Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality globally, accounting for 723,000
mortalities or 8.8% of all cancer-associated mortalities in 2012
(1). Tubular adenocarcinoma is the
most predominant histologic type of gastric cancer, followed by the
papillary and mucinous types (1). The
incidence rate of gastric cancer has substantially declined over
the past few decades; however, the median survival rate remains
poor (2). Furthermore, gastric cancer
is a heterogeneous disease with complex etiology and pathogenesis,
involving a variety of risk factors, including dietary, infectious,
genetic and epigenetic alterations (2).
Over the past few decades, numerous putative
candidate genes and signaling pathways have been reported to serve
a crucial role in the development and progression of gastric
cancer. These include the tumor protein p53,
phosphoinositide-3-kinase, AT-rich interactive domain-containing
protein 1A, Wnt/β, transforming growth factor β and Notch signaling
pathways (3). Therefore,
understanding the underlying molecular mechanisms of gastric cancer
may provide novel insights into the pathogenesis of gastric cancer
and help identify novel potential biomarkers and therapeutic
targets for treatment.
The sirtuins (SIRTs) are a family of
nicotinamide-adenine dinucleotide (NAD)+-dependent
protein deacetylases. Humans encode seven SIRT orthologues,
SIRT1-SIRT7, which exhibit varying intracellular distribution
(4). These SIRTs are known to serve
an important role in stress resistance, genome stability, energy
metabolism and aging (4). Previously,
a number of studies have indicated the role of SIRTs in tumor
development, survival and tumor metabolism (5). SIRT4 utilizes NAD+ for
adenosine diphosphate-ribosylation to downregulate the activity of
glutamate dehydrogenase and suppress insulin secretion from
pancreatic β-cells (6). Previous
studies demonstrated that mitochondrial SIRT4 may function as a
tumor suppressor by regulating the metabolism of glutamine, which
indicates that it may exhibit a therapeutic potential in cancer
(7,8).
Additionally, previous studies have identified an association of
SIRT4 expression in colon and esophageal cancer with a reduction in
adverse outcomes associated with these tumors (9–11).
Furthermore, our previous study demonstrated that a reduced
expression level of SIRT4 protein is associated with gastric cancer
(12). However, the function of SIRT4
in gastric cancer cells remains unknown.
The present study reported that overexpression of
SIRT4 inhibits the proliferation of gastric cancer cells via G1
cell cycle arrest by inhibiting the expression of cyclin D, cyclin
E and phosphorylated extracellular signal-regulated kinase (p-ERK).
In summary, the results of the present study demonstrate the tumor
suppressive function of SIRT4 in gastric cancer and indicate its
potential as a therapeutic target for this disease.
Materials and methods
Cell lines and culture conditions
Human gastric cancer cell lines SGC-7901 and MNK45
were obtained from the Shanghai Institute of Cell Biology of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in high glucose Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (both from Gibco; Thermo
Fisher Scientific, Inc.) and penicillin (100 U/ml) /streptomycin
(0.1 mg/ml) at 37°C and 5% CO2.
Construction and transfection of the
SIRT4 overexpression vector
pHBLV-CMVIE-ZsGreen-T2A-Puro, the lentivirus vector
that induces overexpression of SIRT4 and the empty vector were
purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China).
The final titer of the lentivirus and the negative control virus
was 2×108 PFU/ml. The transfection MOI was 10. Stable
overexpression of SIRT4 was achieved by infecting SGC-7901 and
MNK45 cells with lentiviruses for 72 h followed by culturing in
high glucose Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) with 2 µg/ml puromycin (Beyotime Institute
of Biotechnology, Haimen, China) at 37°C and 5% CO2 for
2 weeks.
Cell proliferation assay
To observe cell proliferation activity, cells were
seeded in 96-well plates at a density of 1,000 cells/well.
Following incubation for 12, 36, 60, 84 or 108 h, detection of each
well was enabled by adding 10 µl Cell Counting Kit-8 reagent
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) and the
absorbance was read by a spectrophotometer at 450 nm following
culturing in the CO2 incubator at 37°C for 2 h.
Colony formation assay
Logarithmic growth phase cells were plated at a
density of 100 cells/well in six-well plates and cultured for 2
weeks. Cells were then fixed in 100% methanol for 15 min at 37°C
and stained using Giemsa stain at 37°C for 30 min to permit direct
counting of the number of colonies formed with the naked eye.
Flow cytometry analysis of cell
cycle
The cell cycle assay was performed using flow
cytometry. Briefly, cells (1×106) were washed twice with
ice-cold PBS, treated with trypsin and subsequently washed with PBS
containing 3% fetal bovine serum. Prior to analysis, cells were
stained using a propidium iodide cell cycle detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) at room temperature for 30
min. Analysis was performed using a FACScan flow cytometer (BD
Biosciences). The cell cycle results were analyzed using the ModFit
analysis software program (version 4.0; Verity Software House,
Inc., Topsham, ME, USA).
Western blot analysis
Cells were lysed using ice-cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) supplemented with protease. The cellular lysates
were collected and the protein content was determined using a
Bicinchoninic Acid assay kit (Beyotime Institute of Biotechnology).
For western blot analysis, 40 µg of protein was resolved on 12%
SDS-PAGE and then transblotted to methanol-activated polyninylidene
difluoride membranes. The resulting blots were blocked with 10%
fat-free milk for 1 h in TBS with 0.1% Tween and incubated with the
appropriate primary antibodies at 4°C overnight. Subsequently, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:1,000; catalog no. ab97200;
Abcam) at room temperature for 30 min. Finally, protein bands were
detected using an enhancement chemiluminescent substrate (EMD
Millipore, Billerica, MA, USA) and quantification was performed
using ImageJ software (version 2.1.4.7; National Institutes of
Health, Bethesda, MD, USA). The following primary antibodies were
used for this western blot analysis: Rabbit anti-human SIRT4
polyclonal antibody (1:1,000; catalog no. HPA029691; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), rabbit anti-human cyclin D
monoclonal antibody (1:1,000; catalog no. 60816-1-IG, ProteinTech
Group, Inc., Chicago, IL, USA), rabbit anti-human cyclin E
monoclonal antibody (1:1,000; catalog no. Ab33911; Abcam,
Cambridge, MA, USA), rabbit anti-human ERK polyclonal antibody
(1:1,000; catalog no. 9102; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-human p-ERK polyclonal antibody
(1:1,000; catalog no. 4370; Cell Signaling Technology, Inc.),
rabbit anti-human β-actin polyclonal antibody (1:1,000; catalog no.
ab11971; Abcam) and rabbit anti-human GAPDH polyclonal antibody
(1:1,000; catalog no. AB-P-R 001; Hangzhou Goodhere Biotechnology
Co., Ltd., Hangzhou, China).
Statistical analysis
Statistical analysis was performed using the
statistical software program SPSS version 20.0 (IBM Corp., Armonk,
NY, USA). All in vitro experiments were performed in
triplicate. Data from three or more independent experiments are
presented as the mean ± standard deviation. Student's t-test was
performed to determine the differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
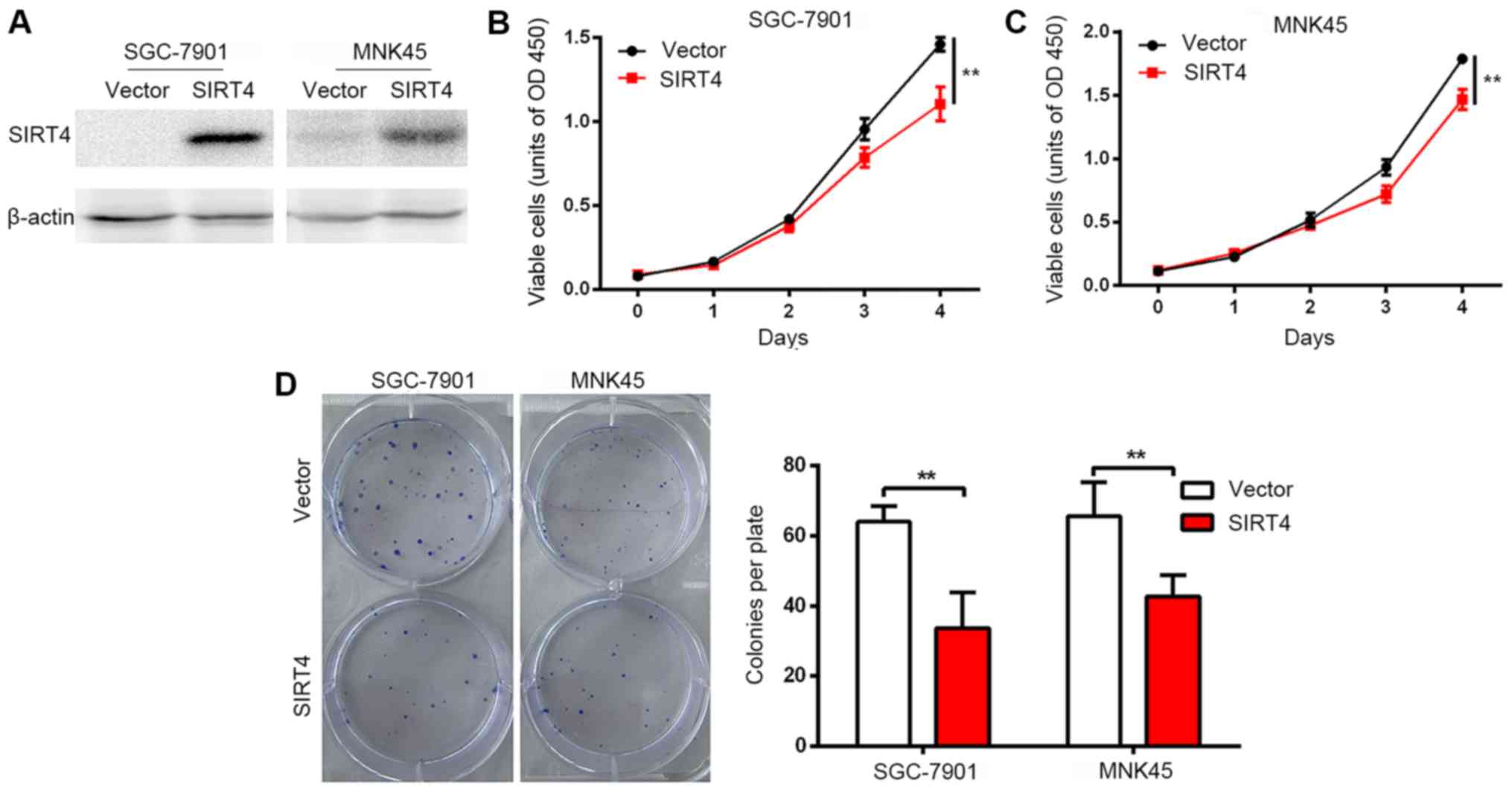
Overexpression of SIRT4 inhibits
proliferation of human gastric cancer cells
Stable strains of human gastric cancer cell lines
SGC-7901 and MNK45 were constructed by lentiviral infection and
overexpression of SIRT4 was confirmed by western blot analysis
(Fig. 1A). A significant inhibition
in the proliferation rates of SGC-7901 and MNK45 cells was observed
following SIRT4 overexpression (Fig. 1B
and C). Furthermore, a colony formation assay revealed that
SIRT4 overexpression significantly reduced the number of colonies
formed by SGC-7901 and MNK45 cells in vitro (Fig. 1D). These results indicated that SIRT4
inhibits the cell growth and proliferation rates of gastric cancer
cells in vitro.
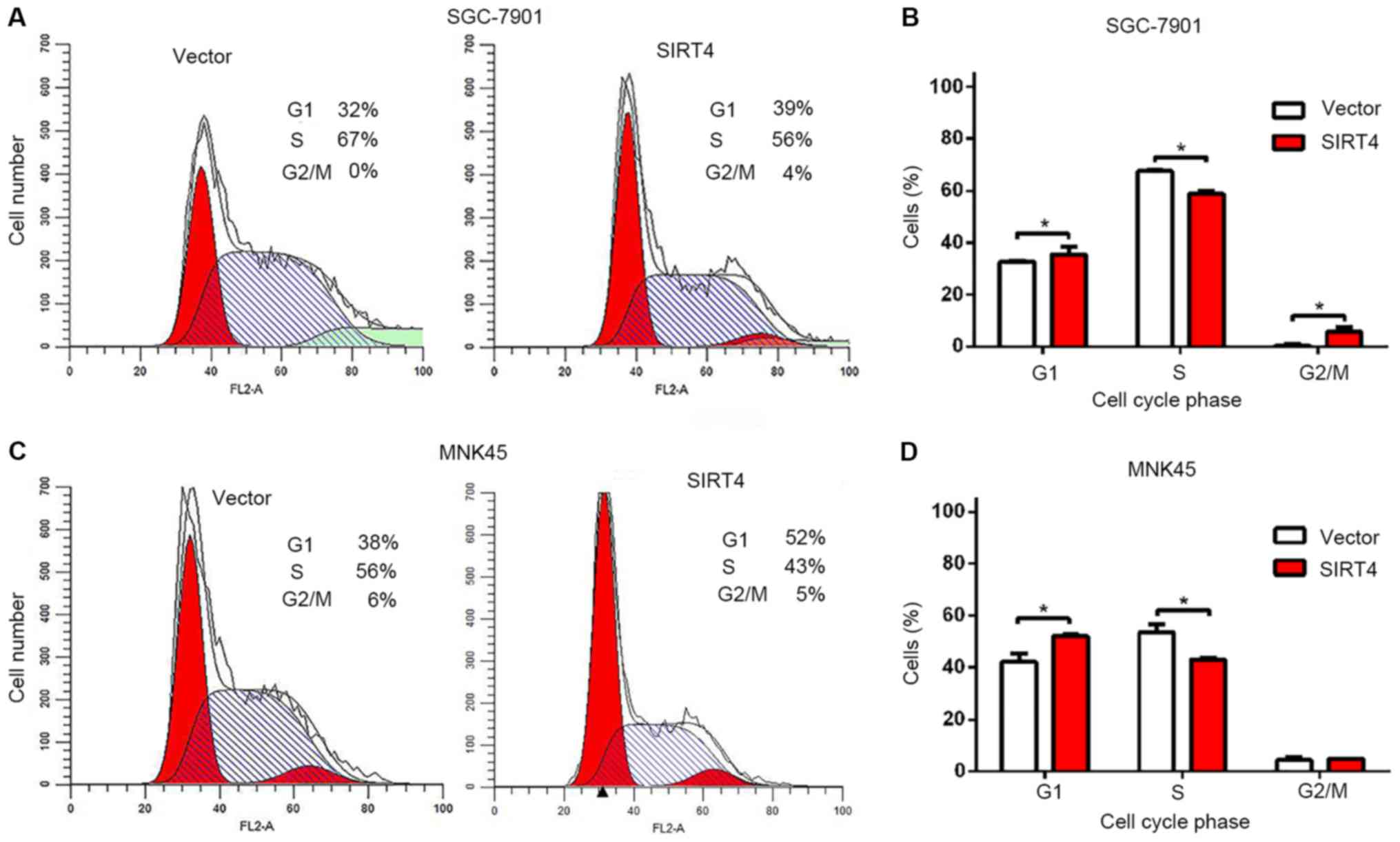
Overexpression of SIRT4 induces G1
cell cycle arrest in gastric cancer cells
To further determine whether SIRT4 inhibits the
proliferation of human gastric cancer cells by arresting the cell
cycle, the cell cycle profiles of SIRT4-overexpressing SGC-7901 and
MNK45 cells were analyzed using flow cytometry and propidium iodide
staining. Overexpression of SIRT4 significantly increased the
proportion of cells in the in G1 phase and reduced the number of
cells in the S phase of the cell cycle, compared with the controls
(Fig. 2). Furthermore, overexpression
of SIRT4 significantly increased the proportion of SGC-7901 cells
in the G2 phase (Fig. 2B). Cell
growth inhibition by SIRT4 overexpression was associated with
significant cell cycle arrest at the G1 phase, which indicates that
overexpression of SIRT4 suppresses cell proliferation by G1 cell
cycle arrest and induces specific inhibition of cell cycle
progression. By contrast, overexpression of SIRT4 did not affect
apoptosis of gastric cancer cells (data not shown).
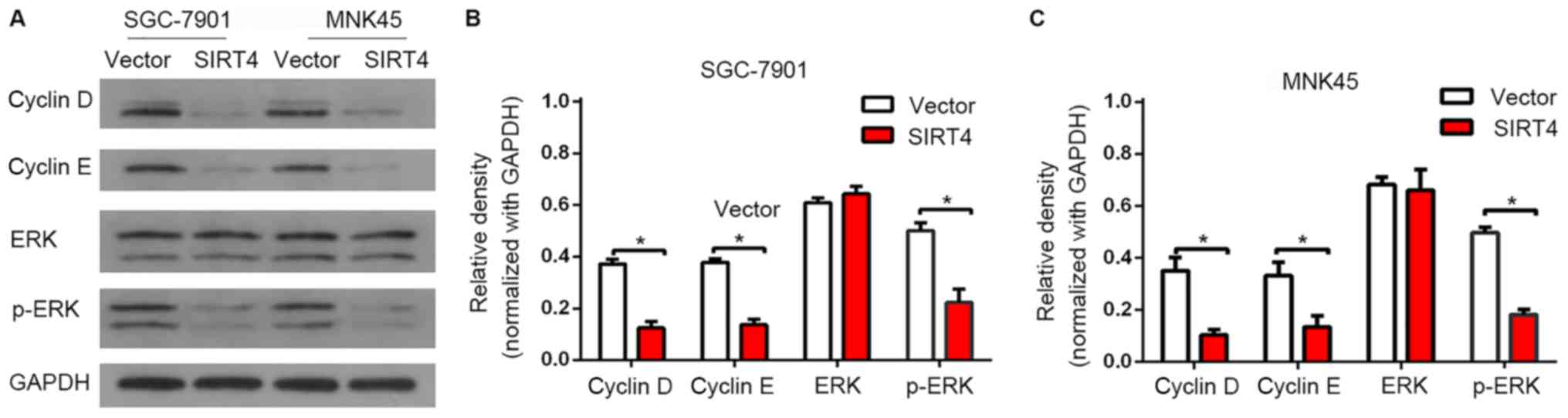
SIRT4 regulates the expression of cell
cycle G1-associated proteins
To validate the results of flow cytometry, the
expression of G1 cell cycle regulatory proteins were detected by
western blot analysis. It was identified that SIRT4 significantly
inhibited the expression of cyclin D and cyclin E (Fig. 3). Additionally, SIRT4 overexpression
was associated with a significant decrease in the expression level
of p-ERK, which indicates a reduced level of activated ERK. These
results indicated that SIRT4-induced G1 cell cycle arrest is
associated with the suppression of ERK, cyclin D and cyclin E.
Discussion
Numerous SIRT family members serve a role in tumor
development and different SIRTs are localized in different
subcellular compartments, and can thus modulate different targets
in the cell (13). For example, SIRT1
is highly expressed in gastric (14),
colon (15), prostate (16) and skin cancer (17), which indicates that it serves a role
in promoting tumor formation in these tissues. By contrast, other
studies demonstrated that SIRT1 expression is reduced in breast
cancer (18) and its expression in a
mouse model has been revealed to prevent the formation of
intestinal tumors (19). Furthermore,
similar observations have been reported for SIRT2, which has been
identified to be downregulated in breast cancer (20), glioma (21) and skin cancer (22), but overexpressed in acute myeloid
leukemia (23) and prostate cancer
(24). Therefore, it remains unclear
whether the observations made for one tumor type can be
extrapolated to conclude the role of SIRTs in other tumor
types.
A limited number of studies investigated the
biological functions and significance of SIRT4 in tumors. Jeong
et al (7) demonstrated that
SIRT4 inhibits the formation of tumor by suppressing glutamine
metabolism. Overexpression of SIRT4 inhibited the growth of HeLa
cells and SIRT4-knockout MEF cells in nude mice reduced their
ability to form large tumors. Furthermore, SIRT4-knockout mice
spontaneously produced lung, liver, breast and lymphoma cancer.
Csibi et al (8) indicated that
overexpression of SIRT4 reduces the growth of the human colon
cancer cell line DLD-1 and the human prostate cancer cell line
DU145. Additionally, Jeong et al (25) identified that SIRT4 inhibits the
growth of Myc-induced B cell lymphoma.
Our previous study and another study revealed that
SIRT4 expression in colon and esophageal cancer is associated with
a reduction in adverse outcomes (9,10).
Furthermore, we previously reported that SIRT4 expression was
associated with pathological parameters in gastric cancer,
including pathological stage, T stage and UICC stage (12). The present study revealed that SIRT4
inhibits the proliferation of gastric cancer cells in vitro.
The experimental results indicate that SIRT4 serves a crucial role
as a tumor suppressor in gastric cancer.
To further analyze the underlying mechanism involved
in the inhibition of cell proliferation in gastric cancer cells
following overexpression of SIRT4, the cell cycle distribution was
analyzed by flow cytometry. Overexpression of SIRT4 arrested the
cell cycle at the G1 phase in SGC-7901 and MNK45 cells.
Additionally, it was identified that overexpression of SIRT4
significantly reduced the expression of cyclin D, cyclin E and
p-ERK in gastric cancer cells. A previous study indicated that
increased expression of cyclin D in cancer cells results in
uncontrolled cell growth (26). ERK
has been reported to regulate the G1 cell cycle phase via
modulation of cyclin D (27). Cyclin
E has also been reported to exhibit a critical role in regulating
the G1 to S phase transition (28,29). The
observations of the present study indicated that SIRT4-induced G1
cell cycle arrest is associated with the suppression of ERK, cyclin
D and cyclin E.
To the best of our knowledge, no previous study has
reported the function of SIRT4 in gastric cancer cells. The present
in vitro study demonstrated that SIRT4 overexpression could
significantly inhibit the cell proliferation of gastric cancer
cells and arrest the cell cycle by suppressing ERK, cyclin D and
cyclin E. In summary, the results of the present study highlight
the tumor suppressive role of SIRT4 in gastric cancer and indicate
its potential as a therapeutic target for this disease.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Project of Wenzhou Science and Technology Bureau (grant nos.
Y20160404 and Y20160411) and the Zhejiang Natural Science
Foundation (grant no. LY18H160055).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, JL, YL and XC performed the experiments. GZ and
GH performed the statistical analysis and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zang ZJ, Cutcutache I, Poon SL, Zhang SL,
McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A, et al:
Exome sequencing of gastric adenocarcinoma identifies recurrent
somatic mutations in cell adhesion and chromatin remodeling genes.
Nat Genet. 44:570–574. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finkel T, Deng CX and Mostoslavsky R:
Recent progress in the biology and physiology of sirtuins. Nature.
460:587–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan H, Su L and Chen WY: The emerging and
diverse roles of sirtuins in cancer: A clinical perspective. Onco
Targets Ther. 6:1399–1416. 2013.PubMed/NCBI
|
|
6
|
Haigis MC, Mostoslavsky R, Haigis KM,
Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos
GD, Karow M, Blander G, et al: SIRT4 inhibits glutamate
dehydrogenase and opposes the effects of calorie restriction in
pancreatic beta cells. Cell. 126:941–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong SM, Xiao C, Finley LW, Lahusen T,
Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, et al:
SIRT4 has tumor-suppressive activity and regulates the cellular
metabolic response to DNA damage by inhibiting mitochondrial
glutamine metabolism. Cancer Cell. 23:450–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Csibi A, Fendt SM, Li C, Poulogiannis G,
Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T,
et al: The mTORC1 pathway stimulates glutamine metabolism and cell
proliferation by repressing SIRT4. Cell. 153:840–854. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyo M, Yamamoto H, Konno M, Colvin H,
Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M, et
al: Tumour-suppressive function of SIRT4 in human colorectal
cancer. Br J Cancer. 113:492–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang G, Cheng J, Yu F, Liu X, Yuan C, Liu
C, Chen X and Peng Z: Clinical and therapeutic significance of
sirtuin-4 expression in colorectal cancer. Oncol Rep. 35:2801–2810.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakahara Y, Yamasaki M, Sawada G, Miyazaki
Y, Makino T, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S,
Mimori K, et al: Downregulation of SIRT4 expression is associated
with poor prognosis in esophageal squamous cell carcinoma.
Oncology. 90:347–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang G, Cui F, Yu F, Lu H, Zhang M, Tang
H and Peng Z: Sirtuin-4 (SIRT4) is downregulated and associated
with some clinicopathological features in gastric adenocarcinoma.
Biomed Pharmacother. 72:135–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roth M and Chen WY: Sorting out functions
of sirtuins in cancer. Oncogene. 33:1609–1620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH,
Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, et al: Expression of
DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stünkel W, Peh BK, Tan YC, Nayagam VM,
Wang X, Salto-Tellez M, Ni B, Entzeroth M and Wood J: Function of
the SIRT1 protein deacetylase in cancer. Biotechnol J. 2:1360–1368.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huffman DM, Grizzle WE, Bamman MM, Kim JS,
Eltoum IA, Elgavish A and Nagy TR: SIRT1 is significantly elevated
in mouse and human prostate cancer. Cancer Res. 67:6612–6618. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hida Y, Kubo Y, Murao K and Arase S:
Strong expression of a longevity-related protein, SIRT1, in Bowen's
disease. Arch Dermatol Res. 299:103–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang RH, Sengupta K, Li C, Kim HS, Cao L,
Xiao C, Kim S, Xu X, Zheng Y, Chilton B, et al: Impaired DNA damage
response, genome instability, and tumorigenesis in SIRT1 mutant
mice. Cancer Cell. 14:312–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Firestein R, Blander G, Michan S,
Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S,
de Cabo R, Fuchs C, et al: The SIRT1 deacetylase suppresses
intestinal tumorigenesis and colon cancer growth. PLoS One.
3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HS, Vassilopoulos A, Wang RH, Lahusen
T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, et al: SIRT2
maintains genome integrity and suppresses tumorigenesis through
regulating APC/C activity. Cancer Cell. 20:487–499. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiratsuka M, Inoue T, Toda T, Kimura N,
Shirayoshi Y, Kamitani H, Watanabe T, Ohama E, Tahimic CG, Kurimasa
A and Oshimura M: Proteomics-based identification of differentially
expressed genes in human gliomas: Down-regulation of SIRT2 gene.
Biochem Biophys Res Commun. 309:558–566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ming M, Qiang L, Zhao B and He YY:
Mammalian SIRT2 inhibits keratin 19 expression and is a tumor
suppressor in skin. Exp Dermatol. 23:207–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dan L, Klimenkova O, Klimiankou M, Klusman
JH, van den Heuvel-Eibrink MM, Reinhardt D, Welte K and Skokowa J:
The role of sirtuin 2 activation by nicotinamide
phosphoribosyltransferase in the aberrant proliferation and
survival of myeloid leukemia cells. Haematologica. 97:551–559.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou H, Chen W, Zhao L, Zuo Q, Zhang G,
Zhang X, Wang H, Gong H, Li X, Wang M, et al: Cortactin is
associated with tumour progression and poor prognosis in prostate
cancer and SIRT2 other than HADC6 may work as facilitator in situ.
J Clin Pathol. 65:1088–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong SM, Lee A, Lee J and Haigis MC:
SIRT4 suppresses tumor formation in genetic models of Myc-induced B
cell lymphoma. J Biol Chem. 289:4135–4144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hall M and Peters G: Genetic alterations
of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human
cancer. Adv Cancer Res. 68:67–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chambard JC, Lefloch R, Pouyssegur J and
Lenormand P: ERK implication in cell cycle regulation. Biochim
Biophys Acta. 1773:1299–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohtsubo M, Theodoras AM, Schumacher J,
Roberts JM and Pagano M: Human cyclin E, a nuclear protein
essential for the G1-to-S phase transition. Mol Cell Biol.
15:2612–2624. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohtsubo M and Roberts JM: Cyclin-dependent
regulation of G1 in mammalian fibroblasts. Science. 259:1908–1912.
1993. View Article : Google Scholar : PubMed/NCBI
|