Introduction
Lung cancer, which results in an estimated 1.59
million mortalities worldwide annually is one of the most frequent
malignancies (1). Non-small cell lung
cancer (NSCLC), which exhibits high mortality and a low 5-year
survival rate, accounts for between 80 and 85% of lung cancer cases
(2–4).
At present, cisplatin-, paclitaxel- and gefitinib-based
chemotherapies are widely used as the first-line chemotherapy for
advanced NSCLC (5). However,
differences in the therapeutic effect among individuals and the
development of multidrug resistance present challenges to
successful chemotherapy in clinical application (6–8). More
seriously, invasion and metastasis, which account for the principal
causes of mortality in patients with cancer, are common in NSCLC
following initial diagnosis (9,10).
Therefore, further elucidation of the molecular mechanisms
underlying tumorigenesis, invasion and metastasis is required to
develop an efficient strategy for the therapy of NSCLC.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNA molecule which are >200 nt in length and
participate in the regulation of gene expression (11). Previous studies have demonstrated that
lncRNAs were able to regulate various biological progresses such as
tumorigenesis, differentiation and inflammation (12,13). In
addition, overexpression or downregulation of lncRNAs is associated
with processes in tumors including proliferation, apoptosis,
invasion and metastasis in numerous types of cancer (14,15). For
example, lncRNA metastasis-associated lung adenocarcinoma
transcript 1, which was overexpressed in various cancer cells, was
identified as a key regulator of cell proliferation and the cell
cycle (16). Additionally, it has
been reported that the small nucleolar RNA host gene 12 (SNHG12)
lncRNA enhances cell proliferation, invasion and migration by
increasing the angiomotin gene expression level in human
osteosarcoma (17). However, the
biological function and mechanism of SNHG12 in NSCLC remain
unknown. Previous studies have indicated that all RNA transcripts
that possess microRNA-binding sites are able to interact with each
other and regulate expression levels, suggesting interactions
between microRNAs and lncRNAs in tumorigenesis (18–22).
In the present study, the expression level and
function were investigated in NSCLC, and it was identified that
SNHG12 was markedly overexpressed in NSCLC tissues and was
associated with poor prognosis and survival of patients with NSCLC.
Furthermore, further mechanical analysis revealed that SNHG12
suppresses NSCLC cell proliferation, invasion and migration by
targeting microRNA-218 (miR-218) and then influences the Slug/zinc
finger E-box-binding homeobox 2 (ZEB2) signaling pathway.
Patients and methods
Tissue specimens and cell lines
In total, 40 NSCLC tissue samples and adjacent
normal tissue samples (3×2×1 cm) were obtained from patients with
NSCLC who were undergoing surgery and without radiotherapy or
chemotherapy. The patients' sex distribution (male, n=21; female,
n=19) and age distribution in years (≥60, n=18; <60, n=22;
range, 28–77; mean ± standard deviation, 58.88±12.65) were
recorded. The present study was approved by the Ethic Review
Committees of The Affiliated Hospital of Hebei University (Baoding,
China) and written informed consent was obtained from all patients.
A 48-month follow-up survival survey based on the medical records
of the patients was performed. Overall survival (OS) time was
defined as the interval between resection and mortality or the last
follow-up visit. Pathological evaluations of tissues were performed
by pathologists at the Department of Pathology of The Affiliated
Hospital of Hebei University. The tissues obtained were stored at
−80°C until further use.
NSCLC cell lines A549 (cat. no. CCL-185) and H1299
(cat. no. CRL-5803) were purchased from the American Type Culture
Collection (Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and antibiotics at 37°C in a humidified
atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was obtained from tumor tissue samples or
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RT was
performed using a TaqMan™ MicroRNA Reverse Transcription kit (cat.
no. 4366596; Thermo Fisher Scientific, Inc.). qPCR was performed
using IQ SYBR-Green Supermix (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) on an iCycler IQ Multi-Color Detection system (Bio-Rad
Laboratories, Inc.). The thermocycling conditions for PCR were as
follows: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for
34 sec; followed by dissociation. Stem-loop primers used to detect
microRNAs were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). U6 small nucleolar RNA and GAPDH were used for
normalization. Primers sequences were as follows: SNHG12,
5′-TCTGGTGATCGAGGACTTCC-3′ (forward) and
5′-ACCTCCTCAGTATCACACACT-3′ (reverse); human (hsa)-miR-218,
5′-GGAGTGGCGAATGGTAGTGGAGT-3′ (forward) and
5′-ACCAGGCTGGACAGTAGAGCG-3′ (reverse); GAPDH,
5′-TCTCTGCTCCTCCTGTTC-3′ (forward) and
5′-GGTTGAGCACAGGGTACTTTATTGA-3′ (reverse); and U6,
5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′
(reverse). Relative expression levels of RNA were calculated using
the 2−ΔΔCq method (23)
with CFX Manager software (version 3.1; Bio-Rad Laboratories,
Inc.).
In situ hybridization (ISH)
ISH was used to detect SNHG12 in 40 paired NSCLC and
adjacent normal samples. A digoxigenin (DIG)-UTP-labeled antisense
RNA probe (Beijing View Solid Biotechnology, Beijing, China) which
was derived from nucleotides 28–257 of the SNHG12 coding sequence
was obtained by in vitro transcription using a DIG RNA
Labeling kit (Roche Diagnostics, Basel, Switzerland). The sense RNA
probe derived from nucleotides 28–257 of the SNHG12 coding sequence
was labeled with DIG-UTP and used as a negative control. ISH was
performed using the ISH kit (Boster Biological Technology,
Pleasanton, CA, USA), according to the manufacturer's protocol.
Cell transfection
The plasmid for SNHG12 inhibitor (SNHG12-inhi) and
the plasmid for hsa-miR-218 mimic (miR-218-mimic) were constructed
by OBiO Technology (Shanghai) Corp., Ltd. (Shanghai, China).
SNHG12-inhi or miR-218-mimic was transfected into A549 and H1299
cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Cell viability analysis
Cell viability was determined using an MTT assay.
First, A549 and H1299 cells were seeded into a 96-well plate at a
density of 2,000 cells/well. After 12 h incubation at 37°C, cells
were transfected with SNHG12-inhi or negative control-inhi
(NC-inhi; OBiO Technology (Shanghai) Corp., Ltd.). At various times
(24, 48, 72 and 96 h), cells from each well were treated with 20 µl
MTT solution (5 mg/ml; Amresco, LLC, Solon, OH, USA). Following
incubation for 4 h at 37°C, 200 µl dimethylsulfoxide was added to
each well to dissolve the precipitated formazan product. Finally,
the absorbance of each well was determined at 570 nm. All
experiments were performed three times.
Colony formation assay
Cells were seeded in a 6-well plate at a density of
500 cells/well and incubated for 12 h at 37°C. Following
transfection with SNHG12-inhi or NC-inhi, cells were incubated for
a further 6–8 days. Finally, cells were washed with PBS, fixed with
4% paraformaldehyde for 10 min at 25°C and stained with 0.1%
crystal violet for 30 min at room temperature, and images were
captured. Colonies were counted by eye and colonies containing ≥50
cells were scored.
Cell apoptosis evaluation
Cells were seeded in a 6-well plate and transfected
with SNHG12-inhi or NC-inhi. Cells were trypsinized, washed and
collected for further staining. In total, 2×105
cells/well, suspended in 400 µl 1X binding buffer, were stained
with 5 µl fluorescein isothiocyanate-Annexin V and 5 µl propidium
iodide in the dark at room temperature for 15 min. Cells were
analyzed immediately using flow cytometry (FACScan®; BD
Biosciences, San Jose, CA, USA).
Transwell assay
Transwell chambers assays were used to determine
cell migration and invasion. For the determination of cell
migration, 4×104 cells suspended in 200 µl serum-free
DMEM were seeded into the upper chamber. For cell invasion
assessment, 200 µl cell suspension (1×105 cells, without
serum) were added into the upper chamber which was pre-coated with
Matrigel (BD Biosciences). The lower chamber was filled with 800 µl
DMEM containing 10% FBS. Cells were incubated for 24–48 h at 37°C.
The non-invasive cells which remained in the upper chamber were
removed with a cotton swab, whereas the invasive cells which were
located in the bottom of membrane were fixed with methanol and
stained with 0.1% crystal violet. Finally, images of invasive cells
from each well were captured under a phase contrast microscope
(CX43; Olympus Corporation, Tokyo, Japan) in three independent
fields.
Wound healing assay
In total, 5×105 cells were seeded in a
6-well plate and incubated for 12 h at 37°C. When the cell density
reached ~90%, cells were scratched with a 10 µl pipette tip. Cells
were washed with PBS to remove the free-floating cells and
transfected with SNHG12-inhi or NC-inhi. Cells were incubated for a
further 24 h at 37°C in DMEM with 1% FBS and images were captured
under an inverted light microscope (CKX53; Olympus, Japan) for
migration analysis.
Western blot analysis
Cells were transfected with different plasmids
(NC-inhi, SNHG12-inhi, miR-NC, miR-218-mimic) for 48 h at 37°C and
collected for total protein extraction. Total protein was obtained
from the cells by lysing with radioimmunoprecipitation buffer
(Beyotime Institute of Biotechnology, Haimen, China). The
concentration of total protein was determined using a BCA protein
assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). The
protein (20 µg) was separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat milk powder suspended in
TBS containing 0.25% Tween-20 for 2 h at room temperature,
membranes were incubated with primary antibodies against cleaved
caspase 3 (cat. no. 9661) cleaved caspase 9 (cat. no. 52873),
matrix metalloproteinase 9 (MMP-9; cat. no. 13667S), epithelial
(E-) cadherin (cat. no. 14472), vimentin (cat. no. 5741), Slug
(cat. no. 9585), ZEB2 (cat. no. 3396) and β-actin (cat. no. 3700;
all Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C for 8
h. The dilution for cleaved caspase-3, cleaved caspase-9, MMP-9,
E-cadherin, vimentin, Slug and ZEB2 was 1:100, and the dilution for
β-actin was 1:1,000. Subsequently, membranes were incubated further
with horseradish peroxidase-labeled anti-rabbit IgG secondary
antibodies (cat. no. 7047; 1:1,000; Cell Signaling Technology,
Inc.) and protein bands were imaged and quantified using an
enhanced chemiluminescence detection system.
Dual-luciferase assay
In total, 50 genes were simultaneously predicted by
the miRcode database (http://www.mircode.org/), and small nuclear RNA host
gene 12 (SNHG12) was detected as a candidate gene associated with
NSCLC based on its associated Gene Ontology (GO; http://www.geneontology.org/) terms and because it
harbors miR-218 binding sites. The wild-type (WT) and mutant
3′-untranslated region of human SNHG12 were the amplified from
human genomic DNA and individually cloned into the luciferase
vector of pmiR-RB-REPORT™ [OBiO Technology (Shanghai) Corp., Ltd.].
A549 cells were co-transfected with 200 ng mutant or WT
pmiR-RB-REPORT™-SNHG12 plasmid, and 100 ng miR-218-mimic or miR-NC
plasmid using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
pRL-SV40 plasmid (Promega Corporation, Madison, WI, USA) carrying
Renilla luciferase was also co-transfected into the cells
for standardizing transfection efficiency. After 48 h, cells were
collected for luciferase activity analysis using a Dual-Luciferase
Reporter assay system (Promega Corporation), according to the
manufacturer's protocol. The firefly luciferase activity was
normalized to Renilla luciferase activity.
Statistical analysis
All results are presented as the mean ± standard
deviations. The statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The
significance among groups was determined using one-way analysis of
variance. Kaplan-Meier analysis was performed to compare overall
survival rates based on the expression levels of SNHG12 in 40
patients with NSCLC. Survival curve was assessed by a log-rank
test. The median level of SNHG12 was used as the cut-off values.
Patients with HCC were divided into a high SNHG12 expression group
and a low SNHG12 expression group. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
SNHG12 is significantly overexpressed,
and is markedly associated with progression and prognosis of
NSCLC
Previous studies have identified that lncRNA was
regulated in a wide range of pathological processes such as
tumorigenesis, cell death, invasion and inflammation, but also
dysregulated in a number of types of cancer (24,25). To
investigate whether there was a difference in SNHG12 expression
between NSCLC tissues and adjacent normal tissues, RT-qPCR was used
to determine the SNHG12 expression levels in 40 paired specimens.
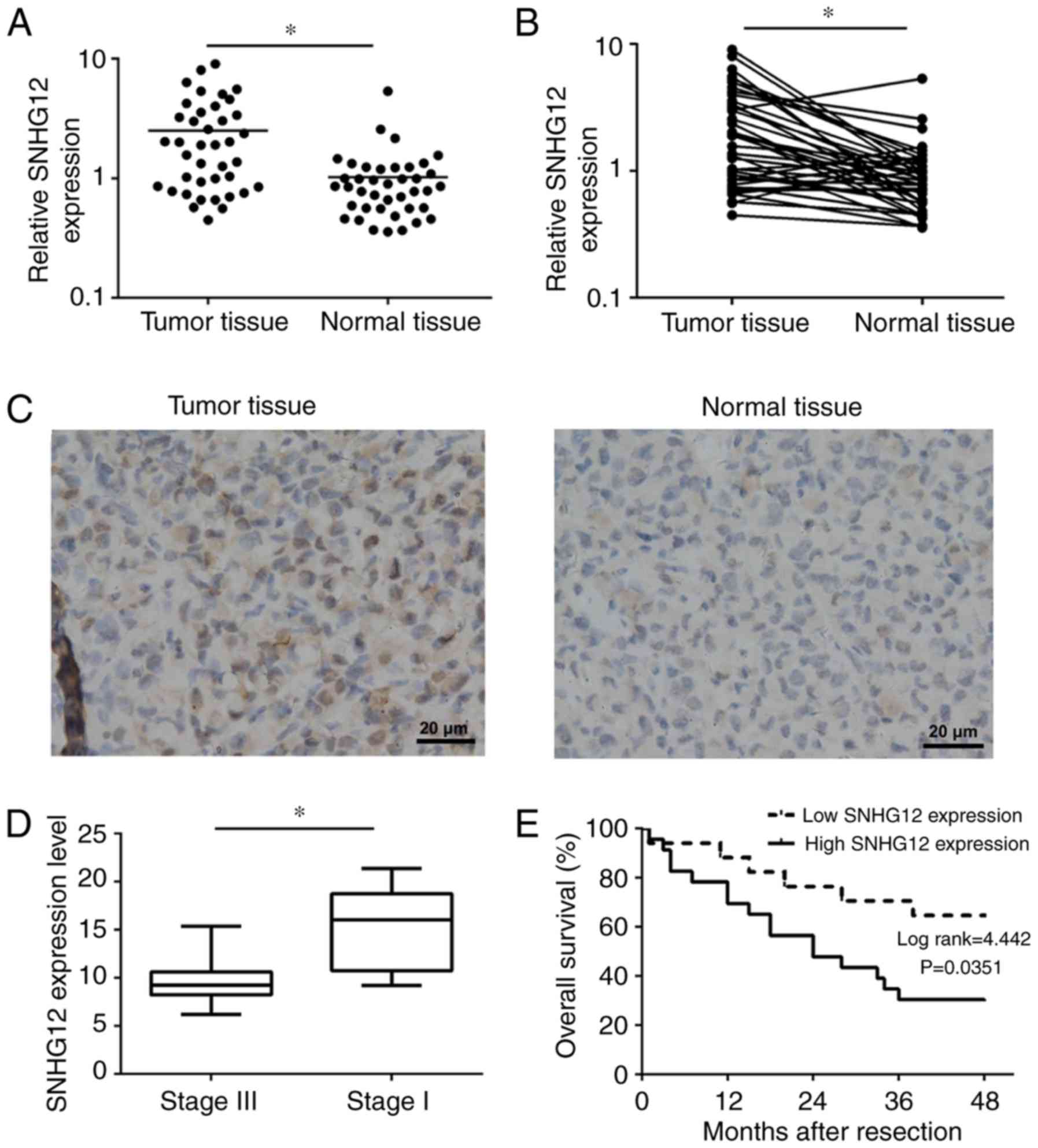
As presented in Fig. 1A and B, SNHG12
was significantly overexpressed in NSCLC tissues (P<0.01).
Furthermore, an ISH assay was also performed to determine SNHG12
expression in tumor tissue sections and adjacent normal tissue
sections. As presented in Fig. 1C,
SNHG12, which was located in the cytoplasm, was markedly
overexpressed in tumor tissue compared with normal tissue. In
addition, the expression level of SNHG12 in advanced NSCLC (stage
III, n=19) was significantly lower compared with that in early
stages (stage I, n=21; P<0.01) (Fig.
1D). Furthermore, for the purpose of determining the
association between SNHG12 expression and OS rate, patients with
NSCLC were divided into a high-expression group (n=23) and a
low-expression group (n=17), according to the median expression
level of all tumor tissues. Compared with the low-expression group,
NSCLC patients from the high-expression group have a significantly
lower OS rate (P=0.0351; Fig. 1E).
These results indicated that SNHG12 was overexpressed in NSCLC and
may be associated with tumor progression and poor prognosis of
NSCLC.
Knockdown of SNHG12 inhibits NSCLC
cell proliferation and induces cell apoptosis
In order to investigate the action of SNHG12 on
NSCLC cell proliferation and apoptosis, A549 and H1299 cells were
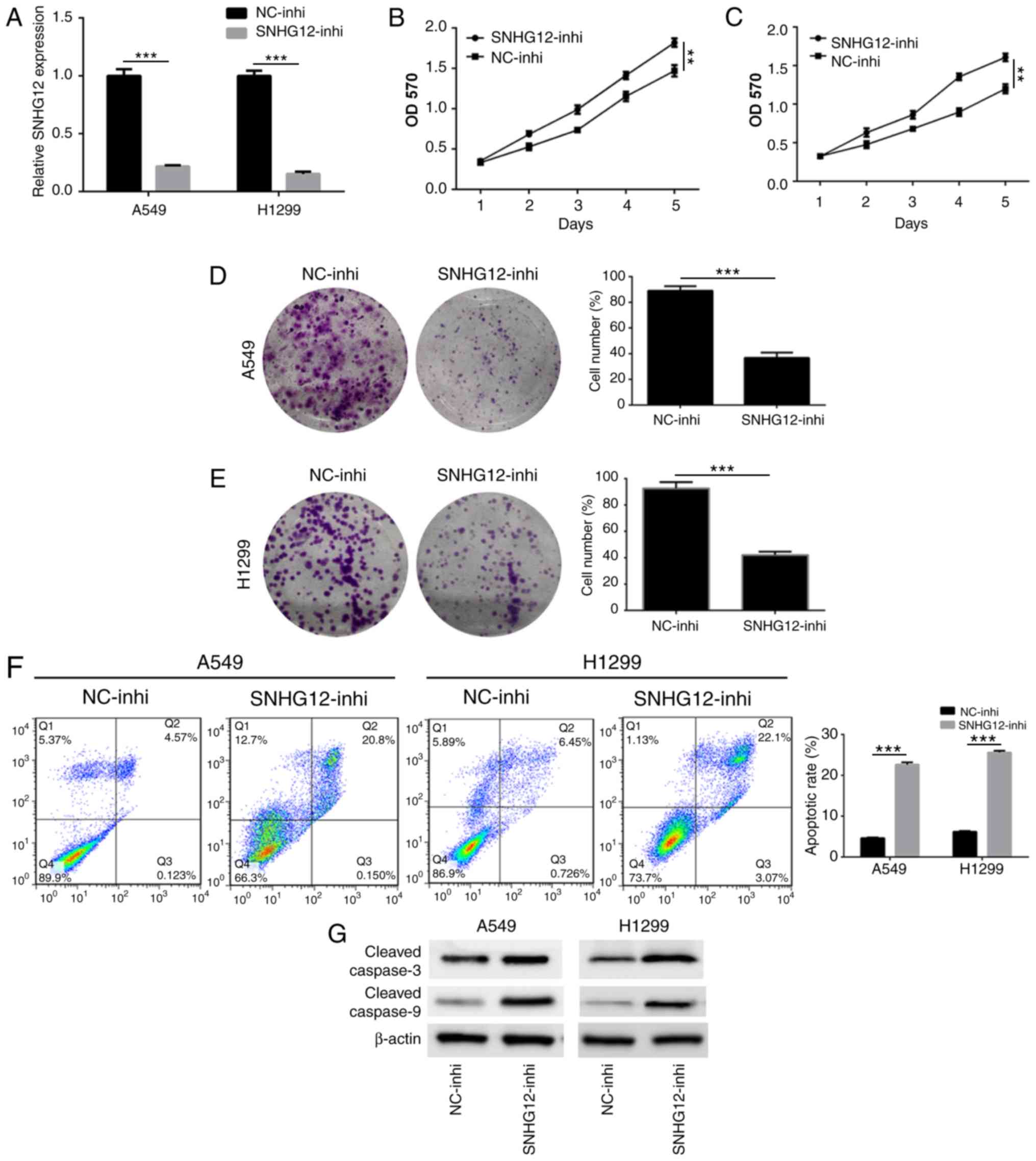
transfected with SNHG12-inhi and NC-inhi. An RT-qPCR assay was used
to assess the effect of SNHG12 knockdown. As presented in Fig. 2A, the expression level of SNHG12 in
A549 and H1299 cells was significantly downregulated following
treatment with SNHG12-inhi (P<0.001), indicating that SNHG12 was
successfully and effectively knocked down. Results of the MTT assay
showed that the viability of A549 and H1299 cells was significantly
decreased following knockdown of SNHG12 (Fig. 2B and C; P<0.001). In addition, a
colony-formation assay was performed to determine cell
proliferation. As presented in Fig. 2D
and E, compared with the control group, the colony-forming
abilities of A549 and H1299 cells were significantly suppressed
following knockdown of SNHG12 (P<0.001). Furthermore, cell
apoptosis induced by knockdown of SNHG12 was also assessed. As
presented in Fig. 2F, the apoptosis
rate of A549 cells which was induced by SNHG12-inhi was 22.4±1.3%,
which was significantly higher compared with that of the control
group (4.2±0.9%; P<0.001). Compared with the control group
(5.1±0.8%), apoptosis of H1299 cells which induced by SNHG12-inhi
was 26.3±1.2% (P<0.001). To further elucidate the underlying
molecular mechanisms of apoptosis induced by SNHG12, western
blotting was used to investigate the caspase-mediated apoptotic
pathway. As presented in Fig. 2G,
knockdown of SNHG12 significantly enhanced expression of cleaved
caspase 3 and cleaved caspase 9, indicating that knockdown of
SNHG12 induced apoptosis through the caspase signaling pathway.
These results indicated that downregulation of SNHG12 significantly
suppressed proliferation and induced apoptosis in NSCLC cells.
Downregulation of SNHG12 strongly
suppresses NSCLC cell migration and invasion by inhibiting the EMT
process
It was identified previously that SNHG12
participates in various biological processes including cell
migration and invasion (26,27). Therefore, cell migration and invasion
assays were used to investigate the function of SNHG12 in NSCLC
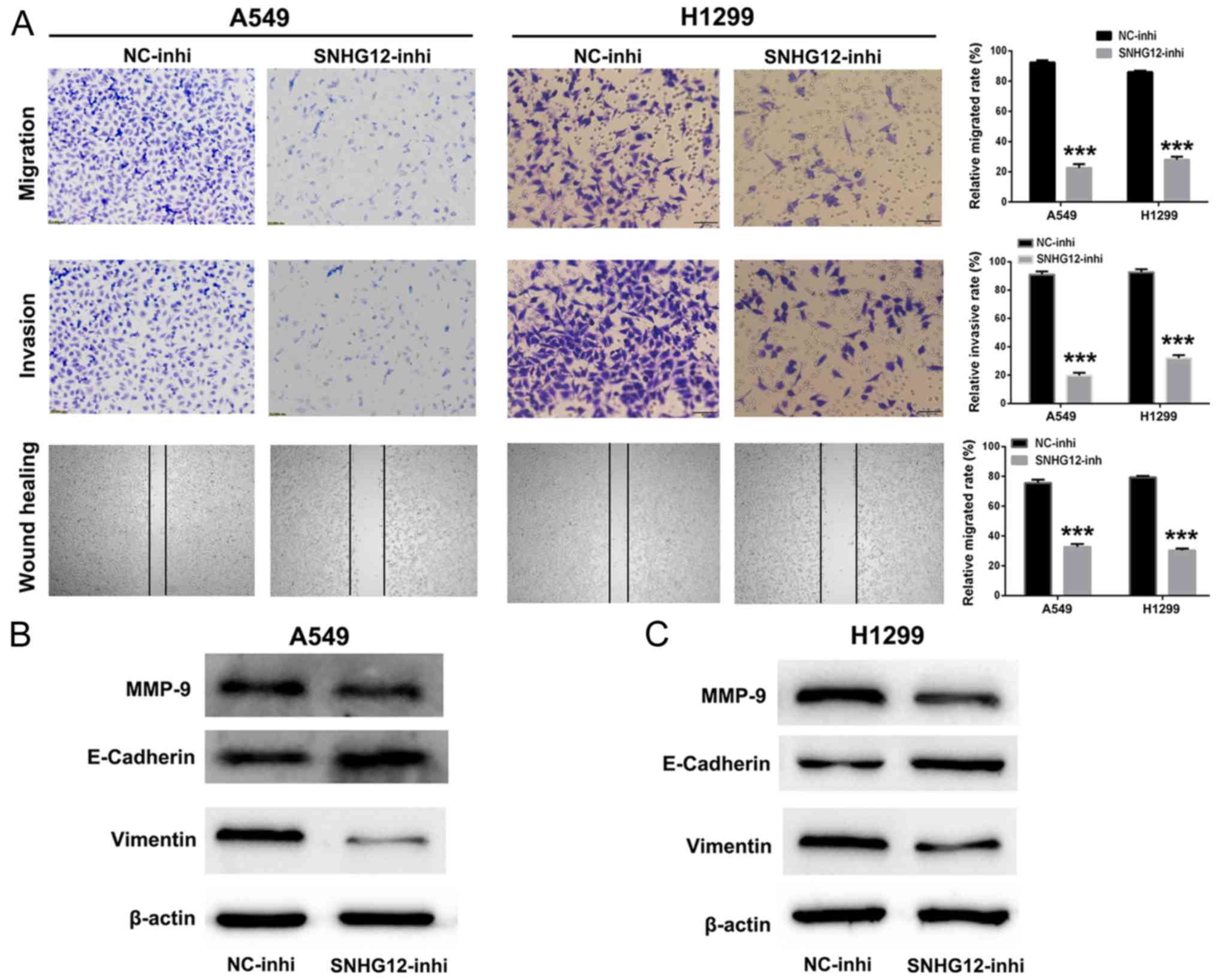
cells. As presented in Fig. 3A,
compared with control group, the numbers of migratory and invasive
A549 and H1299 cells were significantly decreased in the
SNHG12-inhi group (P<0.001). Additionally, the wound healing
assay indicated that the mobility of A549 and H1299 cells treated
with SNHG12-inhi was notably inhibited in comparison with the
control group (P<0.001). These results indicated that knockdown
of SNHG12 exhibited marked suppression of migration and invasion in
NSCLC cells.
Furthermore, western blotting was performed to
investigate further the anti-metastatic mechanism of SNHG12-inhi.
MMPs are associated with the invasive behavior of tumor cells
(28,29). As presented in Fig. 3B and C, the expression level of MMP-9
was decreased in A549 and H1299 cells following treatment with
SNHG12-inhi. EMT has been identified to be an important process for
tumor metastasis (30,31). As presented in Fig. 3B and C, when expression levels of
SNHG12 in A549 and H1299 cells were downregulated by SNHG12-inhi,
the expression level of the mesenchymal marker vimentin was
decreased; in addition, the expression level of the epithelial
marker E-cadherin was upregulated. Collectively, these results
indicated that knockdown of SNHG12 could effectively inhibit
metastasis in NSCLC cells.
SNHG12 interacts directly with miR-218
and serves as its sponge
To further investigate the regulatory mechanism of
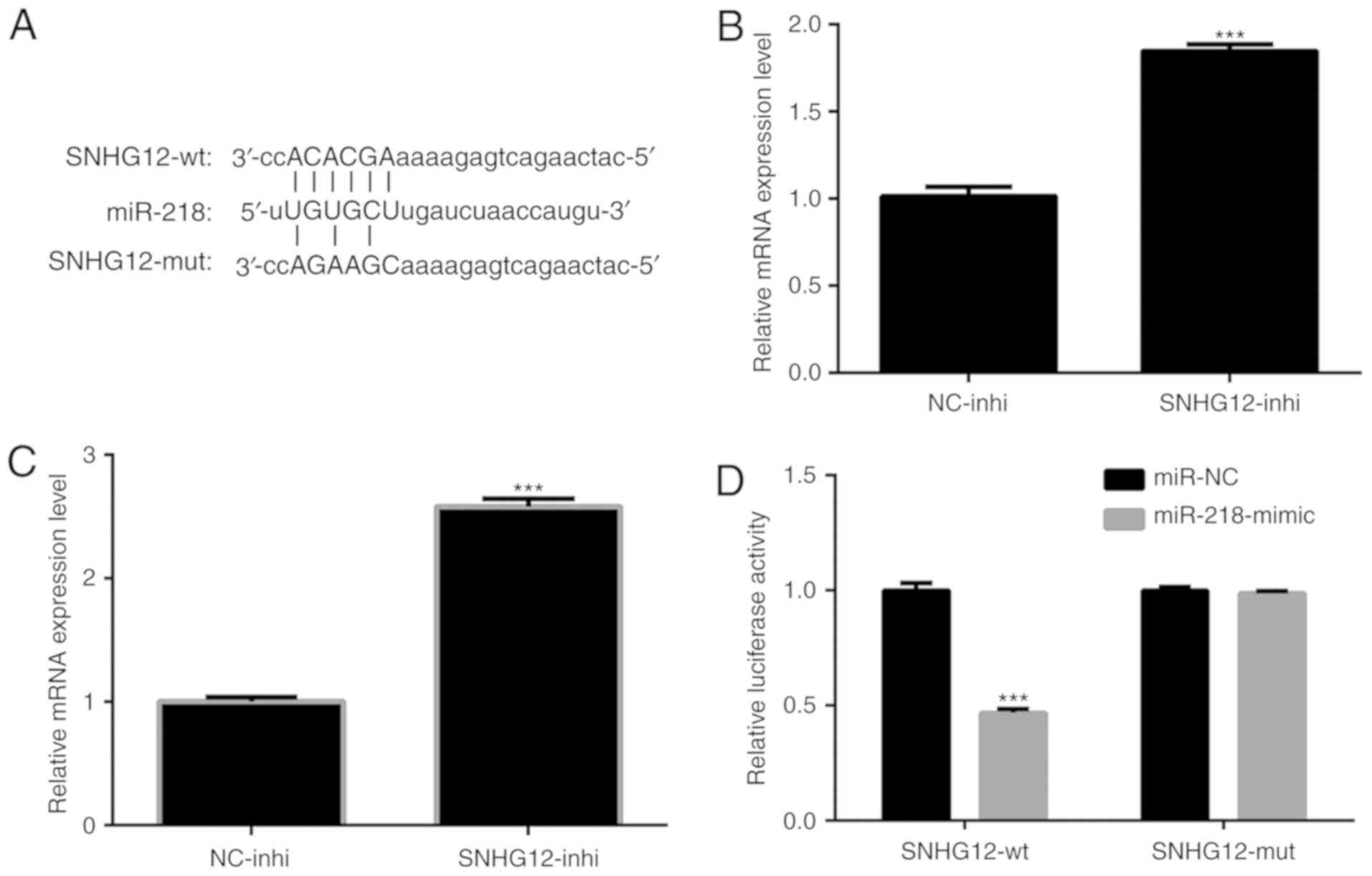
SNHG12 in NSCLC cells, a bioinformatics prediction using miRcode
software (www.mircode.org) was performed. Putative
binding sites for miR-218 on SNHG12 were identified (Fig. 4A). It was determined whether the
expression level of miR-218 was regulated by SNHG12. As presented
in Fig. 4B and C, expression levels
of miR-218 in A549 and H1299 cells were significantly upregulated
by treatment of SNHG12-inhi (P<0.001). To verify that
overexpression of miR-218 was indeed directly regulated by SNHG12,
a dual-luciferase reporter assay was performed.
pmiR-RB-REPORT™-SNHG12-WT and pmiR-RB-REPORT™-SNHG12-MUT were
respectively transfected into A549 cells together with
miR-218-mimic or miR-NC. The results indicated that the luciferase
activity of pmiR-RB-REPORT™-SNHG12-WT was significantly decreased
by miR-218-mimic (Fig. 4D;
P<0.001). In contrast, the luciferase activity of
pmiR-RB-REPORT™-SNHG12-MUT was not affected (Fig. 4D). These results indicated
interactions between miR-218 and the binding sites of SNHG12.
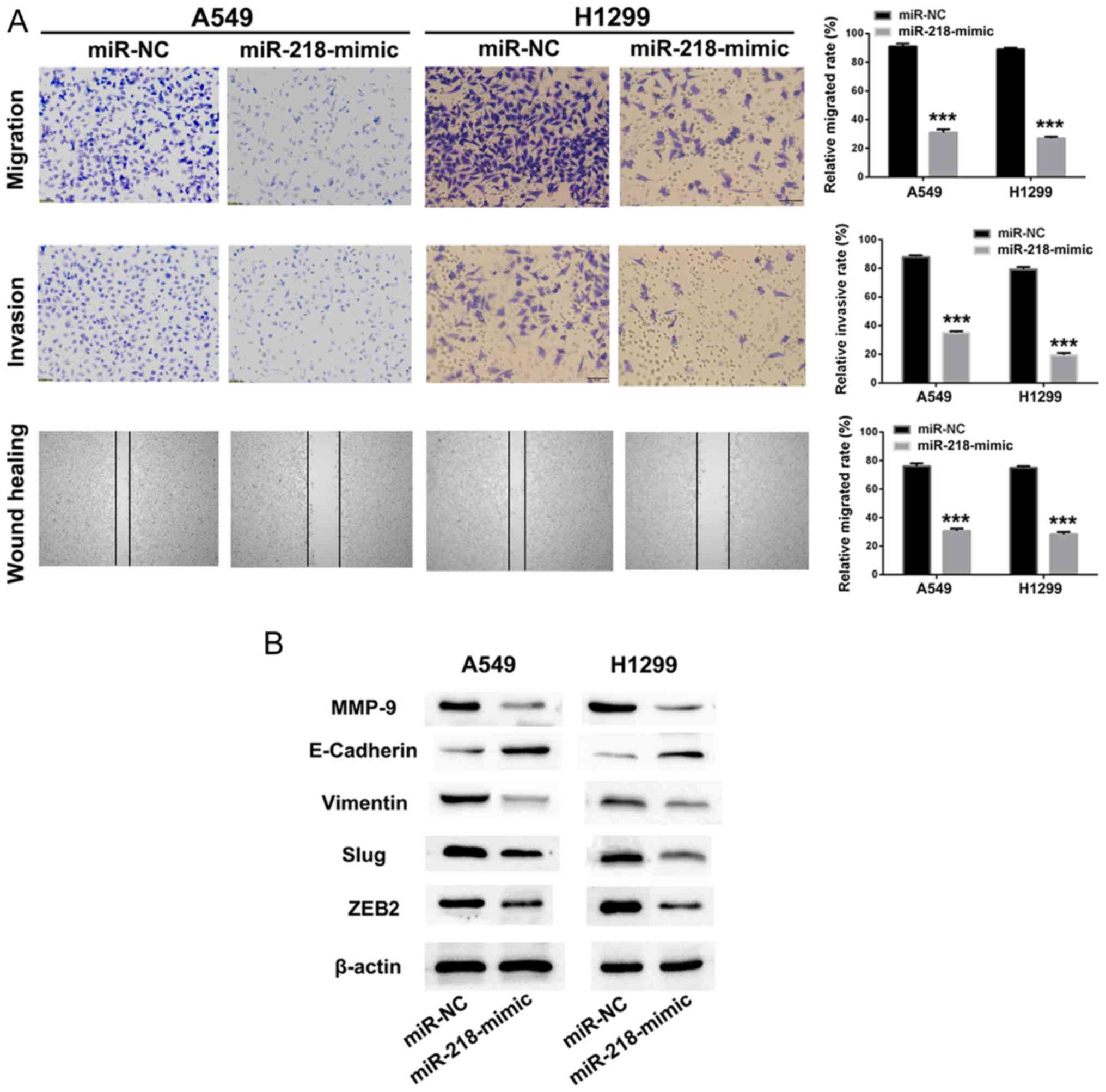
Knockdown of SNHG12 upregulates the
expression level of miR-218, and suppresses NSCLC cells migration
and invasion via the Slug/ZEB2 signaling pathway
As presented in Fig. 4B
and C, knockdown of SNHG12 upregulated the expression level of
miR-218 in A549 and H1299 cells. Knockdown of SNHG12 led to
effective suppression of migration and invasion of NSCLC cells.
Thus, we hypothesized that overexpression of miR-218 could also
impair NSCLC cell migration and invasion. miR-218-mimic or miR-NC
was transfected into A549 and H1299 cells, and cell migration,
invasion and mobility of A549 and H1299 cells were identified to be
significantly inhibited by treatment with miR-218-mimic, in
comparison with the miR-NC group (Fig.
5A; P<0.001).
EMT is a progressive process in which epithelial
cells gradually obtain a mesenchymal (fibroblast-like) cell
phenotype, leading to increased metastasis and invasion (32). It has been identified that EMT is
associated with the progression of lung cancer towards metastasis
and invasion (33). Downregulation of
E-cadherin is a hallmark of the EMT process, and transcription
factors such as Slug, ZEB1 and ZEB2, which are regarded as
regulators of E-cadherin, are the focus of investigation in tumor
metastasis (34–36). Therefore, western blot assays were
used to investigate whether the suppression of metastasis by
SNHG12/miR-218 involves the Slug/ZEB2 signaling pathway. As
presented in Fig. 5B, the
metastasis-associated protein MMP-9 in A549 and H1299 cells was
downregulated following treatment with miR-218-mimic. When treated
with miR-218-mimic, the expression level of the mesenchymal marker
vimentin in A549 and H1299 cells was decreased. Furthermore, the
expression level of E-cadherin, a transcriptional regulator of Slug
and ZEB2, was upregulated following treatment with miR-218-mimic,
resulting in suppression of EMT. These results implied that
knockdown of SNHG12 promoted expression of miR-218 and inhibited
cell migration and invasion via the Slug/ZEB2 EMT signaling
pathway.
Conclusion
In conclusion, the results of the present study
indicated that SNHG12 was overexpressed in NSCLC, and is associated
with tumor progression and poor prognosis. Knockdown of SNHG12
could directly increase the expression level of miR-218 and thus
effectively suppressed NSCLC cell proliferation, migration and
invasion by inhibiting the EMT process via the Slug/ZEB2 signaling
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YW conceived and designed the study. SL and
YY acquired and analyzed the data. YW and YS interpreted the data
and wrote the manuscript. HZ and YW critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethic Review
Committees of The Affiliated Hospital of Hebei University and
written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grønberg BH, Lund-Iversen M, Strøm EH,
Brustugun OT and Scott H: Associations between TS, TTF-1, FR-α,
FPGS, and overall survival in patients with advanced non-small-cell
lung cancer receiving pemetrexed plus carboplatin or gemcitabine
plus carboplatin as first-line chemotherapy. J Thorac Oncol.
8:1255–1264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heist RS and Engelman JA: SnapShot:
Non-small cell lung cancer. Cancer Cell. 21:448.e22012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Song L, Liu X, Yang X, Li X, He T,
Wang N, Yang S, Yu C, Yin T, et al: Artificial virus delivers
CRISPR-Cas9 system for genome editing of cells in mice. ACS Nano.
11:95–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammad IS, He W and Yin L: Understanding
of human ATP binding cassette superfamily and novel multidrug
resistance modulators to overcome MDR. Biomed Pharmacother.
100:335–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song L, Liang X, Yang S, Wang N, He T,
Wang Y, Zhang L, Wu Q and Gong C: Novel
polyethyleneimine-R8-heparin nanogel for high-efficiency gene
delivery in vitro and in vivo. Drug Deliv. 25:122–131. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patrini D, Panagiotopoulos N, Bedetti B,
Mitsos S, Crisci R, Solli P, Bertolaccini L and Scarci M: Surgical
approach in oligometastatic non-small cell lung cancer. Ann Transl
Med. 6:932018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng H and Perez-Soler R: Leptomeningeal
metastases in non-small-cell lung cancer. Lancet Oncol. 19:e43–e55.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang JL, Zheng L, Hu YW and Wang Q:
Characteristics of long non-coding RNA and its relation to
hepatocellular carcinoma. Carcinogenesis. 35:507–514. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen QN, Wei CC, Wang ZX and Sun M: Long
non-coding RNAs in anti-cancer drug resistance. Oncotarget.
8:1925–1936. 2017.PubMed/NCBI
|
|
15
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tripathi V, Shen Z, Chakraborty A, Giri S,
Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A and
Prasanth KV: Long noncoding RNA MALAT1 controls cell cycle
progression by regulating the expression of oncogenic transcription
factor B-MYB. PLoS Genet. 9:e10033682013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ruan W, Wang P, Feng S, Xue Y and Li Y:
Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes cell proliferation and migration by upregulating
angiomotin gene expression in human osteosarcoma cells. Tumour
Biol. 37:4065–4073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuo YL, Li XM and Luo J: Long noncoding
RNA UCA1 modulates breast cancer cell growth and apoptosis through
decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci.
19:3403–3411. 2015.PubMed/NCBI
|
|
20
|
Cao C, Zhang T, Zhang D, Xie L, Zou X, Lei
L, Wu D and Liu L: The long non-coding RNA SNHG6-003, functions as
a competing endogenous RNA to promote the progression of
hepatocellular carcinoma. Oncogene. 36:1112–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Ji G, Ke X, Gu H, Jin W and Zhang
G: MiR-141 inhibits gastric cancer PROLIFERATION BY interacting
with long noncoding RNA MEG3 and down-regulating E2F3 expression.
Dig Dis Sci. 60:3271–3282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Li J, Jia S, Wu M, An J, Zheng Q,
Zhang W and Lu D: miR675 upregulates long noncoding RNA H19 through
activating EGR1 in human liver cancer. Oncotarget. 6:31958–31984.
2015.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lan T, Ma W, Hong Z, Wu L, Chen X and Yuan
Y: Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in
hepatocellular carcinoma. J Exp Clin Cancer Res. 36:112017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang P, Chen D, Ma H and Li Y: LncRNA
SNHG12 contributes to multidrug resistance through activating the
MAPK/Slug pathway by sponging miR-181a in non-small cell lung
cancer. Oncotarget. 8:84086–84101. 2017.PubMed/NCBI
|
|
28
|
Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai
F, Deng H, Chen Y, Liu M and Yi Z: Cucurbitacin E inhibits breast
tumor metastasis by suppressing cell migration and invasion. Breast
Cancer Res Treat. 135:445–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan X, Banerjee P, Guo HF, Ireland S,
Pankova D, Ahn YH, Nikolaidis IM, Liu X, Zhao Y, Xue Y, et al:
Epithelial-to-mesenchymal transition drives a pro-metastatic Golgi
compaction process through scaffolding protein PAQR11. J Clin
Invest. 127:117–131. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krebs AM, Mitschke J, Lasierra Losada M,
Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D,
Reichardt W, Bronsert P, et al: The EMT-activator Zeb1 is a key
factor for cell plasticity and promotes metastasis in pancreatic
cancer. Nat Cell Biol. 19:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo
C, Wang X, Liu H, Deng L, Li C, et al: NEDD9 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Int J Cancer.
134:2294–2304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagaraju GP, Long TE, Park W, Landry JC,
Taliaferro-Smith L, Farris AB, Diaz R and El-Rayes BF: Heat shock
protein 90 promotes epithelial to mesenchymal transition, invasion,
and migration in colorectal cancer. Mol Carcinog. 54:1147–1158.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|