD, Saletore Y, Zumbo P, Elemento O,
Mason CE and Jaffrey SR: Comprehensive analysis of mRNA methylation
reveals enrichment in 3′UTRs and near stop codons. Cell.
149:1635–1646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stahlhut Espinosa CE and Slack FJ: The
role of microRNAs in cancer. Yale J Biol Med. 79:131–140.
2006.PubMed/NCBI
|