Introduction
The efficacy of traditional chemoradiotherapies
remains limited for pancreatic cancer (1–3), which is
expected to be the second most lethal malignancy in the USA by 2020
(4,5).
Distant tumour metastases, frequently in the liver or peritoneum
and rarely in the bone, indicate a poor prognosis (6,7). Thus,
effective targeted therapies are urgently warranted. Therefore,
individualised drug screening is urgently required for the clinical
treatment of pancreatic cancer patients, particularly those in
advanced or metastatic disease stages (8–11).
Accumulating evidence indicates that patient-derived
xenografts (PDXs) are reliable cancer research tools for
personalised drug screening, and they have been increasingly used
in various types of translational cancer research in recent years
(12). These so-called Avatar models
mimic the morphological and molecular characteristics of the tumour
and predict clinical treatment response (13), as they are formed when tissue from a
patient's tumour is grafted onto a mouse or other animal (14).
Adequate understanding of the genomic landscape of
pancreatic cancer can be beneficial for drug screening (10,15–17), and
the precise molecular profile of the tumour assists in predicting
drug responses (18). Next-generation
sequencing (NGS) is a powerful tool to investigate the genomic
landscape of patient tumours and the mechanism of drug response,
which may provide a broader vision for potential clinical drug
screening (19–21). Therefore, NGS technologies are being
used by pharmaceutical companies throughout the drug discovery
process (22).
In our previous study, a PDX model was established
from pancreatic cancer bone metastasis tumour tissue, and a
416-gene exon panel was sequenced to investigate the molecular
characteristics of the tumour (23).
In the present study, the NGS high-throughput information was
further analysed to search for individualised therapy targets for
pancreatic cancer patients with bone metastasis. Based on the
sequencing results and associated literature, AZD4547, a potent
inhibitor of fibroblast growth factor receptor (FGFR), was selected
for evaluation in the pancreatic cancer PDX model and was examined
as a potential therapy (24).
Materials and methods
Reagents and drugs
AZD4547 (cat. no. S2801) and capecitabine (cat. no.
S1156) were purchased from Selleck Chemicals (Shanghai, China). The
antibodies against FGFR1 (cat. no. ab63601), phosphorylated protein
kinase B (p-Akt; phospho S473, cat. no. ab227748) and Ki-67
(OTI5D7; cat. no. ab156956) were purchased from Abcam (Shanghai,
China).
Establishment of a PDX model and
NGS
Pancreatic cancer bone metastasis (diagnosed as
adenocarcinoma) tissues were used to establish a PDX model
subsequent to being obtained at surgery from a 67-year-old female
patient. Written consent was provided by the patient and ethical
approval was obtained from the Ethics Committee of The First
Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China). In total, 50 (range, 4–6 weeks) female BALB/c
nude mice (12–16 g) were purchased from Shanghai Laboratory Animal
Center (Shanghai, China) for establishing PDX models. The mice were
kept at 26–28°C, with 40–60% humidity at a 10 h/14 h light/dark
cycle. Mice were kept in a SPF environment. Tumour tissues were
harvested from PDX models for NGS investigation in a 416-gene exon
panel, as conducted by Geneseeq Technology, Inc (Nanjing, China).
The protocol for establishment of the PDX model and NGS was as
previously described (23).
Treatment protocol
From the 3rd mouse generation, PDX tumours were
permitted to grow to a volume of 150–200 mm3, and then
mice were randomised (6 mice with tumors were set per group and
housed in per rearing cage). AZD4547 and capecitabine were
administered daily for 4 weeks at the following doses: 50 mg/kg
oral AZD4547, 1.0 mM/kg oral capecitabine (1 ml saline administered
orally for control group). Mice were weighed for signs of toxicity
and tumour size was evaluated once per week. Animals were monitored
periodically for their weight with an electronic balance and tumour
growth was measured with a Vernier caliper once per week. Tumour
volume was calculated according to the formula V=LD ×
(SD)2/2, where V represents the tumour volume, and LD
and SD are the longest and shortest tumour diameters, respectively.
Relative tumour growth inhibition (TGI) (%) was calculated using
the formula (1-T/C), where T represents the relative tumour volume
of the treated mice and C represents the relative tumour volume of
the control mice. Euthanasia was conducted on the mice prior to the
single tumour volume reaching 1,500 mm3. The usage of
experimental animals was according to the Principles of Laboratory
Animal Care (NIH no. 85-23, 1985 version). All animal studies were
according to the Institutional Animal Care and Use Committee of
Zhejiang University and the approval ID was SYXK(ZHE)2005-0072.
Fluorescence immunohistochemistry
Mice with similar tumour sizes were anaesthetised
with chloral hydrate (4%) at 300 mg/kg by intraperitoneal
injection. The vasculature was perfused with 4% paraformaldehyde in
0.1 mol/l PBS by inserting an 18-gauge cannula into the left
ventricle aorta. Next, the xenograft tumour was removed and stored
in 4% paraformaldehyde in 0.1 mol/l PBS for 2 h at 4°C. Subsequent
to a rinse in PBS, tumour tissues were incubated in 30% sucrose
overnight at 4°C and frozen with liquid nitrogen for 1 min for
cryostat sectioning (8–10 µm) after being embedded in optimal
cutting temperature compound. Cryostat sections were fixed in
acetone for ~10 min. The slides were allowed to air dry for 30 min
and were washed 3 times for 5 min each in PBS. Samples were
subsequently incubated in 5% bovine serum albumin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) in PBS for 30
min at room temperature to block non-specific antibody binding.
Following blocking with a non-specific antibody, the slides were
incubated in two primary antibodies [FGFR1 (dilution, 1:100) and
p-Akt (dilution, 1:25)] overnight at room temperature. The slides
were then incubated for 1 h at 37°C with fluorescent (Cy3- or
FITC-conjuncted) secondary antibodies (goat anti-rat; dilution,
1:50; cat. no. E670005; Sangon Biotech Co., Ltd., Shanghai, China).
All slides were counterstained with DAPI (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at room temperature for 20 min.
Tissue sections were imaged using an Olympus BX51 fluorescence
microscope (magnification, ×200; Olympus Corporation, Tokyo,
Japan).
Immunohistochemistry
Tumour specimens were fixed in 10% neutral formalin
at 4°C for 6 h, then embedded in paraffin, sectioned (5-µm thick)
and placed on slides for marker analysis. Sections were incubated
with the primary antibody (Ki-67, dilution, 1:150) overnight at
4°C, after blocking for non-specific antibody (goat anti-rat, 1:50,
Shanghai cat. no., C516337; Sangon Biotech Co., Ltd.) binding at
4°C overnight. The streptavidin-biotin-peroxidase complex method
(Lab Vision, Fremont, CA) was used for immunohistochemistry
(25). Images of the slides were
captured using an Olympus BX60 (Olympus Corporation).
Statistical analysis
The results are presented as the mean ± standard
deviation. Calculations and statistics were performed with Excel
2010 (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of
variance (ANOVA) was used to analyse the significance of
differences among groups. Bonferroni's correction was the post hoc
test used following one-way ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
NGS of pancreatic cancer bone
metastasis in a PDX model highlights FGFR1 as a potential
therapeutic target
Based on a 416-gene exon NGS panel, our
previous study focused on gene polymorphisms/mutations, while the
present study focused on gene amplification (Table I). FGFR1, MYC, PIK3CA, RECQL4 and SOX2
genes were found to be amplified, among which FGFR1 was amplified
with the most significant fold-change (3.1-fold), while the fold
change for the remaining were the following: RECQL4 3.0, SOX2 2.7,
MYC 2.3, and PIK3CA 2.2. Therefore, AZD4547, a potent inhibitor of
FGFR, was selected as a potential therapy to be evaluated in our
PDX model.
 | Table I.Gene amplification identified by
next-generation sequencing. |
Table I.
Gene amplification identified by
next-generation sequencing.
| Gene | Fold-change |
|---|
| FGFR1 | 3.1 |
| MYC | 2.3 |
| PIK3CA | 2.2 |
| RECQL4 | 3.0 |
| SOX2 | 2.7 |
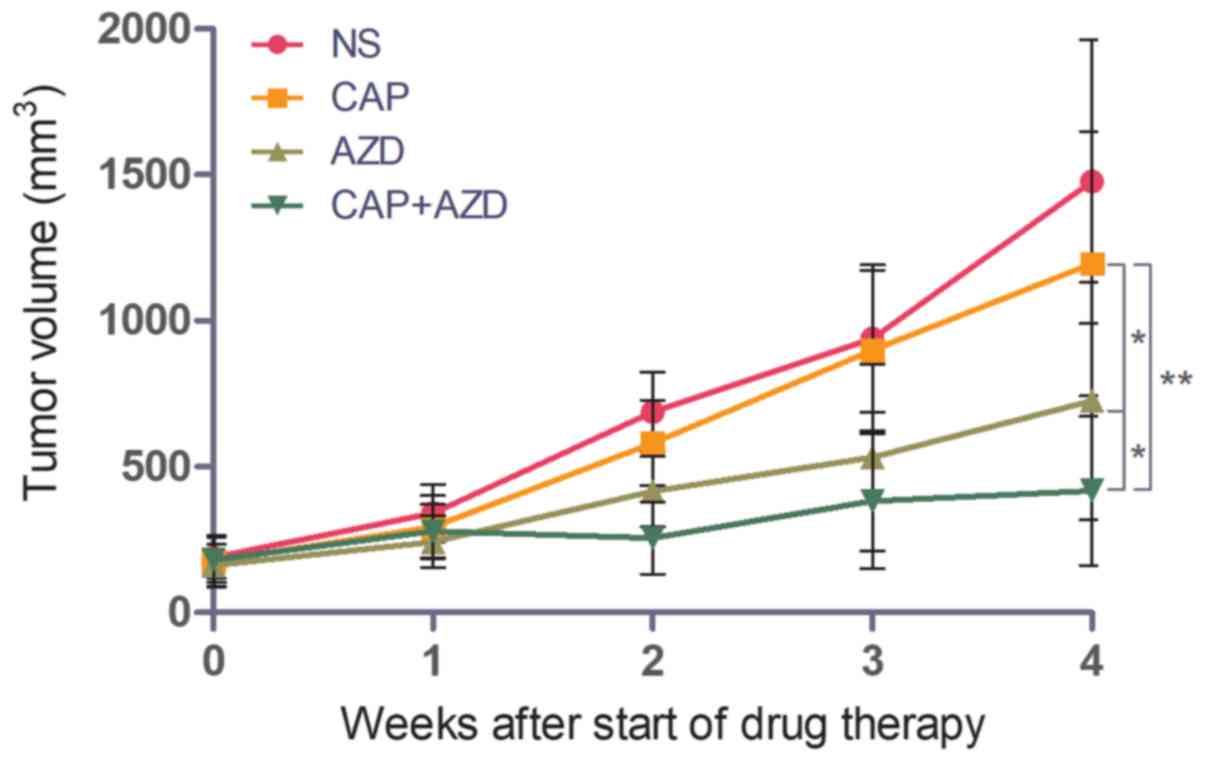
Growth of pancreatic cancer bone
metastasis in a PDX model is lower in AZD4547-treated mice
To test whether the PDX model of pancreatic cancer
bone metastasis was sensitive to FGFR inhibition, the ability of
AZD4547 to inhibit tumour growth was evaluated. Capecitabine, a
chemotherapy drug, was used as a positive control. When the tumour
volume reached 150–200 mm3, saline, AZD4547 (50 mg/kg),
capecitabine (1.0 mM/kg) or the two drugs together was administered
orally once per day for 28 days. The mice were sacrificed and
excised tumours were measured. Mice treated with AZD4547 alone
exhibited increased tumour growth inhibition (TGI, 43.1%) compared
with those treated with capecitabine alone (TGI, 12.9%), while the
combination of the two demonstrated a synergistic effect, with a
TGI of 70.5% (Fig. 1).
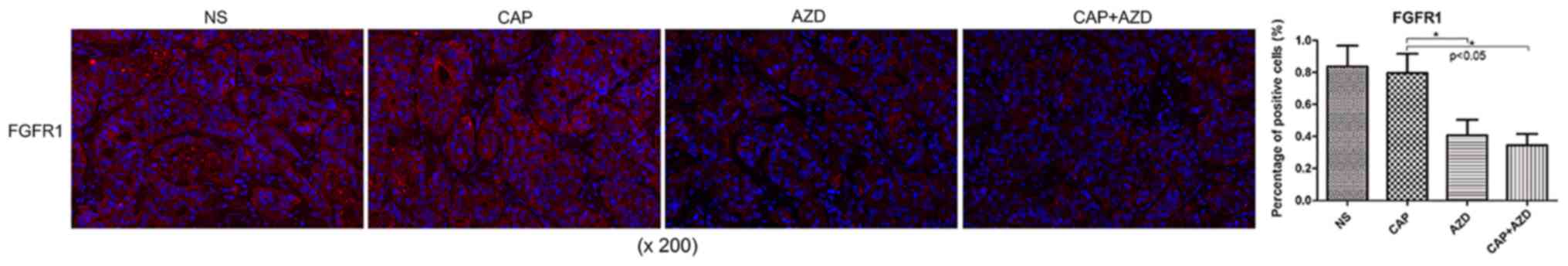
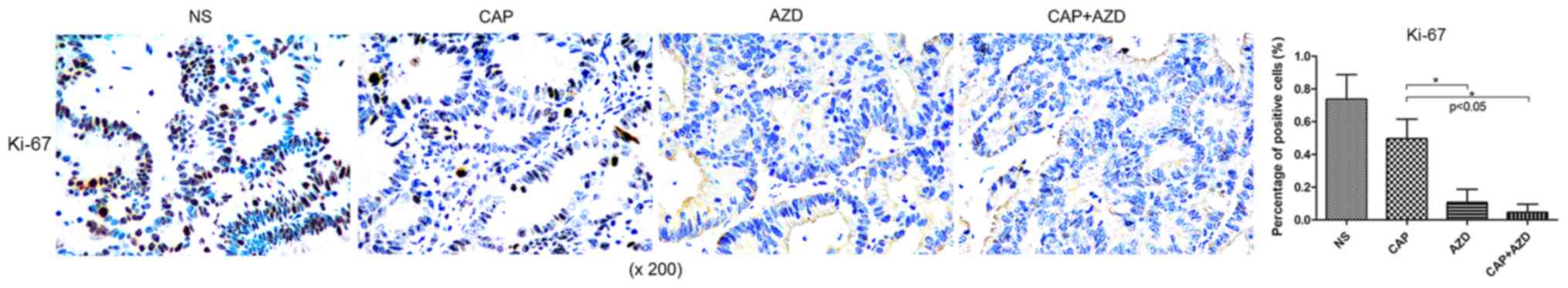
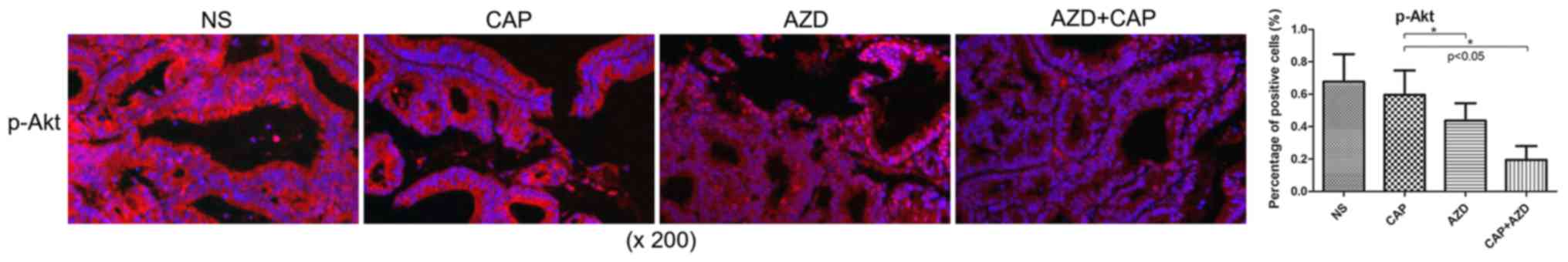
Expression of FGFR1 and downstream
targets is reduced in pancreatic cancer bone metastasis in a PDX
model in AZD4547-treated mice
Prior to drug treatment, PDX tumour tissue was
identified by fluorescence immunohistochemistry to exhibit high
expression of FGFR1, which was significantly suppressed following
AZD4547 treatment (Fig. 2). AKT
pathway has been reported to be regulated by upstream FGFR1
(26). Using immunohistochemistry, it
was found that Ki-67, a cell proliferation marker, and p-Akt were
significantly reduced in the AZD4547-treated groups (Figs. 3 and 4).
Therefore, AZD4547 may be effective at reducing tumour growth in
this pancreatic cancer PDX model by inhibiting the function of
FGFR1.
Discussion
Multiple clinical studies have shown that NGS and
PDX may replace or compliment personalised medicine in identifying
novel therapeutic targets and biomarkers (27). Our previous study described a PDX
model of pancreatic cancer bone metastasis which was confirmed
presenting with clinical patients stable tumor characteristics
(23). Based on these previous
sequencing results, the FGFR1 gene was found to be amplified by
3.1-fold compared with RECQL4, SOX2, MYC, and PIK3CA. The FGFR
family of receptor tyrosine kinases have been implicated in tumour
progression and metastasis in human pancreatic cancer (28). In the present study, four other genes,
including RECQL,4 were also revealed to be amplified; however,
inhibitors for these proteins were not readily available.
Therefore, AZD4547, a novel selective small-molecule inhibitor of
FGFR (29), was selected as a
potential therapy to be evaluated in the PDX model.
Several clinical trials of AZD4547 in the treatment
of bladder cancer, gliomas, myeloma and lung cancer have been
recently registered in clinicaltrials.gov, including NCT02546661,
NCT02824133, NCT02465060, NCT02664935, NCT02965378 and NCT02154490
(updated to December 12, 2017). However, to the best of our
knowledge, there are no studies evaluating the effect of AZD4547 in
pancreatic cancer at a preclinical or clinical stage. In the
present study, the antitumour efficacy of AZD4547 in a bone
metastatic pancreatic cancer was demonstrated.
In the present study, the PDX model of pancreatic
cancer bone metastasis was confirmed to exhibit high expression of
FGFR1 prior to drug evaluation. It was demonstrated that AZD4547
exhibited higher efficacy in reducing growth than capecitabine, a
chemotherapy drug. The combination of the two exhibited a
significant synergistic effect, with a TGI of 70.5%. Furthermore,
it was found that AZD4547 inhibited tumour cell proliferation and
reduced the expression of FGFR1 targets, such as p-Akt (29). As amplification of FGFR1 has been
identified in 2.6% of pancreatic ductal adenocarcinoma patients
(30), AZD4547 may provide targeted
treatment in this subpopulation of pancreatic cancer patients.
However, as western blot analysis of total and FGFR1 and Akt in PDX
tumour tissues was not performed in the present study, further
investigation is required to confirm the mechanism of action of
AZD4547 and to clarify whether resistance to the drug develops over
time. Future studies will focus on monitoring the changes of
associated signalling pathways during drug resistance in order to
find targets for reversing drug resistance.
In conclusion, in the present study, AZD4547, a FGFR
inhibitor, was revealed to supress proliferation and reduce
expression of FGFR1 targets in an FGFR1-amplified pancreatic cancer
PDX model. This inhibitor may prove to be an effective treatment in
patients with FGFR1-amplified pancreatic cancer. In addition, it
was successfully demonstrated that PDX-NGS-based drug screening is
a novel, promising tool for individualised drug screening to
improve the clinical treatment of pancreatic cancer patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81772537 and 81374014), the
Zhejiang Provincial Science and Technology Projects of Traditional
Chinese Medicine (grant nos. 2017ZB089 and 2016ZA128) and the
Zhejiang Provincial Science and Technology Projects (grant nos.
LGF18H160041, 2017C33212, 2017C33213 and 2015C33264).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ and XW contributed to the design of the study. ZG
contributed to main experiments and manuscript preparation. DS
contributed to literature research, revision of manuscript,
language retouching, and data collection. HL contributed to the
data collection and processing.
Ethics approval and consent to
participate
Written consent was provided by the patient and
ethical approval was obtained from the Ethics Committee of The
First Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China). All animal studies were according to the
Institutional Animal Care and Use Committee of Zhejiang University
(approval no., SYXK(ZHE)2005-0072).
Patient consent for publication
The patient involved in this study confirmed that
all medical records could be used for medical research and for
publication in any formal printed or online open accessed
journals.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mattie M, Christensen A, Chang MS, Yeh W,
Said S, Shostak Y, Capo L, Verlinsky A, An Z, Joseph I, et al:
Molecular characterization of patient-derived human pancreatic
tumor xenograft models for preclinical and translational
development of cancer therapeutic. Neoplasia. 15:1138–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sohal DP, Mangu PB, Khorana AA, Shah MA,
Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH,
Engebretson A, et al: Metastatic pancreatic cancer: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 34:2784–2796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative -intent resection of pancreatic
cancer: A randomized controlled trial. JAMA. 297:267–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang L, Holtzinger A, Jagan I, BeGora M,
Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et
al: Ductal pancreatic cancer modeling and drug screening using
human pluripotent stem cell- and patient-derived tumor organoids.
Nat Med. 21:1364–1371. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iguchi H, Yasuda M, Matsuo T, Sumii T and
Funakoshi A: Clinical features and management of pancreatic cancer
with bone metastases. Nippon Shokakibyo Gakkai Zasshi. 101:872–878.
2004.(In Japanese). PubMed/NCBI
|
|
7
|
Pneumaticos SG, Savidou C, Korres DS and
Chatziioannou SN: Pancreatic cancer's initial presentation: Back
pain due to osteoblastic bone metastasis. Eur J Cancer Care (Engl).
19:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boj SF, Hwang CI, Baker LA, Chio II, Engle
DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al:
Organoid models of human and mouse ductal pancreatic cancer. Cell.
160:324–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heestand GM and Kurzrock R: Molecular
landscape of pancreatic cancer: Implications for current clinical
trials. Oncotarget. 6:4553–4561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
The Lancet Oncology: Pancreatic cancer:
Cause for optimism? Lancet Oncol. 17:8452016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aparicio S, Hidalgo M and Kung AL:
Examining the utility of patient-derived xenograft mouse models.
Nat Rev Cancer. 15:311–316. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson JI, Decker S, Zaharevitz D,
Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M,
Arbuck S, Hollingshead M and Sausville EA: Relationships between
drug activity in NCI preclinical in vitro and in vivo models and
early clinical trials. Br J Cancer. 84:1424–1431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hidalgo M, Bruckheimer E, Rajeshkumar NV,
Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, Strawn S, Wick
MJ, Martell J and Sidransky D: A pilot clinical study of treatment
guided by personalized tumorgrafts in patients with advanced
cancer. Mol Cancer Ther. 10:1311–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanley RH: Molecular pathology. Mol
Oncol. 6:177–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verweij J, de Jonge M, Eskens F and
Sleijfer S: Moving molecular targeted drug therapy towards
personalized medicine: Issues related to clinical trial design. Mol
Oncol. 6:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garay JP and Gray JW: Omics and therapy-A
basis for precision medicine. Mol Oncol. 6:128–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakradhar S: Colorectal cancer: 5 big
questions. Nature. 521:S162015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macconaill LE and Garraway LA: Clinical
implications of the cancer genome. J Clin Oncol. 28:5219–2528.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belchis DA, Tseng LH, Gniadek T, Haley L,
Lokhandwala P, Illei P, Gocke CD, Forde P, Brahmer J, Askin FB, et
al: Heterogeneity of resistance mutations detectable by
next-generation sequencing in TKI-treated lung adenocarcinoma.
Oncotarget. 7:45237–45248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jee J, Rasouly A, Shamovsky I, Akivis Y,
Steinman SR, Mishra B and Nudler E: Rates and mechanisms of
bacterial mutagenesis from maximum-depth sequencing. Nature.
534:693–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woollard PM, Mehta NA, Vamathevan JJ, Van
Horn S, Bonde BK and Dow DJ: The application of next-generation
sequencing technologies to drug discovery and development. Drug
Discov Today. 16:512–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan Z, Lan H, Chen X, Jiang X, Wang X and
Jin K: Individualized drug screening based on next generation
sequencing and patient derived xenograft model for pancreatic
cancer with bone metastasis. Mol Med Rep. 16:4784–4790. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Zhang L, Su X, Li M, Xie L,
Malchers F, Fan S, Yin X, Xu Y, Liu K, et al: Translating the
therapeutic potential of AZD4547 in FGFR1-amplified non-small cell
lung cancer through the use of patient-derived tumor xenograft
modes. Clin Cancer Res. 18:6658–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shteyngart B, Chaiwiriyakul S, Wong J and
Cantor JO: Preferential binding of lysozyme to elastic fibres in
pulmonary emphysema. Thorax. 53:193–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Zhu LX, Cheng X, Lin Y, Yan P and
Peng B: Promotion of dental pulp cell migration and pulp repair by
a bioceramic putty involving FGFR-mediated signaling pathways. J
Dent Res. 94:853–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garralda E, Paz K, López-Casas PP, Jones
S, Katz A, Kann LM, López-Rios F, Sarno F, Al-Shahrour F, Vasquez
D, et al: Integrated next-generation sequencing and avatar mouse
models for personalized cancer treatment. Clin Cancer Res.
20:2476–2484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katoh M and Nakagama H: FGF receptors:
Cancer biology and therapeutics. Med Res Rev. 34:280–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gavine PR, Mooney L, Kilgour E, Thomas AP,
Al-Kadhimi K, Beck S, Rooney C, Coleman T, Baker D, Mellor MJ, et
al: AZD4547: An orally bioavailable, potent, and selective
inhibitor of the fibroblast growth factor receptor tyrosine kinase
family. Cancer Res. 72:2045–2056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehnen NC, von Mässenhausen A, Kalthoff H,
Zhou H, Glowka T, Schütte U, Höller T, Riesner K, Boehm D,
Merkelbach-Bruse S, et al: Fibroblast growth factor receptor 1 gene
amplification in pancreatic ductal adenocarcinoma. Histopathology.
63:157–166. 2013. View Article : Google Scholar : PubMed/NCBI
|