Introduction
Lung cancer is a commonly diagnosed cancer, and it
is the leading cause of cancer deaths worldwide (1). Lung cancer is divided into two major
histological types: Small cell lung cancer (SCLC) and non-small
cell lung cancer (NSCLC). NSCLC represents 80–85% of all lung
cancers, and the most common subtype of NSCLC is adenocarcinoma
(2). Recent advances in the
molecular biology of lung cancer have revealed many genetic and
epigenetic alterations, and some of them have been found to be
druggable oncogenic drivers, such as EGFR mutations, BRAF
V600E mutations and fusions of ALK, ROS1 or RET
(3,4). The proto-oncogene KRAS is one of
the common driver mutations in lung adenocarcinoma, and it is
mutated in approximately 25% of these patients (5). KRAS encodes a small GTP-binding
protein that is involved in many cellular processes by regulating
multiple signaling cascades (6).
Wild-type KRAS has intrinsic GTPase activity because it catalyzes
the hydrolysis of GTP bound to GDP; however, KRAS mutations
impair GTPase activity, resulting in the dysregulation of its
downstream pathways and effectors when it is in the GTP-bound form.
Given that meta-analyses have shown that KRAS mutations are
associated with an unfavorable prognosis in patients with NSCLC
(7,8), targeting oncogenic KRAS-driven
NSCLC is highly important. Moreover, there are no clinical trials
indicating therapeutic efficacy against KRAS-mutated NSCLC
(9). For instance, a randomized
phase II study indicated that compared with docetaxel, the
mitogen-activated protein kinase kinase (MEK) inhibitor trametinib
did not improve progression-free survival for previously treated
patients with KRAS-mutated NSCLC (10). Additionally, a phase III study of the
MEK inhibitor selumetinib plus docetaxel did not show preferred
clinical activity compared with docetaxel alone in NSCLC patients
with KRAS mutations (11).
Thus, the KRAS mutation remains undruggable, and developing
therapeutic strategies against oncogenic KRAS-driven NSCLC
is urgently needed.
We previously assessed the growth-inhibitory effect
of short hairpin RNA (shRNA)-mediated knockdown of mutant
KRAS in combination with various molecular inhibitors; we
found that mutant KRAS knockdown sensitized NSCLC cells to a
p38 inhibitor (12). In the current
study, we adopted MEK inhibitors as alternatives to mutant
KRAS knockdown in combination with p38 inhibitors to
evaluate the impact of dual MEK and p38 inhibition on the tumor
growth of KRAS-mutated NSCLC cells.
Materials and methods
Cell lines and reagents
KRAS mutant NSCLC cell lines NCI-H23,
NCI-H157, NCI-H460 and NCI-H1792 were kindly provided by Drs John
D. Minna and Adi F. Gazdar of the University of Texas Southwestern
Medical Center at Dallas. The cancer cells were cultured in
RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 5% fetal bovine serum. The reagents selumetinib (Selleck
Chemicals, Houston, TX, USA), LY2228820 (Selleck Chemicals),
PD0325901 (Sigma-Aldrich), and p38 MAP Kinase Inhibitor V
(Calbiochem, San Diego, CA, USA) were purchased from commercial
suppliers.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of MAPK14 and
GAPDH were determined by real-time RT-PCR as previously
described (13). TaqMan probe and
primer sets for these genes were purchased from Applied Biosystems
(Carlsbad, CA, USA). Total RNA was extracted using an RNeasy mini
kit (QIAGEN, Valencia, CA, USA), and cDNA was synthesized using 2
µg of total RNA with Superscript VILO MasterMix (Invitrogen,
Carlsbad, CA, USA) and the oligo (dT) primer system (Invitrogen).
qPCR was performed using a LightCycler 480 system (Roche
Diagnostics, Tokyo, Japan). For quantitative analysis, the
GAPDH gene was used as an internal reference gene to
normalize the input cDNA. The comparative Ct method was used to
compute the relative expression values.
Use of synthetic small interfering
RNA
siRNAs targeting MAPK14 were obtained from
the siGENOME library (Dharmacon Inc., Lafayette, CO, USA). An siRNA
against Tax was used as a non-targeting control as
previously described (13). The
cells were transfected with 10 nM siRNA using Lipofectamine RNAiMAX
transfection reagent (Invitrogen) according to the manufacturer's
protocol. After 48 h, the cells were harvested to verify target
gene silencing.
Cell proliferation/viability
assays
Eighteen h after plating 1.5×105
trypan-negative cells per well on 6-well plates, the cells were
treated with the inhibitors or DMSO alone. After 24, 48 and 72 h,
trypan-negative cells were counted by a TC10 Automated Cell Counter
(Bio-Rad, Richmond, CA, USA). In addition, 18 h after plating 5,000
trypan-negative cells per well on 96-well plates, these cells were
treated with the inhibitors or DMSO alone. After 48 or 72 h, the
cell viabilities were evaluated by a CellTiter-Glo luminescent cell
viability assay (Promega, Madison, WI, USA).
Colony formation assay
Colony formation assays were performed as described
previously (12). Briefly, 24 h
after siRNA transfection, the cells were harvested, and 1,000
trypan blue-negative cells were then replated for colony formation
in liquid culture. After 24 h, the cells were treated with the
inhibitors or DMSO alone. The culture media with the inhibitors was
exchanged every 3 days during culture, and the colonies were
stained with methylene blue 14 days after the initial
treatment.
DNA fragment detection by ELISA
After plating in 96-well plates in replicates of 6,
10,000 trypan blue-negative cells were treated with the inhibitors
or DMSO alone. Forty-eight h after the treatment, the cells were
assayed by the cytoplasmic histone-associated DNA fragment method
using a Cell Death Detection ELISA Plus Kit (Roche Diagnostics,
Tokyo, Japan) according to the manufacturer's protocol.
Apoptotic cell detection by Annexin
V-fluorescein staining
Four days after siRNA transfection, the cells were
double-stained using an Annexin V-FLUOS kit (Roche Diagnostics) and
Hoechst 33342 solution (Molecular Probes, Eugene, OR, USA) as
previously described (13). The
stained cells were viewed immediately using a fluorescence
microscope (Keyence, Osaka, Japan; Model BZ-8100), and the cells
positive for annexin-V were considered apoptotic. The cells
visualized by Hoechst staining were counted in 12 randomly selected
microscopic fields, and the percentage of apoptotic cells was
calculated by dividing the number of Annexin V-positive cells by
the total number of cells. The results were obtained from two
independent experiments.
Western blotting
Western blotting was performed as described
previously (14). Briefly, after
serum starvation for 12 h, cells were treated with selumetinib or
U0126 for 12 h, and then whole cell lysates were prepared using
RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
separated on SDS/polyacrylamide gels, and electroblotted onto
nitrocellulose membranes (Bio-Rad). The membranes were incubated
with a phospho-p38 MAPK (Thr180/Tyr182) XP rabbit antibody, p38
MAPK rabbit antibody, phospho-p44/42 MAPK (Thr202/Tyr204) rabbit
antibody and p44/42 MAPK rabbit antibody, all of which were
purchased from Cell Signaling Technology (Beverly, MA, USA). The
membranes were also incubated with anti-Actin mouse monoclonal
antibody (Sigma-Aldrich), which was used as a loading control. The
membranes were developed with peroxidase-labeled anti-mouse or
anti-rabbit IgG antibodies (Amersham Pharmacia, Piscataway, NJ,
USA) and Super Signal chemiluminescence substrate (Thermo
Scientific, Rockford, IL, USA). The densitometry data were obtained
from three independent experiments using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data were statistically analyzed with GraphPad
Prism 7 for Mac OS X (GraphPad Software, San Diego, CA).
Differences between three or more unmatched groups were analyzed by
one-way ANOVA with Bonferroni's multiple comparisons or
nonparametric Kruskal-Wallis test with Dunn's multiple comparisons.
Furthermore, differences between three or more matched groups were
analyzed by two-way repeated measures ANOVA with Dunnett's multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
In our previous study, mutant KRAS knockdown
sensitized NSCLC cells to a p38 inhibitor (12). This prompted us to investigate
whether MEK inhibitors, as alternatives to mutant KRAS
knockdown, could efficiently impair KRAS-mutated NSCLC cell
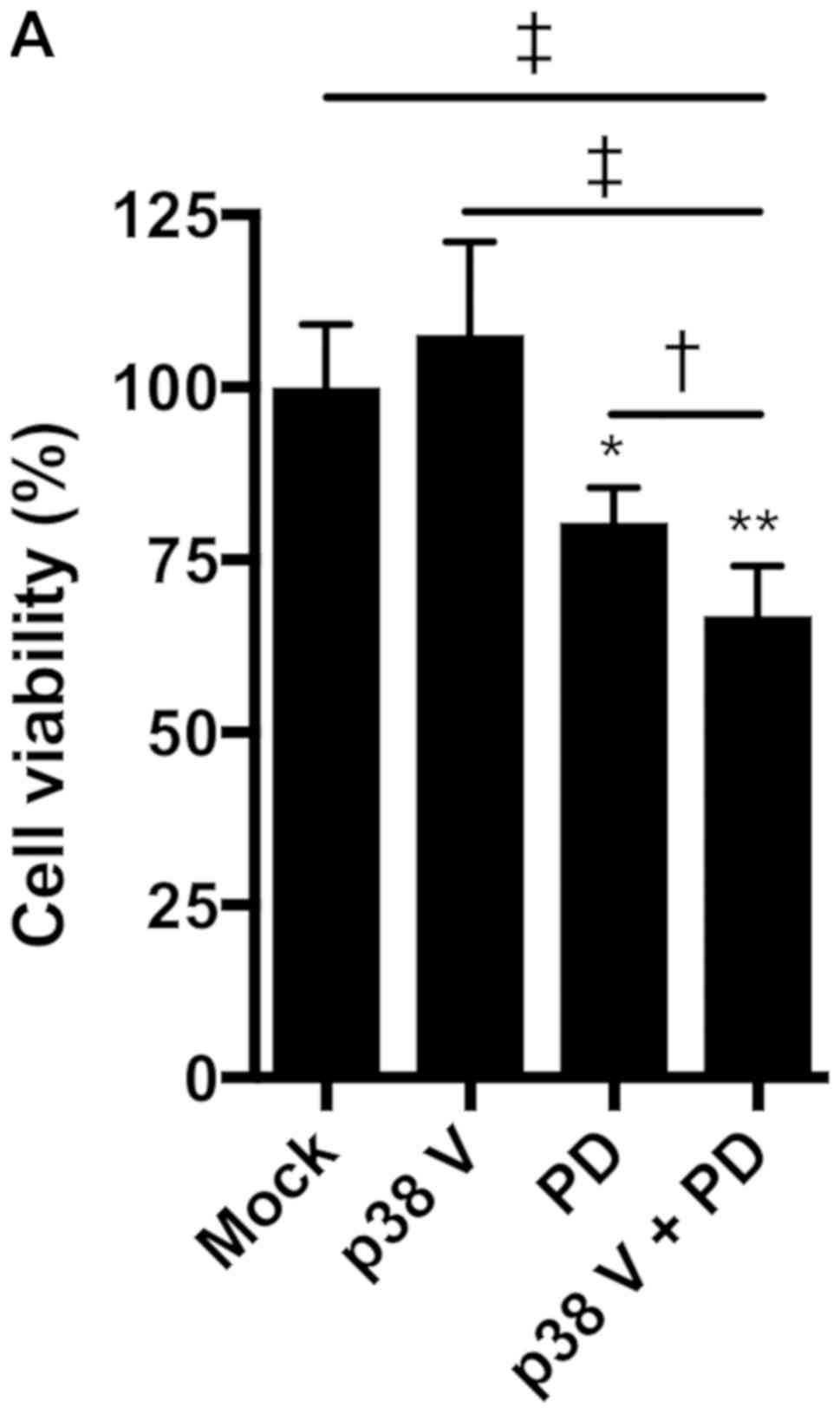
growth in combination with p38 inhibitors. Treatment with the p38
inhibitor p38 V (p38 V) alone had no significant effect on the
viability of NCI-H1792 NSCLC cells harboring KRAS mutations;
however, the MEK inhibitor PD0325901 significantly reduced cell
viability, and combined treatment with PD0325901 plus p38 V further
enhanced this inhibitory effect (Fig.
1A). To confirm this finding, we tested another pair: The MEK
inhibitor selumetinib and the p38 inhibitor LY2228820. LY2228820
alone modestly impaired cell viability, while selumetinib enhanced
its effect in a dose-dependent manner (Fig. 1B). In addition, compared to LY2228820
or selumetinib treatment alone, combined treatment with selumetinib
plus LY2228820 significantly reduced NCI-H1792 cell proliferation
(Fig. 1C).
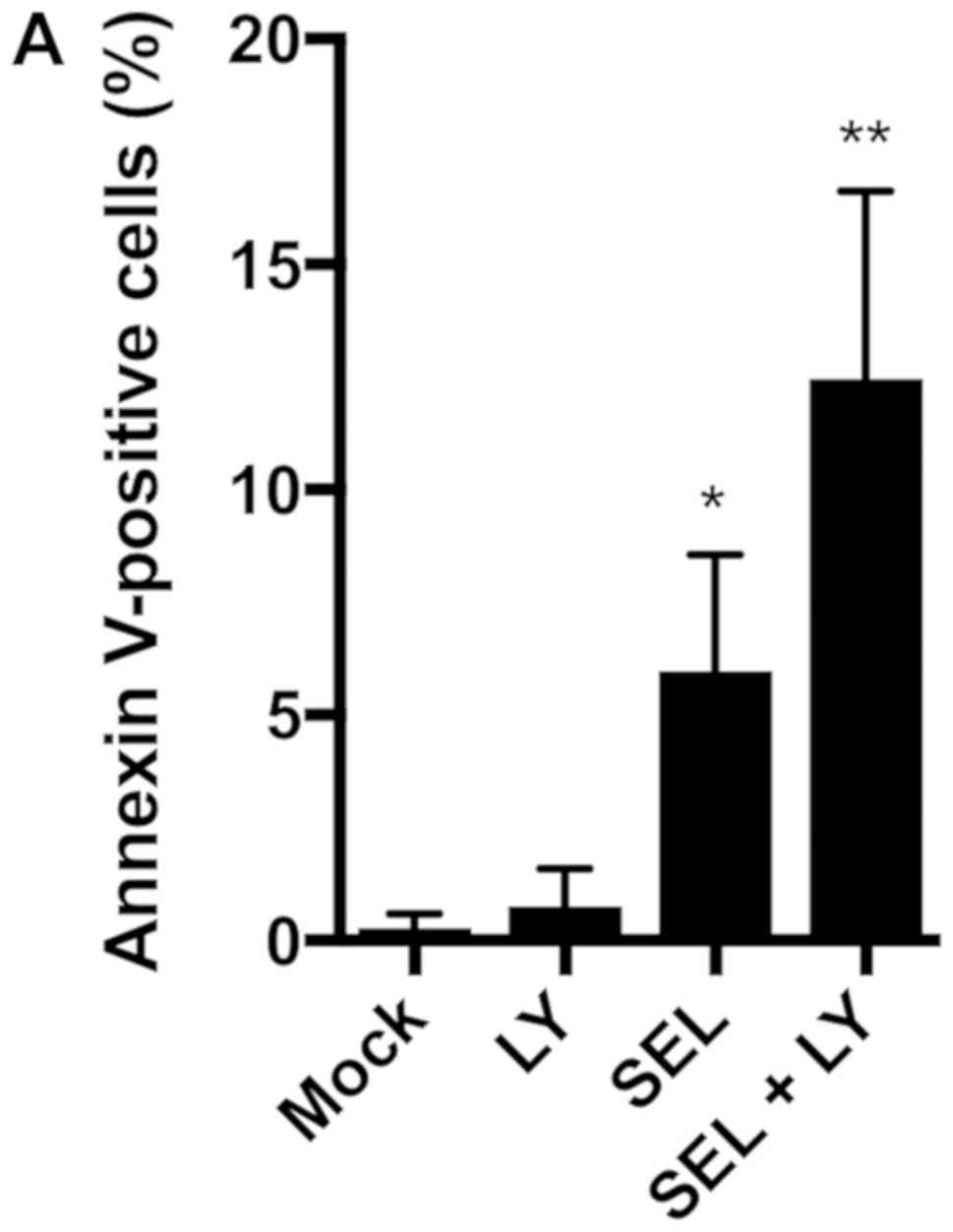
We next assessed whether the combination of MEK and
p38 inhibitors induces apoptosis in NCI-H1792 cells. Selumetinib
alone, but not LY2228820 alone, increased the number of Annexin
V-positive apoptotic cells, and the combination of selumetinib plus
LY2228820 further increased the number of apoptotic cells (Fig. 2A). The DNA fragmentation assays
confirmed that the induction of apoptosis was enhanced by the
combination of both inhibitors (Fig.
2B).
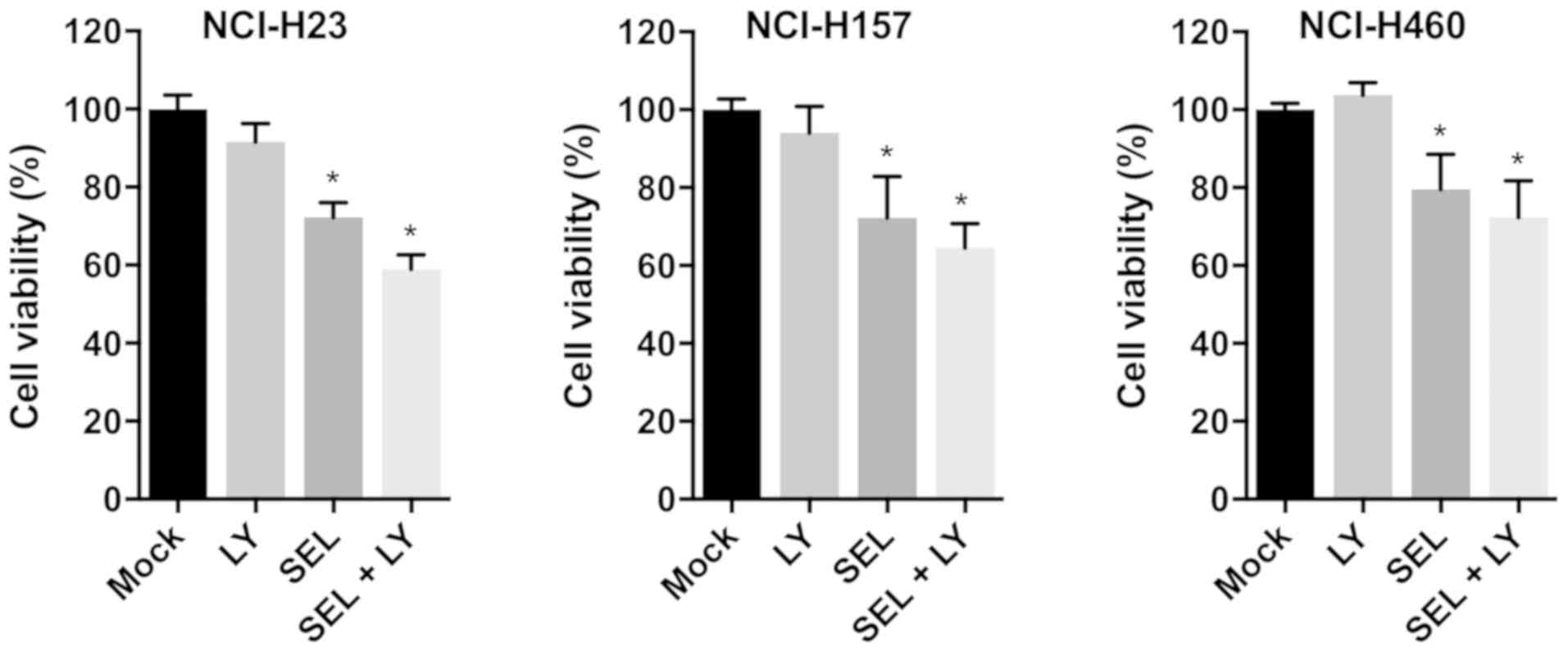
To evaluate whether combined treatment with MEK and
p38 inhibitors impaired cell growth in other KRAS-mutated
NSCLC cell lines, we examined the growth-inhibitory effect of
selumetinib and LY2228820 in NCI-H23, NCI-H157 and NCI-H460 cells.
In all cell lines, compared with single inhibitor treatments,
combined treatment with both reagents reduced cell viabilities
(Fig. 3). Thus, the combined
treatment of MEK and p38 inhibitors appears to be universally
effective for inhibiting the growth of KRAS-mutated NSCLC
cells.
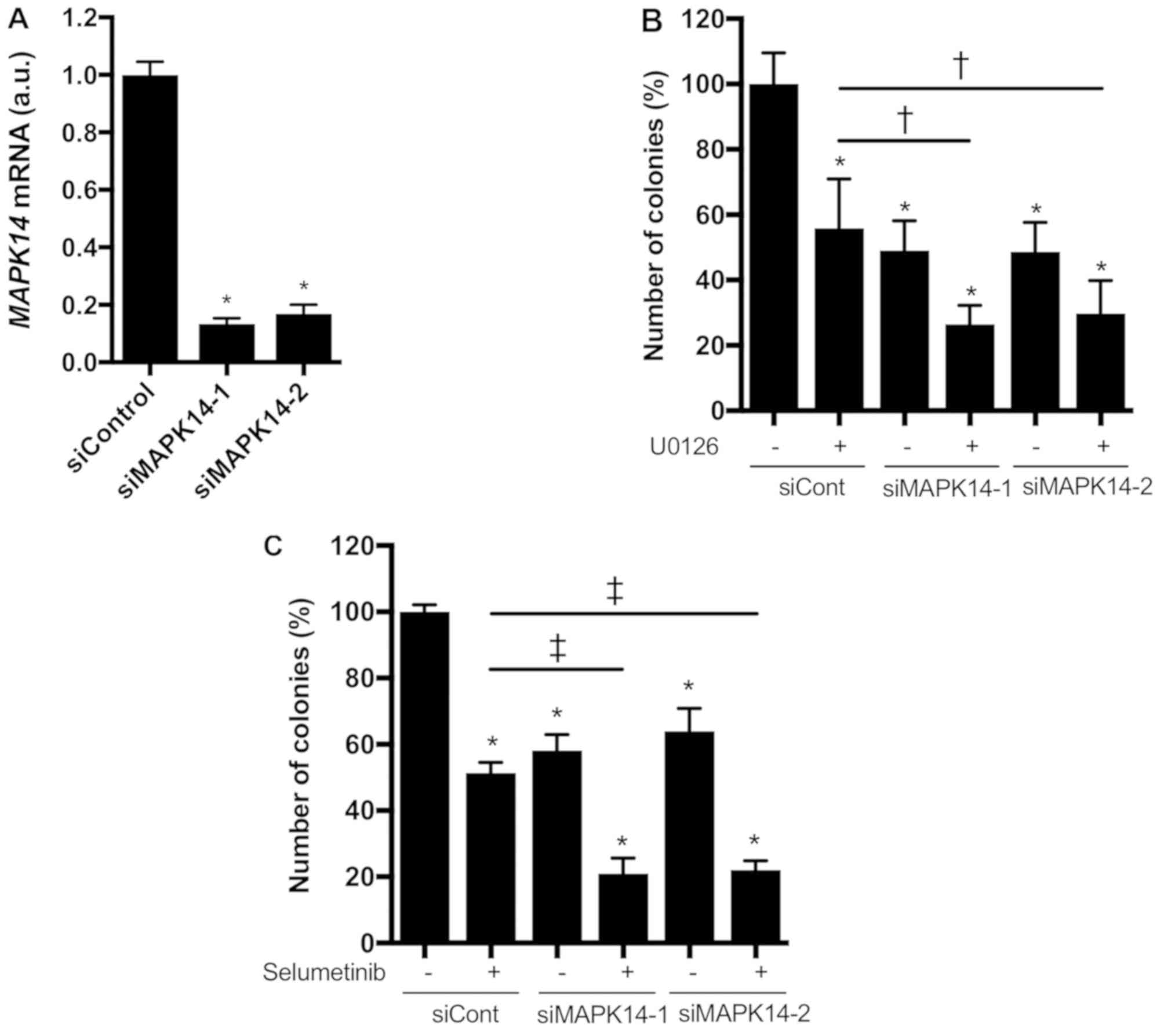
We further investigated whether knocking down
MAPK14, which encodes p38α, enhanced the growth-inhibitory
effect of MEK inhibitors in KRAS-mutated NSCLC cells.
MAPK14 siRNAs markedly reduced MAPK14 mRNA expression
in NCI-H1792 cells (Fig. 4A). In
addition, the colony formation of NCI-H1792 cells was significantly
inhibited by the MAPK14 siRNAs or MEK inhibitors (U0126 or
selumetinib), and these inhibitory effects were more prominent upon
combined treatment with MAPK14 siRNAs plus MEK inhibitors
(Fig. 4B and C). These findings
indicate that p38α loss confers hypersensitivity to MEK inhibitors
in KRAS-mutated NSCLC cells.
To elucidate the regulatory mechanism by which dual
MEK and p38 inhibition efficiently inhibits cell growth, we
examined the effects of MEK inhibition on the expression levels of
p38 and extracellular regulating kinase (ERK). The MEK inhibitors
(U0126 and selumetinib) increased phosphorylated p38 levels but
decreased total p38 levels, which were accompanied by decreased
phosphorylated ERK levels in NCI-H1792 cells (Fig. 5A-C). These results support the idea
that MEK inhibition efficiently sensitizes KRAS-mutated
NSCLC cells to p38 inhibitors. We also assessed whether the MEK
inhibitor-induced p38 protein repression was due to transcriptional
down-regulation. Treatment with selumetinib did not affect
MAPK14 mRNA expression levels in NCI-H1792 cells (Fig. 5D), suggesting that p38 protein
expression is post-transcriptionally modulated by MEK inhibition.
Taken together, the present study indicates that dual inhibition of
MEK and p38 has a synergistic effect on cell growth of
KRAS-mutated NSCLC.
Discussion
Mitogen-activated protein kinase (MAPK) signaling
pathways are highly conserved among eukaryotes and play essential
roles in the transduction of extracellular signals to diverse
cellular responses (15). MAPK
signaling comprises three major pathways, ERK, c-Jun N-terminal
kinase (JNK) and p38 MAPK, all of which mediate a large number of
molecules through regulating gene expression and interacting with
each other (15). The ERK pathway
has a central role in NSCLC tumorigenesis (16), which is supported by the fact that
this pathway comprises several oncogenic drivers of NSCLC, such as
KRAS and BRAF (5). The
p38 MAPK pathway is also involved in tumor development and
maintenance by regulating numerous molecules that control tumor
growth and survival, but it appears to have opposing roles as
either a tumor inhibitor or a tumor promoter, depending on the
cellular environment (17–19). Thus, the role of p38 MAPK in
tumorigenesis is controversial and has not been fully defined in
NSCLC.
In the present study, we showed that dual MEK and
p38 inhibition has the potential to suppress KRAS-mutated
NSCLC tumor growth. In agreement with our observations, previous
studies have shown that blocking both the ERK and p38 pathways
efficiently suppresses colorectal cancer growth (20,21). In
a study by van Houdt et al, oncogenic KRAS activated p38α to
maintain cell proliferation during MEK inhibition in
KRAS-mutated colorectal cell lines (21). These authors found that MEK/ERK
inhibition induced oncogenic KRAS-dependent p38 phosphorylation in
colorectal cancer cells (21), which
is consistent with our findings that MEK inhibitors increased
phosphorylated p38 levels in KRAS-mutated NSCLC cells.
Additionally, it was found that phosphorylated p38 levels were
elevated in oncogenic RAS-transformed keratinocytes when EGFR
signaling was abrogated, and blocking both EGFR and p38, but not
EGFR or p38 alone, impaired cell proliferation (22); these results suggest that oncogenic
RAS-driven tumors depend on p38 pathway activation to survive when
the signaling pathways downstream of EGFR are blocked.
Collectively, these findings suggest that oncogenic KRAS-driven
NSCLC tumors could switch survival signaling to the p38 pathway
when the ERK pathway is unavailable.
Intriguingly, in addition to increased
phosphorylated p38 expression levels, the total p38 expression
levels were decreased by MEK inhibitor treatment in
KRAS-mutated NSCLC cells. This effect may underlie our
observation that the growth-inhibitory effects of the combination
of siRNA-mediated p38α knockdown with MEK inhibitors were more
prominent than those of combined treatment with MEK and p38
inhibitors. These data support the idea that targeting MEK
efficiently sensitizes KRAS-mutated NSCLC tumors to p38
inhibitors. We also found that treatment with a MEK inhibitor did
not affect MAPK14 expression, indicating that MEK
inhibitor-induced p38 down-regulation may be a post-transcriptional
event. Further investigation is needed to clarify the precise
mechanism regarding how MEK regulates p38 expression.
Despite the recent development of therapeutic
strategies such as molecular targeted therapy and immunotherapy in
NSCLC (3,4,23),
targeting oncogenic KRAS-driven NSCLC remains a challenge. While
many molecular targeted monotherapy drugs have failed to improve
outcomes in KRAS-mutated NSCLC, combinatorial approaches
with MEK inhibitors plus other inhibitors, such as AKT, CDK4/6,
HSP90 or FGFR1 inhibitors, have recently provided hopeful results
in preclinical and clinical studies (9,24–28). Our
findings may provide a novel combinatorial approach of dual MEK and
p38 inhibition, and further studies including in vivo
experiments will elucidate its therapeutic significance in
oncogenic KRAS-driven NSCLC.
Acknowledgements
The authors would like to thank Dr Junichi Okada, Dr
Shuichi Okada and Dr Masanobu Yamada of the Division of
Endocrinology and Metabolism, Department of Internal Medicine,
Gunma University Graduate School of Medicine, and Dr Yasuhiko Koga
and Dr Haruka Aoki-Saito of the Department of Respiratory Medicine,
Gunma University Graduate School of Medicine for technical support
and advice.
Funding
This study was supported by Grants-in-Aid for
Scientific Research (C) (grant no. 26461181) from the Japan Society
for the Promotion of Science.
Availability of data and materials
All data generated or analyzed in the current study
are included in this article. Data sharing is not applicable to
this article as no public datasets were generated or analyzed
during the current study.
Authors' contributions
Conceptualization, methodology, funding acquisition
and writing of the original draft was performed by NS.
Investigation, validation and acquisition of data was performed by
NS, YM, YT, NK and TM. Analysis and interpretation of data was
performed by NS, YM, YT, NK, TM, RS, KK and TH. Resources were
obtained by NS, KK and TH; writing, reviewing and editing the
manuscript was performed by NS, YM, YT, NK, TM, RS, KK and TH;
supervision and project administration was provided by NS and
TH.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houston KA, Henley SJ, Li J, White MC and
Richards TB: Patterns in lung cancer incidence rates and trends by
histologic type in the United States, 2004–2009. Lung Cancer.
86:22–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI
|
|
4
|
Saito M, Shiraishi K, Kunitoh H,
Takenoshita S, Yokota J and Kohno T: Gene aberrations for precision
medicine against lung adenocarcinoma. Cancer Sci. 107:713–720.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kris MG, Johnson BE, Berry LD, Kwiatkowski
DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson
SL, Su PF, et al: Using multiplexed assays of oncogenic drivers in
lung cancers to select targeted drugs. JAMA. 311:1998–2006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pylayeva-Gupta Y, Grabocka E and Bar-Sagi
D: RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer.
11:761–774. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng D, Yuan M, Li X, Chen L, Yang J, Zhao
X, Ma W and Xin J: Prognostic value of K-RAS mutations in patients
with non-small cell lung cancer: A systematic review with
meta-analysis. Lung Cancer. 81:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ying M, Zhu XX, Zhao Y, Li DH and Chen LH:
KRAS mutation as a biomarker for survival in patients with
non-small cell lung cancer, a meta-analysis of 12 randomized
trials. Asian Pac J Cancer Prev. 16:4439–4445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matikas A, Mistriotis D, Georgoulias V and
Kotsakis A: Targeting KRAS mutated non-small cell lung cancer: A
history of failures and a future of hope for a diverse entity. Crit
Rev Oncol Hematol. 110:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blumenschein GR Jr, Smit EF, Planchard D,
Kim DW, Cadranel J, De Pas T, Dunphy F, Udud K, Ahn MJ, Hanna NH,
et al: A randomized phase II study of the MEK1/MEK2 inhibitor
trametinib (GSK1120212) compared with docetaxel in KRAS-mutant
advanced non-small-cell lung cancer (NSCLC)†. Ann Oncol.
26:894–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jänne PA, van den Heuvel MM, Barlesi F,
Cobo M, Mazieres J, Crinò L, Orlov S, Blackhall F, Wolf J, Garrido
P, et al: Selumetinib plus docetaxel compared with docetaxel alone
and progression-free survival in patients with KRAS-mutant advanced
non-small cell lung cancer: The SELECT-1 randomized clinical trial.
JAMA. 317:1844–1853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sunaga N, Shames DS, Girard L, Peyton M,
Larsen JE, Imai H, Soh J, Sato M, Yanagitani N, Kaira K, et al:
Knockdown of oncogenic KRAS in non-small cell lung cancers
suppresses tumor growth and sensitizes tumor cells to targeted
therapy. Mol Cancer Ther. 10:336–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunaga N, Kaira K, Imai H, Shimizu K,
Nakano T, Shames DS, Girard L, Soh J, Sato M, Iwasaki Y, et al:
Oncogenic KRAS-induced epiregulin overexpression contributes to
aggressive phenotype and is a promising therapeutic target in
non-small-cell lung cancer. Oncogene. 32:4034–4042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sunaga N, Imai H, Shimizu K, Shames DS,
Kakegawa S, Girard L, Sato M, Kaira K, Ishizuka T, Gazdar AF, et
al: Oncogenic KRAS-induced interleukin-8 overexpression promotes
cell growth and migration and contributes to aggressive phenotypes
of non-small cell lung cancer. Int J Cancer. 130:1733–1744. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heigener DF, Gandara DR and Reck M:
Targeting of MEK in lung cancer therapeutics. Lancet Respir Med.
3:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loesch M and Chen G: The p38 MAPK stress
pathway as a tumor suppressor or more? Front Biosci. 13:3581–3593.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiacchiera F, Grossi V, Cappellari M,
Peserico A, Simonatto M, Germani A, Russo S, Moyer MP, Resta N,
Murzilli S and Simone C: Blocking p38/ERK crosstalk affects
colorectal cancer growth by inducing apoptosis in vitro and in
preclinical mouse models. Cancer Lett. 324:98–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Houdt WJ, de Bruijn MT, Emmink BL,
Raats D, Hoogwater FJ, Borel Rinkes IH and Kranenburg O: Oncogenic
K-ras activates p38 to maintain colorectal cancer cell
proliferation during MEK inhibition. Cell Oncol. 32:245–257.
2010.PubMed/NCBI
|
|
22
|
Wright LN, Ryscavage A, Merlino G and
Yuspa SH: Modeling the transcriptional consequences of epidermal
growth factor receptor ablation in Ras-initiated squamous cancer.
Clin Cancer Res. 18:170–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miura Y and Sunaga N: Role of
immunotherapy for oncogene-driven non-small cell lung cancer.
Cancers (Basel). 10(pii): E2452018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tolcher AW, Khan K, Ong M, Banerji U,
Papadimitrakopoulou V, Gandara DR, Patnaik A, Baird RD, Olmos D,
Garrett CR, et al: Antitumor activity in RAS-driven tumors by
blocking AKT and MEK. Clin Cancer Res. 21:739–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao Z, Le Blanc JM, Wang C, Zhan T, Zhuang
H, Wang P, Yuan Z and Lu B: Coadministration of trametinib and
palbociclib radiosensitizes KRAS-mutant non-small cell lung cancers
in vitro and in vivo. Clin Cancer Res. 22:122–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park KS, Oh B, Lee MH, Nam KY, Jin HR,
Yang H, Choi J, Kim SW and Lee DH: The HSP90 inhibitor, NVP-AUY922,
sensitizes KRAS-mutant non-small cell lung cancer with intrinsic
resistance to MEK inhibitor, trametinib. Cancer Lett. 372:75–81.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manchado E, Weissmueller S, Morris JP IV,
Chen CC, Wullenkord R, Lujambio A, de Stanchina E, Poirier JT,
Gainor JF, Corcoran RB, et al: A combinatorial strategy for
treating KRAS-mutant lung cancer. Nature. 534:647–651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitai H, Ebi H, Tomida S, Floros KV,
Kotani H, Adachi Y, Oizumi S, Nishimura M, Faber AC and Yano S:
Epithelial-to-mesenchymal transition defines feedback activation of
receptor tyrosine kinase signaling induced by MEK inhibition in
KRAS-mutant lung cancer. Cancer Discov. 6:754–769. 2016. View Article : Google Scholar : PubMed/NCBI
|