Introduction
Brain tumors are generally divided into two major
categories, primary tumors originating from intracranial tissue and
metastatic tumors originating from other parts (1). Prevalence of metastatic brain tumors is
10-fold that of primary brain tumors, and brain metastases occur in
20–40% of tumor patients (2,3). Brain tumors seriously endanger human
life and health. Brain tumors mostly occur in young adults and
gliomas is the most common type (4).
Genetic factors, radiation exposure, intracranial injury, and viral
factors are correlated with the etiology of brain tumors (5). Due to the high metabolic state of
tumors and the abnormal disorder of blood vessels, oxygen level
required by tumor cells is significantly higher than oxygen supply,
which in turn causes hypoxia of the tumor tissues. It has been
reported that the existence of the hypoxic microenvironment may
cause the recurrence of tumors and increase the degree of
malignancy (6,7).
Hyperbaric oxygen (HBO) is an adjuvant therapy that
plays an important role in the treatment of malignant tumors
(8). HBO is pure oxygen with a
higher pressure than normal atmospheric pressure, and its mechanism
of action mainly depends on increasing the oxygen capacity in
cytoplasm and further increasing oxygen content in cells, resulting
in a decrease in cerebrovascular blood flow due to cerebral
vasoconstriction, and a corresponding decrease in intracranial
pressure (9). Inflammatory factors
can cause tissue damage after brain injury by causing excessive
release of inflammatory mediators, and HBO can improve brain
metabolism and restore brain function (10). In the development of brain tumors,
tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are
polypeptide cytokines involved in inflammation and have a wide
range of biological activities (11). Elevated levels of IL-6 and TNF-α in
serum can cause the body to undergo a stress reaction after brain
injury in order to destroy the blood-brain barrier, and cause
monocytes and neutrophils to enter the brain (12).
The current study aimed to investigate the effect of
HBO on postoperative rehabilitation of brain tumors and the effects
on TNF-α and IL-6 in patients, so as to provide references for the
treatment of brain tumors.
Patients and methods
General information
This is a retrospective study. A total of 132 brain
tumor patients who were admitted to the People's Hospital of Rizhao
(Rizhao, China) from October 2014 to October 2017 were selected.
Those patients included 64 males and 68 females, with an age range
of 13–72 years. There were 62 patients in the observation group and
70 patients in the control group. Patients in the control group
were treated with conventional drugs, and patients in the
observation group were treated with HBO on the basis of
conventional drug therapy. There was no statistically significant
difference in general data between the groups (P>0.05). All the
cases were diagnosed by imaging and postoperative pathological
examinations. The patients were excluded from pregnancy, lactation,
bleeding after brain surgery and contraindications, tumors in other
parts of the body, cerebral thrombosis, liver and kidney
dysfunction, and other diseases. The study was approved by the
Ethics Committee of People's Hospital of Rizhao. All patients or
their family members signed informed consent. General information
is shown in Table I.
 | Table I.General information. |
Table I.
General information.
| Indexes | Observation
(n=62) | Control (n=70) | χ2/t | P-value |
|---|
| Age (years) |
| ≥43 | 29 (46.77) | 35 (50.00) | 0.137 | 0.730 |
|
<43 | 33 (53.23) | 35 (50.00) |
|
|
| Sex |
| Male | 35 (56.45) | 29 (41.43) | 2.971 | 0.116 |
|
Female | 27 (43.55) | 41 (58.57) |
|
|
| Course of disease
(years) |
| ≥2 | 16 (25.81) | 20 (28.57) | 0.127 | 0.845 |
|
<2 | 46 (74.19) | 50 (71.43) |
|
|
| Blood glucose
(mmol/l) | 5.64±2.13 | 5.82±2.09 | 0.489 | 0.625 |
| Hemoglobin (g/l) | 12.24±1.25 | 12.32±1.07 | 0.396 | 0.693 |
| Blood calcium
(mmol/l) | 2.33±0.25 | 2.28±0.31 | 1.012 | 0.314 |
| Hematocrit (%) | 37.25±5.24 | 37.92±5.13 | 0.741 | 0.460 |
| Albumin (g/l) | 39.86±3.56 | 40.18±4.21 | 0.468 | 0.640 |
| Insulin-like growth
factor-I (ng/ml) | 232.53 ±19.28 | 235.46±18.86 | 0.882 | 0.380 |
| Sialic acid
(mg/l) | 252.26±46.84 | 254.31±45.67 | 0.254 | 0.800 |
Reagents and equipment
TNF-α and IL-6 ELISA kits were purchased from Wuhan
Boster Biological Technology, Ltd., Wuhan, China. The Anthus PHOMO
automatic microplate reader was purchased from Shanghai Zhongsheng
Science Development Co., Ltd. (Beijing, China). The KJ-2V4M
Ultrasonic Transcranial Doppler Blood Flow Analyzer was purchased
from Nanjing Kejin Industrial Co., Ltd. (Nanjing, China). Medical
air pressurized cabin was purchased from Guizhou Fenglei Oxygen
Capsules Co., Ltd. Vitamin C was purchased from Guangdong Hengjian
Pharmaceutical Co., Ltd., Jiangmen, China (state approval no.
H44021171).
Two treatment methods
In the control group, routine treatment methods such
as brain neurotrophic drugs, dehydration drugs, hemostatic drugs,
and awakening agents were used, and the observation group was
supplemented with HBO treatment. Medical air pressurized cabin was
used with treatment pressure 0.18 Mpa. Pressure was increased for
25 min and patients were asked to wear mask to absorb pure oxygen
for 60 min, rest for 10 min and decompression for 25 min, once a
day. Routine use of vitamin C (0.1 g/time, 3 times per day) was
performed during BO treatment. Treatment efficacy was reviewed
after 10 HBO treatments.
Detection of serum TNF-α and IL-6
Peripheral venous blood (3 ml) was extracted from
each patient at 1 day before treatment and 1 day after treatment.
Blood was centrifuged at 2,300 × g for 8 min to collect serum.
Serum TNF-α and IL-6 levels were measured by ELISA according to the
instructions of the kit.
Observation indicators
Cerebral arterial flow velocity and spasticity were
measured by cranial color Doppler ultrasono-graphy. Neurological
function deficit (NFD) and activities of daily living (ADL) were
used to evaluate the clinical recovery of the patients. Clinical
efficacy was compared and analyzed.
Statistical analysis
SPSS 17.0 statistical software was used for analysis
(Shanghai Cabit Information Technology Co., Ltd., Shanghai, China).
Chi-square test was used for the comparisons of countable data,
Student's t-test was used for comparison of measurement data, and
paired t-test was used for comparison before and after treatment in
the same group. P<0.05 was considered to indicate a
statistically significant difference.
Result
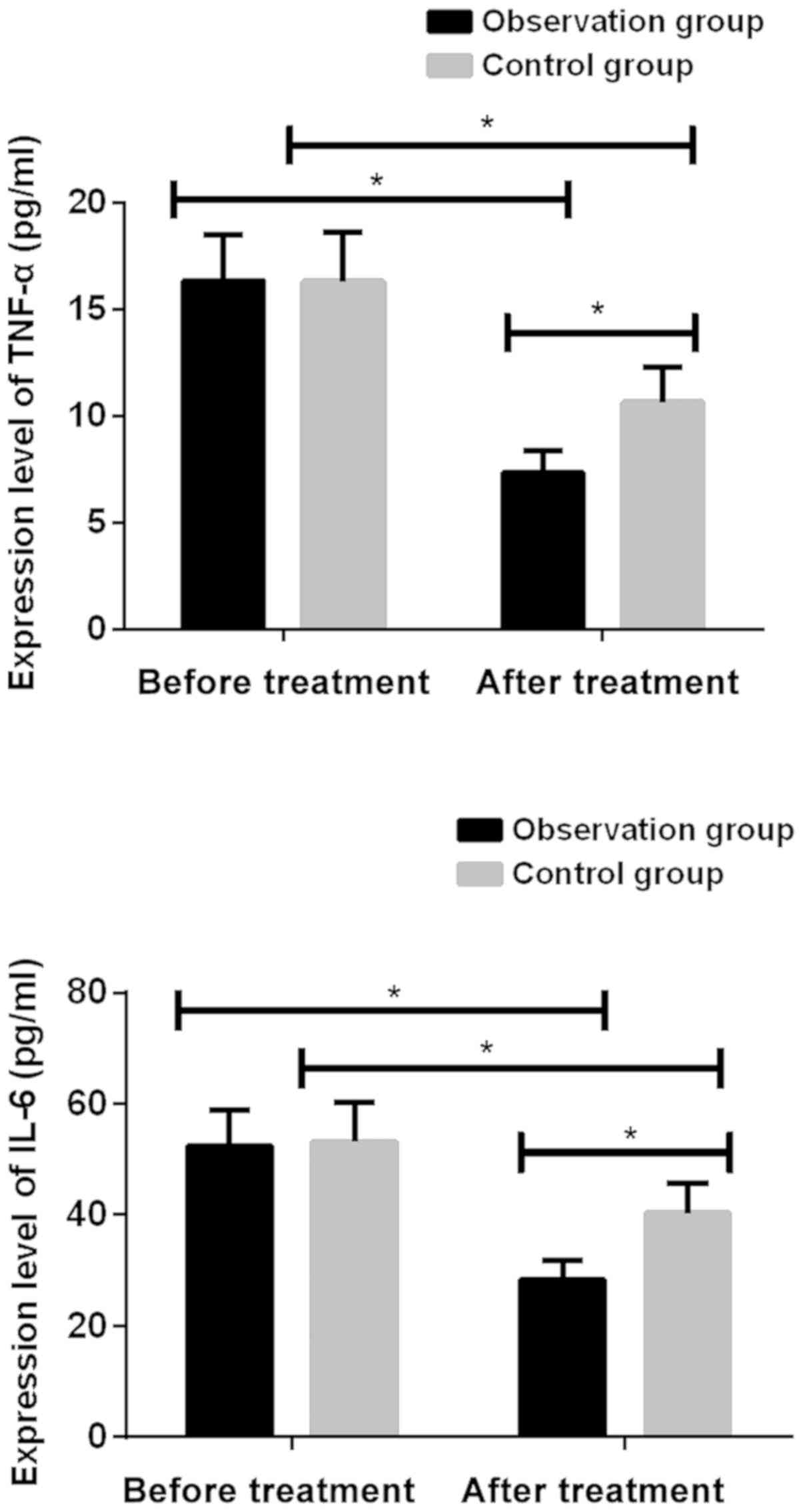
Serum TNF-α and IL-6 levels in the two
groups before and after treatment
Levels of TNF-α and IL-6 in the observation group
were not significantly different from those in the control group
before treatment (P>0.05). Levels of TNF-α and IL-6 in both
groups were significantly reduced after treatment (P<0.001).
After treatment, levels of TNF-α and IL-6 were significantly lower
in the observation group than in the control group (P<0.001)
(Fig. 1; Table II).
 | Table II.Serum TNF-α and IL-6 levels in the
groups before and after treatment. |
Table II.
Serum TNF-α and IL-6 levels in the
groups before and after treatment.
| Groups | Cases | Treatment time | TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|
| Observation | 62 | Before | 16.35±2.15 | 52.35±6.58 |
|
|
| After |
7.35±1.04a |
28.35±3.42a |
|
|
| t value | 29.67 | 25.48 |
|
|
| P-value | <0.001 | <0.001 |
| Control | 70 | Before | 16.32±2.28 | 53.08±7.12 |
|
|
| After | 10.65±1.64 | 40.27±5.46 |
|
|
| t value | 16.89 | 11.94 |
|
|
| P-value | <0.001 | <0.001 |
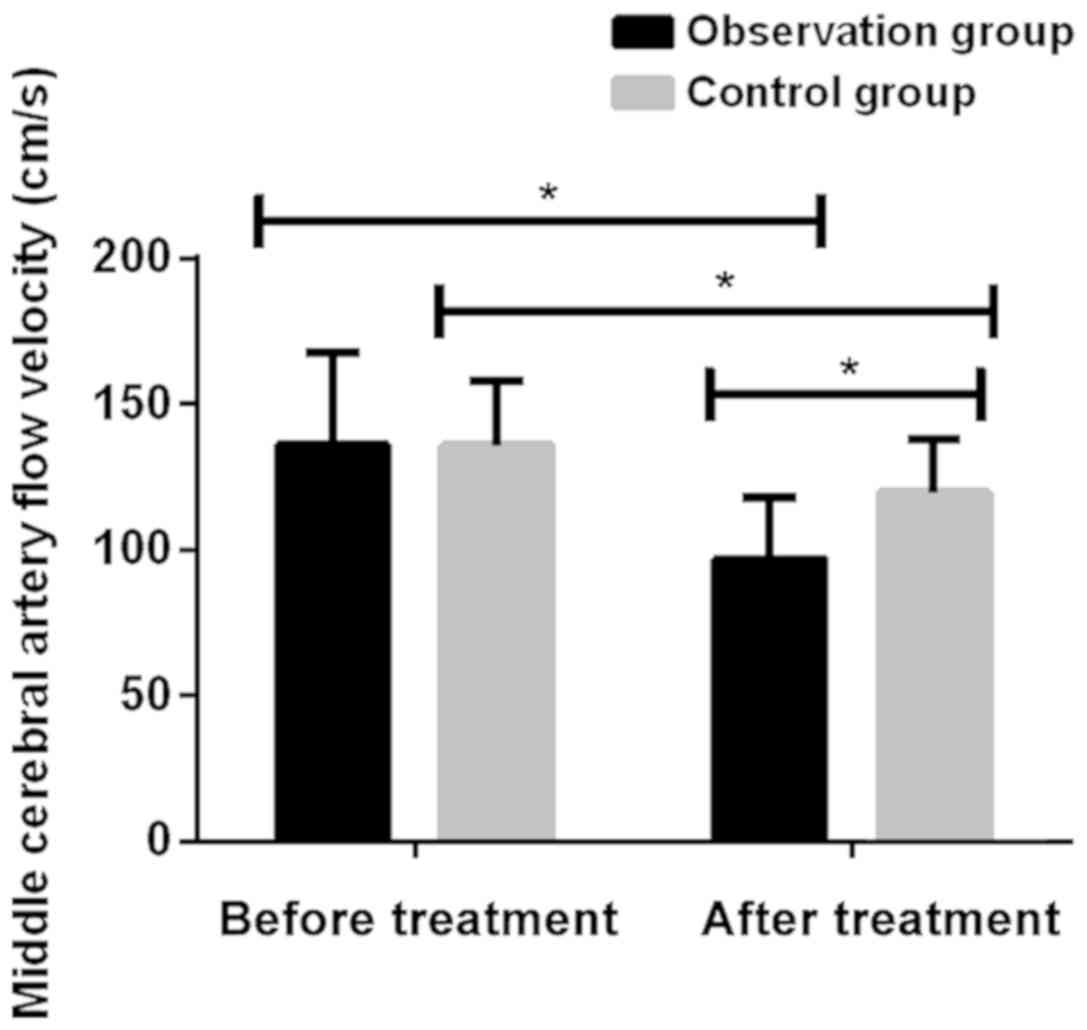
Comparison of flow velocity of middle
cerebral artery by transcranial Doppler before and after treatment
in two groups of patients
There was no significant difference in the flow rate
of cerebral arteries between the observation and the control groups
before treatment (P>0.05). Flow rate of cerebral arteries in
both groups were significantly reduced after treatment
(P<0.001). Cerebral arterial flow velocity in the observation
group after treatment of 96.74±20.86 cm/sec was significantly lower
than that in the control group 119.52±18.27 cm/sec (P<0.001)
(Fig. 2; Table III).
 | Table III.Comparison of cerebral arterial flow
velocity in the two groups before and after treatment. |
Table III.
Comparison of cerebral arterial flow
velocity in the two groups before and after treatment.
| Groups | Cases | Treatment time | Cerebral arterial
flow velocity (cm/sec) | t value | P-value |
|---|
| Observation | 62 | Before | 135.89±31.42 | 8.174 | <0.001 |
|
|
| After |
96.74±20.86a |
|
|
| Control | 70 | Before | 135.78±21.84 | 4.778 | <0.001 |
|
|
| After | 119.52±18.27 |
|
|
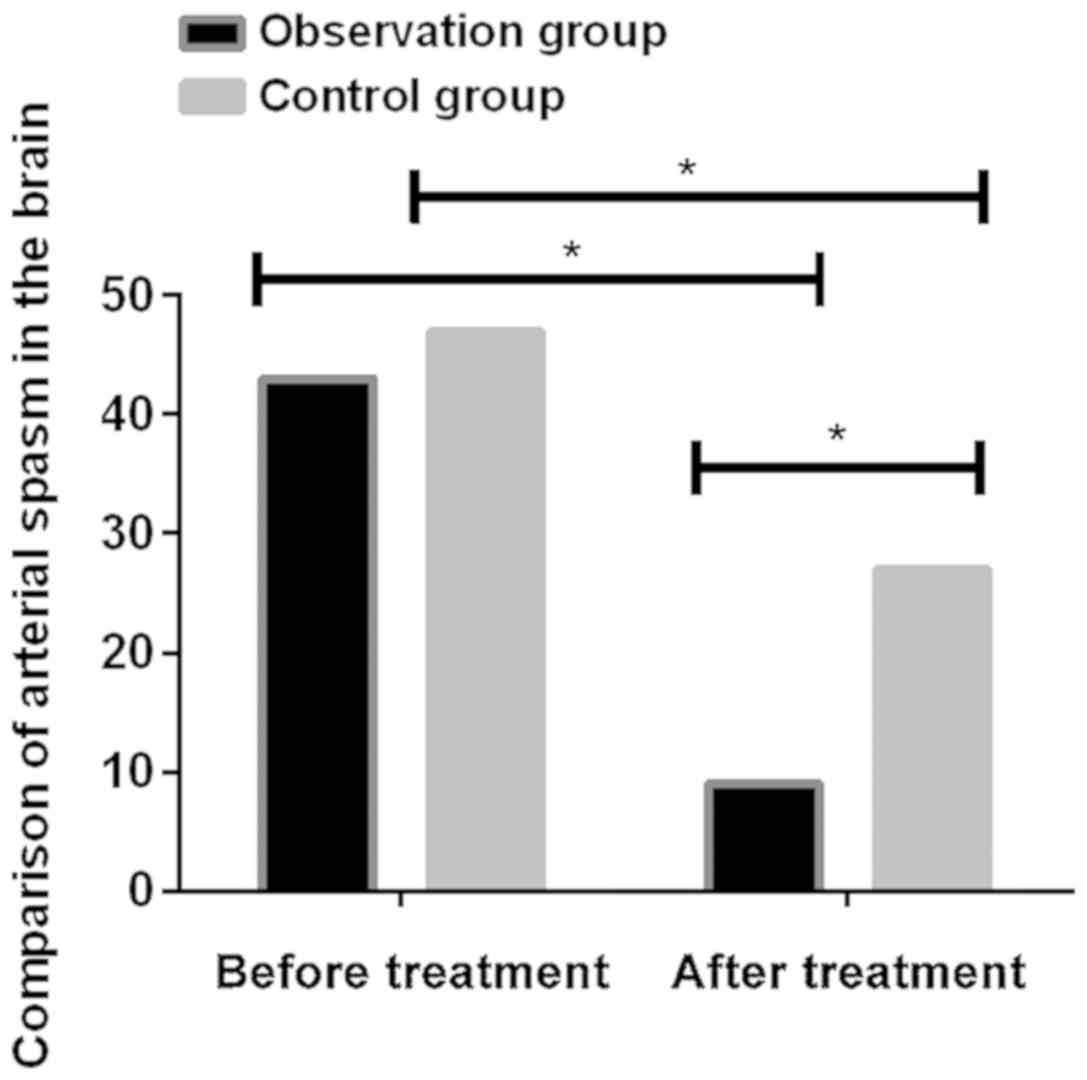
Comparison of cerebral arterial spasm
between the two groups before and after treatment
Before treatment, there was no significant
difference in the incidence of arterial spasm between the
observation and the control groups (P>0.05). Incidence of
arterial spasm was significantly decreased after treatment in both
groups (P<0.001). After treatment, there were 9 patients with
cerebral arterial spasm in the observation group and 27 patients
with cerebral arterial spasm in the control group. Significant
differences were found between the two groups (P<0.05) (Fig. 3; Table
IV).
 | Table IV.Comparison of cerebral arterial spasm
between the two groups before and after treatment. |
Table IV.
Comparison of cerebral arterial spasm
between the two groups before and after treatment.
| Groups | Cases | Treatment time | Cerebral arterial
spasm, n (%) | χ2 | P-value |
|---|
| Observation | 62 | Before | 43 (69.35) | 38.29 | <0.001 |
|
|
| After | 9
(14.52)a |
| Control | 70 | Before | 47 (67.14) | 11.47 | <0.001 |
|
|
| After | 27 (38.57) |
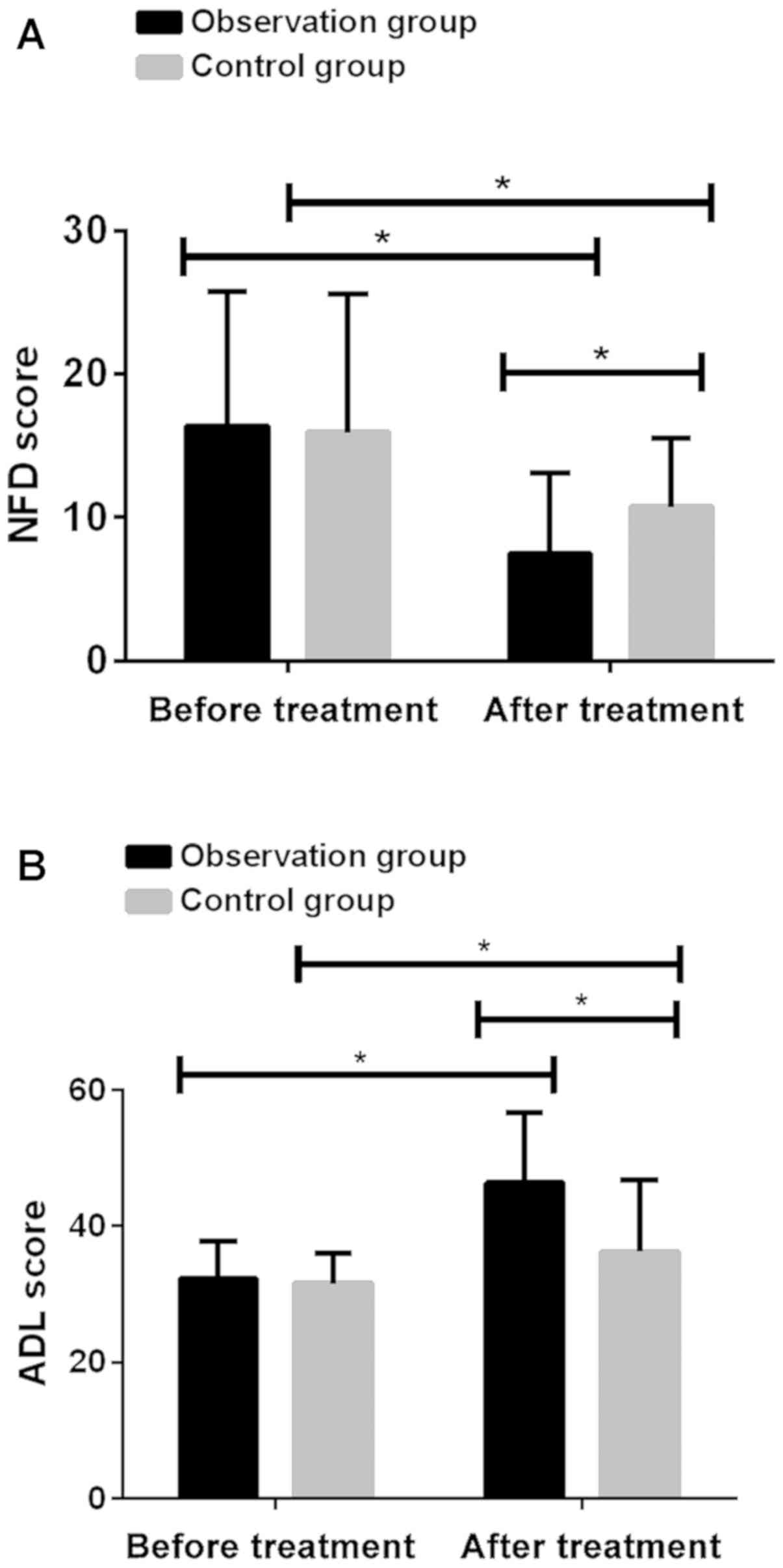
Comparison of NFD and ADL scores
before and after treatment in two groups of patients
Before treatment, there was no significant
difference in NFD and ADL scores between two groups (P>0.05).
After treatment, NFD scores were significantly reduced and ADL
scores were significantly increased (P<0.001). NFD score in the
observation group after treatment (7.52±5.57) was significantly
lower than that in the control group (10.74±4.75; P<0.001) and
ADL score in the observation group (46.38±10.27) was significantly
higher than that in the control group (36.28±10.51; P<0.001)
(Fig. 4; Table V).
 | Table V.Comparison of NFD and ADL scores
before and after treatment in two groups of patients. |
Table V.
Comparison of NFD and ADL scores
before and after treatment in two groups of patients.
| Groups | Cases | Treatment time | NFD score | ADL score |
|---|
| Observation | 62 | Before | 16.35±9.38 | 32.25±5.46 |
|
|
| After |
7.52±5.57a |
46.38±10.27a |
|
|
| t value | 6.373 | 9.566 |
|
|
| P-value | <0.001 | <0.001 |
| Control | 70 | Before | 15.93±9.64 | 31.56±4.39 |
|
|
| After | 10.74±4.75 | 36.28±10.51 |
|
|
| t value | 4.041 | 3.467 |
|
|
| P-value | <0.001 | <0.001 |
Discussion
Brain tumor is a common neurosurgical malignancy in
clinical practice. Continuous growth of intracranial tumors will
compress surrounding tissues, resulting in nerve compression and
cerebral edema (13). Studies have
shown that most patients with brain tumors have symptoms of
intracranial hypertension (14).
Surgical treatment is currently the preferred treatment for brain
tumors in clinical practice, but it cause some trauma to nerve
tissue while removing tumors (15).
Brain function recovery is a long process (16). HBO has a significant effect on
decompression sickness, hypoxic-ischemic encephalopathy, and
anaerobic infections (17–19). HBO has the following characteristics:
it is beneficial to improve tissue hypoxia; it has significant
curative effect on decompression sickness and thrombosis; it can
reduce tissue edema, and brain edema can be controlled; and it can
inhibit the growth of some aerobic and anaerobic bacteria (20,21).
Results of this study showed that levels of TNF-α
and IL-6 in the observation group were significantly lower than
those in the control group after treatment (P<0.001). Findings
reported by Chen et al (22)
are basically consistent with our results, suggesting that HBO
therapy can improve the inflammatory response in patients with
brain tumors. It has been reported that the course of brain tumors
is related to the efficacy of HBO therapy. Early use of HBO has
important implications for the reduction of inflammatory factors,
improvement of hypoxic symptoms, recovery of nerve function and
brain function (23). In this study,
cerebral arterial flow velocity in the observation group after
treatment (96.74±20.86 cm/sec) was significantly lower than that in
the control group (119.52±18.27 cm/sec; P<0.001). After
treatment, there were 9 patients with arterial spasm in the
observation group and 27 patients with arterial spasm in the
control group. The difference was statistically significant
(P<0.05).
After treatment, NFD score in the observation group
after treatment (7.52±5.57) was significantly lower than that in
the control group (10.74±4.75; P<0.001) and ADL score in the
observation group (46.38±10.27) was significantly higher than that
in the control group (36.28±10.51; P<0.001). Similar results
were reported by Xu (24) and Lim
et al (25). Control group
was treated with conventional drugs, and combined use of HBO was
performed in the treatment group and better efficacy was achieved.
HBO is an ideal treatment for postoperative rehabilitation of brain
tumor patients. HBO reduces flow of cerebral arteries in patients
with brain tumors, so symptoms of hypoxia were improved. At the
same time, HBO can also improve the ability of the body to
sterilize and engulf necrotic cells, so as to achieve the
elimination of lesions (26).
Without timely treatment, brain cells around the tumor ‘ischemic
penumbra area’ will die and patients' life and health will be
endangered.
In conclusion, conventional therapy plus HBO is more
effective than conventional therapy alone in postoperative
rehabilitation of brain tumors. It can improve the inflammatory
response of brain tumor patients, reduce the flow rate of cerebral
arteries, and effectively reduce the number of patients with
cerebral arterial spasm. At the same time, it lowers NFD and
improves ADL. Therefore, it should be popularized in clinical
practices.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH drafted the manuscript. SH and GW were mainly
devoted to collecting and interpreting the general data. JL and HC
performed ELISA. SH and CC interpreted cranial color Doppler
ultrasonography result. All authors read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
People's Hospital of Rizhao (Rizhao, China). Signed informed
consents were obtained from the patients or the guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sonoda J and Wharton RP: Drosophila Brain
Tumor is a translational repressor. Genes Dev. 15:762–773. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabrese C, Poppleton H, Kocak M, Hogg
TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et
al: A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winkler F, Kozin SV, Tong RT, Chae SS,
Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E,
et al: Kinetics of vascular normalization by VEGFR2 blockade
governs brain tumor response to radiation: Role of oxygenation,
angiopoietin-1, and matrix metalloproteinases. Cancer Cell.
6:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amariglio N, Hirshberg A, Scheithauer BW,
Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M,
Waldman D, Leider-Trejo L, et al: Donor-derived brain tumor
following neural stem cell transplantation in an ataxia
telangiectasia patient. PLoS Med. 6:e10000292009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kircher MF, de la Zerda A, Jokerst JV,
Zavaleta CL, Kempen PJ, Mittra E, Pitter K, Huang R, Campos C,
Habte F, et al: A brain tumor molecular imaging strategy using a
new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med.
18:829–834. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bondy ML, Scheurer ME, Malmer B,
Barnholtz-Sloan JS, Davis FG, Il'yasova D, Kruchko C, McCarthy BJ,
Rajaraman P, Schwartzbaum JA, et al: Brain Tumor Epidemiology
Consortium. Brain tumor epidemiology: Consensus from the Brain
Tumor Epidemiology Consortium. Cancer 113 (Suppl). 1953–1968. 2008.
View Article : Google Scholar
|
|
7
|
Sadetzki S, Chetrit A, Freedman L, Stovall
M, Modan B and Novikov I: Long-term follow-up for brain tumor
development after childhood exposure to ionizing radiation for
tinea capitis. Radiat Res. 163:424–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marois P: Hyperbaric oxygen treatment. Ann
Neurol. 74:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogawa K, Yoshii Y, Inoue O, Toita T, Saito
A, Kakinohana Y, Adachi G, Iraha S, Tamaki W, Sugimoto K, et al:
Phase II trial of radiotherapy after hyperbaric oxygenation with
chemotherapy for high-grade gliomas. Br J Cancer. 95:862–868. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luna-Oliva L, Ortiz-Gutiérrez RM, Cano-de
la Cuerda R, Piédrola RM, Alguacil-Diego IM, Sánchez-Camarero C and
Martínez Culebras Mdel C: Kinect Xbox 360 as a therapeutic modality
for children with cerebral palsy in a school environment: A
preliminary study. NeuroRehabilitation. 33:513–521. 2013.PubMed/NCBI
|
|
11
|
McIntyre S, Taitz D, Keogh J, Goldsmith S,
Badawi N and Blair E: A systematic review of risk factors for
cerebral palsy in children born at term in developed countries. Dev
Med Child Neurol. 55:499–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nordberg A, Miniscalco C, Lohmander A and
Himmelmann K: Speech problems affect more than one in two children
with cerebral palsy: Swedish population-based study. Acta Paediatr.
102:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan X, Mikolaenko I, Elhassan I, Ni X,
Wang Y, Ball D, Brat DJ, Perry A and Eberhart CG: Notch1 and notch2
have opposite effects on embryonal brain tumor growth. Cancer Res.
64:7787–7793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schneweis S, Grond M, Staub F, Brinker G,
Neveling M, Dohmen C, Graf R, Heiss WD and Shuaib A: Predictive
value of neurochemical monitoring in large middle cerebral artery
infarction. Stroke. 32:1863–1867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao YP, Zhang YQ, Duan HY, Ma Y, Liang H,
Zhang QH, Xue CQ, Luo B and Pan X: Intracranial mixed germ cell
tumor. Zhonghua Yi Xue Za Zhi. 97:661–665. 2017.(In Chinese).
PubMed/NCBI
|
|
16
|
Tian L, Lin Q and Zhang J: To explore the
community rehabilitation assessment scales for patients with stroke
sequelae. Glob J Cardiovasc Cerebrovasc Dis. 3:16–20. 2015.
|
|
17
|
Kranke P, Bennett M, Roeckl-Wiedmann I and
Debus S: Hyperbaric oxygen therapy for chronic wounds. Cochrane
Database Syst Rev. 14:CD0041232004.
|
|
18
|
Bennett MH, Feldmeier J, Hampson N, Smee R
and Milross C: Hyperbaric oxygen therapy for late radiation tissue
injury. Cochrane Database Syst Rev. 5:CD0050052005.
|
|
19
|
Thom SR: Hyperbaric-oxygen therapy for
acute carbon monoxide poisoning. N Engl J Med. 347:1105–1106. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin W, Badr AE, Mychaskiw G and Zhang JH:
Down regulation of COX-2 is involved in hyperbaric oxygen treatment
in a rat transient focal cerebral ischemia model. Brain Res.
926:165–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palzur E, Vlodavsky E, Mulla H, Arieli R,
Feinsod M and Soustiel JF: Hyperbaric oxygen therapy for reduction
of secondary brain damage in head injury: An animal model of brain
contusion. J Neurotrauma. 21:41–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen LF, Tian YF, Lin CH, Huang LY, Niu KC
and Lin MT: Repetitive hyperbaric oxygen therapy provides better
effects on brain inflammation and oxidative damage in rats with
focal cerebral ischemia. J Formos Med Assoc. 113:620–628. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schellart NA, Reits D, van der Kleij AJ
and Stalpers LJ: Hyperbaric oxygen treatment improved
neurophysiologic performance in brain tumor patients after
neurosurgery and radiotherapy: A preliminary report. Cancer.
117:3434–3444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu WZ: Clinical effect of hyperbaric
oxygen therapy in patients with brain tumor and cerebral aneurysm.
Chin J Practical Nervous Diseases. 20:61–62. 2017.
|
|
25
|
Lim SW, Wang CC, Wang YH, Chio CC, Niu KC
and Kuo JR: Microglial activation induced by traumatic brain injury
is suppressed by postinjury treatment with hyperbaric oxygen
therapy. J Surg Res. 184:1076–1084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin L: Effectiveness of postoperative
hyperbaric oxygen interventional therapy for patients with brain
tumor and cerebral aneurysm. Chin J General Practice. 11:684–685.
2013.
|