Introduction
Preoperative chemoradiotherapy (CRT) significantly
reduces the risk of local recurrence and cancer-specific mortality
compared with surgery alone in locally advanced rectal cancer
(LARC) (1,2). Following a German phase III trial in
2004, preoperative CRT with infusional 5-florouracil (5-FU) and
total mesorectal excision surgery has become the standard treatment
for stage II and III rectal cancer in Western countries (2). Recently, new agents such as oral
fluoropyrimidines, oxaliplatin and irinotecan, which were used in
the metastatic disease setting or adjuvant chemotherapy, have been
used by several groups to modify tumor response in clinical trials
of CRT (3). CAO/ARO/AIO-04 phase III
trials showed that adding oxaliplatin to 5-FU improved pathological
complete response (pCR) and disease free survival (DFS) compared
with 5-FU alone (4), whereas
STAR-01, ACCORD 12 and NSABP R-04 phase III trials with 5-FU or
capecitabine plus oxaliplatin did not show significant improvements
in pCR and DFS (5–7). In addition, phase III trials with
irinotecan have not been reported, but early phase I/II trials with
5-FU or capecitabine plus irinotecan showed that pCR rates were
13.7–37% (8–13). Therefore, the use of fluoropyrimidine
plus oxaliplatin or irinotecan in CRT is not recommended outside of
clinical trials.
S-1 is an oral fluoropyrimidine containing tegafur,
gimeracil, and oteracil potassium in a molar ratio of 1:0.4:1
(14). Tegafur is a prodrug of 5-FU,
and gimeracil is a reversible inhibitor of dihydropyrimidine
dehydrogenase that degrades 5-FU (15). Oteracil potassium inhibits the enzyme
orotate phosphoribosyl-transferase, which converts tegafur to 5-FU
and decreases gastrointestinal toxicity of 5-FU (15). S-1 has good anticancer efficacy for
colorectal cancer (CRC) and an acceptable toxicity profile
(16). In addition,
chemoradiotherapy with S-1 was effective and well tolerated in a
previous phase I/II study (17).
Early phase studies of preoperative CRT with S-1 plus irinotecan
(phase II) or oxaliplatin (phase II) regimen showed favorable
toxicity profile and good pCR rates (18,19).
Recently, triplet combination chemotherapy regimen
(FOLFOXRI) has been demonstrated to be superior to doublet regimen
(FOLFIRI) in metastatic CRC, though triplet regimen has more
adverse effects than doublet chemotherapy (20). Several tumors, including CRC, have
intra-tumor genetic heterogeneity, which reflects the presence of
different subclonal populations within the cancer and are likely
associated with clinical course and response to therapy (21,22).
Chemotherapy or chemoradiotherapy, including more agents with
different mechanisms, may improve treatment response in view of
this heterogeneity. Therefore, we hypothesized that chemoradiation
with triplet radiosensitizer of fluoropyrimidines, oxaliplatin and
irinotecan may have a higher response than regimens used in
previous studies. However, the feasibility of chemoradiation with
triplet radiosensitizer of fluoropyrimidines, oxaliplatin and
irinotecan is not well known. Therefore, we designed a new
preoperative CRT with sequential oxaliplatin and irinotecan with
S-1 for LARC and aimed to determine the maximum tolerated dose
(MTD) and recommended dose (RD) of oxaliplatin following irinotecan
in a phase I study.
Materials and methods
Ethics and patient consent
The present study was reviewed and approved by Mie
University Institutional Review Board, and the study was performed
in accordance with the Helsinki Declaration of 1975, as revised in
2000. Patients were required to provide written informed consent
prior to enrollment. The present study was registered at the UMIN
Clinical Trial Registry as UMIN000017674 (further details
accessible at: http://www.umin.ac.jp/ctr/index.htm).
Eligibility criteria
Eligible patients had LARC with T3 to 4 or
involvement of regional nodes as determined by computed tomography
(CT), magnetic resonance imaging (MRI), or endoscopic ultrasound
and had histologically-confirmed adenocarcinoma prior to surgery.
Eligible patients also had an Eastern Cooperative Oncology Group
performance status of 0–1 and a survival expectation of >3
months. Additional eligibility criteria included: age 20–80 years
at enrollment, no severe compromise of main organ functions
(including bone marrow, lung, liver and kidney), and blood specific
biochemistry results (leukocyte count 4,000–12,000/µl, platelet
count ≥100×103/µl, hemoglobin concentration ≥9.0 g/dl,
total bilirubin concentration ≤1.5× upper normal limit, serum
aspartate aminotransferase and alanine aminotransferase levels
<2.5× upper normal limit, and serum creatinine concentration
<1.5× upper normal limit). The exclusion criteria were patients
with potential risk factors for S-1, irinotecan, and
oxaliplatin-related adverse events. The risk factors included
patients with child-bearing potential or lactation; patients
without intention to use contraception; clinically significant
cardiovascular, pulmonary or renal disease; clinical evidence of
gastrointestinal bleeding; bowel obstruction and perforation;
active inflammatory bowel disease; active infection; considerable
pleural effusion, cardiac effusion and ascites; and any other cases
regarded as inadequate for enrollment by the investigator.
Pretreatment evaluation
Complete clinical and radiographic staging was
performed prior to enrollment in the study. Patients underwent the
following evaluations: History and physical examination; CT scan of
the chest, abdomen, and pelvis; chest X-ray; colonoscopy; barium
enema; endoscopic ultrasound; and pelvic MRI. A complete blood
count with differential analysis, serum chemistry tests,
urinalysis, electrocardiogram, carcinoembryonic antigen, and
carbohydrate antigen 19-9 levels were obtained. During treatment,
patients were evaluated at least weekly via a history and physical
examination, and they underwent a complete blood count with
differential analysis, electrolyte analysis, liver function tests,
chemistry panel assays, coagulation panel assays, and
urinalysis.
Treatment
Patients underwent four-field (anterior-posterior,
posterior-anterior, and right and left laterals) approach and
radiation therapy was delivered using a 10-MV linear accelerator.
Patients were treated in the prone position using a dedicated
device to minimize exposure of the small bowel. A CT-based
treatment planning system was mandatory to define the planning
target volume, including the primary tumor, internal iliac lymph
nodes, presacral lymph nodes, obturator lymph nodes, and the
surrounding mesorectum. Radiotherapy was administered in fractions
of 1.8 Gy/day, 5 days/week, for 5 weeks. The total dose of
radiation delivered was 45 Gy. During radiation therapy, patients
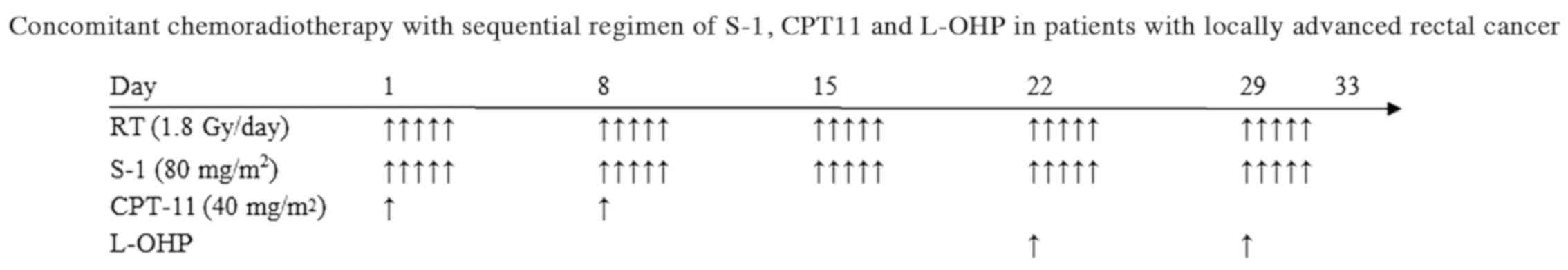
received chemotherapy using the following regimen. The regimen
comprised fix doses of S-1 (80 mg/m2/day) and irinotecan
(40 mg/m2/day). S-1 was given on days 1–5, 8–12, 15–19,
22–26 and 29–33. Irinotecan (40 mg/m2/day) was given on
days 1 and 8, and oxaliplatin was given on days 22 and 29, 2 weeks
after irinotecan administration. Table
I provides a summary of oxaliplatin dose levels. Oxaliplatin
administration was initiated at 40 mg/m2 (level 1), with
a planned dose escalation to dose levels 2 and 3. A treatment
schema is shown in Fig. 1. Patients
were premedicated prior to chemotherapy treatment to minimize
nausea and vomiting, which are associated with 5-HT3 receptor
antagonists. The interval between the completion of CRT and
operation was 6–8 weeks. The assessment of tumor resectability
included a history and physical examination, imaging evaluation
using CT and MRI, and proctosigmoidoscopy. Following thorough
exploration of the peritoneal cavity and distant lesions for
metastases, low anterior resection or abdominoperineal resection
with total mesorectal excision was performed in conjunction with a
colonic J-pouch-anal anastomosis (CAA). A diverting ileostomy was
usually used to prevent from anastomotic leakage.
 | Table I.Summary of S-1/CPT-11/L-OHP dose
levels used in combination with radiation therapy. |
Table I.
Summary of S-1/CPT-11/L-OHP dose
levels used in combination with radiation therapy.
| Level | S-1 (mg/m) | CPT-11
(mg/m2) | L-OHP
(mg/m2) |
|---|
| −1 | 80 | 40 | 30 |
| 1 | 80 | 40 | 40 |
| 2 | 80 | 40 | 50 |
| 3 | 80 | 40 | 60 |
Assessment
Adverse events were assessed at least weekly during
radiation therapy, using the National Cancer Institute common
toxicity criteria (version 3.0). Side effects were managed
aggressively with standard supportive measures. Resectability was
also evaluated and a complete pathological response was defined as
no evidence of malignancy in the specimen.
Study design, definitions, and
endpoints
The primary endpoint of this study was the
determination of the MTD and the RD. The secondary endpoint was
evaluation of the extent and frequency of the adverse events and
the resectability of locally-advanced rectal cancer. The
dose-limiting toxicity (DLT) was defined as Grade 4 hematological
toxicity (leucopenia and neutropenia persisting beyond 4 days and
thrombocytopenia); Grade 3 or higher non-hematological toxicity,
despite adequate supportive care; any single interruption of
radiation therapy of ≥7 days or >2 interruptions per radiation
course; any delay of >14 days in the completion of radiation
therapy; and any treatment-related hospitalization or death.
Perianal dermatitis was defined as adverse effects of radiation and
excluded as a criterion of DLT. Patients with DLT before
oxaliplatin administration were not included in this study. The MTD
was defied as the dose level that produced DLT in at least two out
of three patients, or two out of six patients. If DLT occurred in
one of the first three patients, three additional patients were
assigned to receive the same dose level. If none of the three
patients initially receiving a given dose level exhibited DLT, or
if one out of six patients exhibited DLT, the dose was increased to
the next level. Dose escalation was not allowed in the same
patient. The dose level immediately below the MTD was considered
the RD for phase II studies.
Results
Patient characteristics
From May 2015 through February 2017, nine patients
treated at our institution were enrolled in this study. Two
patients who developed Grade 3 radiation enterocolitis before
oxaliplatin administration were not included in this study because
of delays of >14 days in completing radiation therapy.
Characteristics of patients who completely received this protocol
treatment are summarized in Table
II.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | Patients |
|---|
| Age (range) | 55 (38–76) |
| Sex |
|
Male | 3 |
|
Female | 4 |
| Performance
status |
| 0 | 7 |
| Tumor site |
| Rb | 5 |
|
Rb-P | 2 |
| Clinical stage |
|
cT2N1 | 1 |
|
cT3N0 | 1 |
|
cT3N1 | 1 |
|
cT3N2 | 1 |
|
cT4N1 | 1 |
|
cT4N2 | 2 |
DLT and RD levels
The observed toxicities are described in Table III. Three patients were initially
enrolled at dose level 1. In the S-1+irinotecan regimen on level 1
protocol phase, almost adverse effects were Grade 1 though two
patients had nausea of Grade 2 which required intravenous fluids.
All three patients had Grade 1 perianal dermatitis by first dose of
S-1+oxaliplatin. In the level 1 S-1+ oxaliplatin regimen phase, one
patient experienced Grade 2 diarrhea and one patient developed
Grade 2 anemia from Grade 1 in the irinotecan phase. In addition,
all three patients developed perianal dermatitis by completion of
irradiation. In summary of the level 1 protocol, all three patients
were treated without interruptions to chemotherapy or irradiation
or Grade 3–4 adverse effects defined as DLT. Second, three patients
were enrolled at dose level 2. In phase of S-1+irinotecan regimen
on level 2 protocol, one patient experienced a 3-day interruption
of irradiation because of developing Grade 3 diarrhea after the
second dose of irinotecan. Another patient also had a 4-day
interruption of irradiation with Grade 2 stomatitis, fatigue, and
diarrhea just before the first dose of S-1+oxaliplatin. All three
patients had Grade 1 perianal dermatitis caused by the first dose
of S-1+oxaliplatin as well as the S-1+irinotecan regimen on level 1
protocol phase. In the level 2 S-1+ oxaliplatin regimen phase, two
patients developed Grade 3 diarrhea treated with intravenous fluids
just before completion of irradiation though irradiation was not
interrupted. Grade 1 peripheral neuropathy caused by oxaliplatin
was observed in all three patients. Subsequently, three additional
patients were enrolled at the same dose level 1 according to the
study protocol, because of incidence of Grade 3 non-hematological
toxicity. One patient had Grade 2 diarrhea without interruption of
irradiation and chemotherapy in the S-1+irinotecan regimen phase.
In addition, this patient experienced Grade 3 diarrhea and
stomatitis with cessation of radiation therapy (total 43.2 Gy),
delay of chemotherapy and administration of intravenous fluids in
the S-1+oxaliplatin regimen phase. Because non-hematological
toxicity as DLT was observed in three patients and level 2 was
designated as MTD, this study finished by enrolling seven patients.
Therefore, level 1 was considered the RD for the phase II
study.
 | Table III.Toxicity parameters evaluated in the
study and matched control group. |
Table III.
Toxicity parameters evaluated in the
study and matched control group.
|
| Dose level 1 | Dose level 2 |
|---|
|
|
|
|
|---|
| S-1+CPT11
toxicity | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 |
|---|
| Hematological |
|
Neutropenia | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
|
Anemia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST/ALT
abnormalities | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Non-hematological |
|
Nausea | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Stomatitis | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
|
Fatigue | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
|
Diarrhea | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
|
Peripheral neuropathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin
(perianal dermatitis) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
|
|
| Dose level
1 | Dose level
2 |
|
|
|
|
| S-1+L-OHP
toxicity | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 |
|
| Hematological |
|
Neutropenia | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
Anemia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST/ALT
abnormalities | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
|
Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Non-hematological |
|
Nausea | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Stomatitis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
Fatigue | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
|
Diarrhea | 2 | 1 | 0 | 0 | 0 | 1 | 3 | 0 |
|
Peripheral neuropathy | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Skin
(perianal dermatitis) | 0 | 3 | 0 | 0 | 1 | 3 | 0 | 0 |
Efficacy measures
We evaluated the tumor response to
chemoradiotherapy. Details of the patients' treatment responses are
shown in Table IV. The downstaging
rate in the tumor and nodal stage was 85.7% (6/7 patients) and
57.1% (4/7 patients), respectively. Five patients achieved
radiographic CR (28.5%) and PR (42.8%). Five patients underwent
laparoscopic low anterior resection with colonic J-pouch
reconstruction, one underwent laparoscopic CAA with colonic J-pouch
and total intersphincteric resection, and one underwent
laparoscopic CAA with coloplasty and subtotal intersphincteric
resection. Surgical site infection and anastomotic leakage were not
observed. Two patients had a microscopically-positive
circumferential resectional margin (R1) and five had complete
resection (R0). Finally, two patients had pathological CR
(28.5%).
 | Table IV.Individual patient characteristics
and treatment response. |
Table IV.
Individual patient characteristics
and treatment response.
| Patient | Dose level | Pretreatment
stage | Pathological
stage | Dose-limiting
toxicity | Radiographic
response | Pathological
effects gradea | Surgical
outcome |
|---|
| 1 | 1 | T3N1M0 | T0N0M0 | No | CR | 3b | R0 |
| 2 | 1 | T4N2M0 | T2N0M0 | No | PR | 2 | R0 |
| 3 | 1 | T4N2M0 | T2N1M0 | No | SD | 1b | R0 |
| 4 | 2 | T2N1M0 | T0N0M0 | Yes | CR | 3b | R0 |
| 5 | 2 | T3N0M0 | T3N0M0 | No | SD | 1a | R1 |
| 6 | 2 | T4N2M0 | T3N2M0 | Yes | PR | 1a | R1 |
| 7 | 2 | T3N2M0 | T2N2M0 | Yes | PR | 1a | R0 |
Discussion
CRT is an important element that enhances local
control for patients with LARC such as T3, T4, and lymph node
metastasis (3). Proposed improvement
for CRT have been better local control and less toxicity.
Preoperative CRT improved local control and reduced therapeutic
toxicity compared with postoperative CRT and preoperative therapy
with continuous infusional 5-FU is the standard practice for LARC
(2). Furthermore, many clinical
trials have been performed to improve clinical outcomes in
preoperative CRT (3). Newer
generation agents, including cytotoxic and biological drugs such as
oral fluoropyrimidines, oxaliplatin, irinotecan, antibody against
vascular endothelial growth factor, and anti-epidermal growth
factor receptor, have been used as radiosensitizers in recent
clinical trials (3). Despite the
oncological benefits of these agents shown in clinical trials, the
mechanism of conventional chemotherapeutic agents for
radiosensitization varies among the different agents (23). One mechanism of 5-FU as
radiosensitizer is through killing S phase cells, which are
relatively radioresistant (24).
Oxaliplatin plays a role as a radiosensitizer through various
mechanisms, such as DNA damage by the formation of inter- and
intra-strand crosslinks, induction of G2 and M cell cycle arrest,
and blockage of DNA repair (25,26).
Irinotecan is metabolized to SN38, which inhibits topoisomerase I
and has radiosensitizing properties. It has been suggested that
irinotecan may enhance the lethal effects of ionizing radiation by
attaching to the DNA-topoisomerase I adducts in sites of single
strand DNA breaks (26). These
cytotoxic drugs provide therapeutic efficacy by different
mechanisms for each other and combination therapies with these
agents are standard chemotherapy in CRC. However, intra-tumor
heterogeneity, which reflects the presence of different subclonal
populations within the cancer, likely impacts the response and
resistance to therapy (21,22). Combination therapy with more agents
may be effective countermeasure to drug resistance due to tumor
heterogeneity. In addition, the toxicity of triplet regimen is
greater while anticancer effect of triplet is superior to doublet
in CRC (27). Therefore, we designed
chemoradiation with triplet radiosensitizer of fluoropyrimidines,
oxaliplatin, and irinotecan and adopted a sequential schedule of
oxaliplatin and irinotecan for low toxicity. This sequential
administration could reduce adverse effects than simultaneous
administration and may be feasible not only for young patients but
also for elderly patients.
In this study, we performed CRT with S-1 plus
oxaliplatin following irinotecan for seven patients with LARC. The
prescribed dose (40, 80 mg/m2) of irinotecan and S-1 in
this study was based on previous phase II studies (23). Initial dose of irinotecan was
prescribed as the minimum dose following these studies (28,29)
because this trial was the first study with triple agents: S-1 and
irinotecan followed by S-1 and oxaliplatin. A multicenter phase I
trial (SAMRAI-1) concluded that the RD of irinotecan was 60
mg/m2 in preoperative CRT combined with S-1 and
irinotecan and incidence of Grade 3 or higher diarrhea defined as
DLT was 11.1% (30). Our study
showed Grade 3 enterocolitis of two patients, who were accordingly
excluded from this trial, and Grade 3 diarrhea of one patient in
S-1 plus irinotecan phase despite lower dose and shorter dose
duration compared with SAMRAI-1. This discrepancy may be due to
UGT1A1 genetic polymorphisms, which delay the metabolism of SN-38,
the active metabolite of irinotecan, and are susceptible to
toxicities such as neutropenia and diarrhea. Our study did not
evaluate UGT1A1 genetic polymorphisms because the dose of
irinotecan was far lower than that in doublet regimen of systemic
chemotherapy using irinotecan, whereas SAMRAI-1 excluded a genotype
of UGT1A1*6/*6, UGT1A1*28/*28 or heterozygote for both UGT1A1*6 and
*28. In addition, Grade 3 enterocolitis in two patients might be
associated with adverse effects of irradiation and
chemotherapy.
The RD of oxaliplatin was determined to be 40
mg/m2. DLT occurred in three of seven patients at dose
level 2 and all DLTs were Grade 3 diarrhea. However, the RD of
oxaliplatin identified in this study was lower than that in
previous trials of oral fluoropyrimidines plus oxaliplatin. Phase
III trials with capecitabine plus oxaliplatin were performed with
oxaliplatin 50–60 mg/m2 (23). In addition, oxaliplatin was also
administered with 60 mg/m2 in phase II studies with S-1
plus oxaliplatin (19). The lower RD
of oxaliplatin in this study might be caused by administration
followed by irinotecan considering that all DLTs were Grade 3
diarrhea. Our protocol, combined with S-1 80 mg/m2,
irinotecan 40 mg/m2 and followed by oxaliplatin 40
mg/m2, was safe in this patient group without genotyping
of UGT1A1 polymorphisms. However, if sequential CRT regimen with
oxaliplatin following irinotecan in this study is transposed to
inverse sequential regimen with irinotecan following oxaliplatin,
diarrhea may decrease in the late phase of CRT.
With 5-FU or capecitabine plus oxaliplatin CRT, pCR
rates were 16–17 and 13.3–19.2%, respectively, in phase III trials,
while the pCR rates of 5-FU or capecitabine plus irinotecan CRT
were 13.7–37 and 15–50%, respectively, in phase II studies
(23). In S-1 based CRT regimens of
early phase studies, pCR rates of CRT with S-1 plus oxaliplatin and
irinotecan were 27.3 and 34.7%, respectively (18,19). Our
study showed an acceptable pCR rate of 28.5% though it was a phase
1 trial. Though we anticipated that a CRT regimen with triplet
agents including oxaliplatin, irinotecan, and S-1 could potentially
have better pCR rates than previous CRT studies with S-1 plus
oxaliplatin or irinotecan, our CRT regimen did not show excellent
pCR rates in this study. This result might be affected according to
lower dose of both oxaliplatin and irinotecan than in the SAMRAI-1
study (30) and SHOGUN trial with
S-1 (31).
In conclusion, a new preoperative CRT with
sequential oxaliplatin and irinotecan with S-1 for LARC resulted in
acceptable toxicity and promising efficacy. However, the RDs of
oxaliplatin were lower than in previous CRT studies that combined
oxaliplatin with S-1. To administer higher oxaliplatin dose,
sequential CRT regimen with oxaliplatin followed by irinotecan may
be recommended. Therefore, we are planning a phase I trial of
preoperative CRT with sequential oxaliplatin followed by irinotecan
with S-1 for LARC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HF drafted the manuscript. HF, YOm, SI, TK, HY,
YOku, YOki, SY, JH, MKo, MO and TA contributed to the collection
and analysis of the data. HF, YT, YI and MKu conceived and designed
the study, and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by Mie
University Institutional Review Board (Mie, Japan; approval no.
2892). Patients were required to provide written informed consent
prior to enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rödel C, Hofheinz R and Fokas E: Rectal
cancer: Neoadjuvant chemoradiotherapy. Best Pract Res Clin
Gastroenterol. 30:629–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rödel C, Graeven U, Fietkau R, Hohenberger
W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA,
Lang-Welzenbach M, et al: Oxaliplatin added to fluorouracil-based
preoperative chemoradiotherapy and postoperative chemotherapy of
locally advanced rectal cancer (the German CAO/ARO/AIO-04 study):
Final results of the multicentre, open-label, randomised, phase 3
trial. Lancet Oncol. 16:979–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aschele C, Cionini L, Lonardi S, Pinto C,
Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti
P, et al: Primary tumor response to preoperative chemoradiation
with or without oxaliplatin in locally advanced rectal cancer:
Pathologic results of the STAR-01 randomized phase III trial. J
Clin Oncol. 29:2773–2780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de
La Roche G, Bouché O, et al: Clinical outcome of the ACCORD 12/0405
PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol.
30:4558–4565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Connell MJ, Colangelo LH, Beart RW,
Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC,
Ryan DP, et al: Capecitabine and oxaliplatin in the preoperative
multimodality treatment of rectal cancer: Surgical end points from
National surgical adjuvant breast and bowel project trial R-04. J
Clin Oncol. 32:1927–1934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iles S, Gollins S, Susnerwala S, Haylock
B, Myint S, Biswas A, Swindell R and Levine E:
Irinotecan+5-fluorouracil with concomitant pre-operative
radiotherapy in locally advanced non-resectable rectal cancer: A
phase I/II study. Br J Cancer. 98:1210–1216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta VK, Cho C, Ford JM, Jambalos C, Poen
J, Koong A, Lin A, Bastidas JA, Young H, Dunphy EP and Fisher G:
Phase II trial of preoperative 3D conformal radiotherapy,
protracted venous infusion 5-fluorouracil, and weekly CPT-11,
followed by surgery for ultrasound-staged T3 rectal cancer. Int J
Radiat Oncol Biol Phys. 55:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohiuddin M, Winter K, Mitchell E, Hanna
N, Yuen A, Nichols C, Shane R, Hayostek C and Willett C;: Radiation
Therapy Oncology Group Trial 0012: Randomized phase II study of
neoadjuvant combined-modality chemoradiation for distal rectal
cancer: Radiation therapy oncology group trial 0012. J Clin Oncol.
24:650–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Navarro M, Dotor E, Rivera F,
Sánchez-Rovira P, Vega-Villegas ME, Cervantes A, García JL, Gallén
M and Aranda E: A Phase II study of preoperative radiotherapy and
concomitant weekly irinotecan in combination with protracted venous
infusion 5-fluorouracil, for resectable locally advanced rectal
cancer. Int J Radiat Oncol Biol Phys. 66:201–205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Willeke F, Horisberger K,
Kraus-Tiefenbacher U, Wenz F, Leitner A, Hochhaus A, Grobholz R,
Willer A, Kähler G, Post S and Hofheinz RD: A phase II study of
capecitabine and irinotecan in combination with concurrent pelvic
radiotherapy (CapIri-RT) as neoadjuvant treatment of locally
advanced rectal cancer. Br J Cancer. 96:912–917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gollins S, Sun Myint A, Haylock B, Wise M,
Saunders M, Neupane R, Essapen S, Samuel L, Dougal M, Lloyd A, et
al: Preoperative chemoradiotherapy using concurrent capecitabine
and irinotecan in magnetic resonance imaging-defined locally
advanced rectal cancer: Impact on long-term clinical outcomes. J
Clin Oncol. 29:1042–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malet-Martino M and Martino R: Clinical
studies of three oral prodrugs of 5-fluorouracil (capecitabine,
UFT, S-1): A review. Oncologist. 7:288–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shirao K, Ohtsu A, Takada H, Mitachi Y,
Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H
and Satoh A: Phase II study of oral S-1 for treatment of metastatic
colorectal carcinoma. Cancer. 100:2355–2361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadahiro S, Suzuki T, Tanaka A, Okada K,
Kamijo A, Murayama C, Akiba T and Nakayama Y: Phase I/II study of
preoperative concurrent chemoradiotherapy with S-1 for locally
advanced, resectable rectal adenocarcinoma. Oncology. 81:306–311.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato T, Ozawa H, Hatate K, Onosato W,
Naito M, Nakamura T, Ihara A, Koizumi W, Hayakawa K, Okayasu I, et
al: A Phase II trial of neoadjuvant preoperative chemoradiotherapy
with S-1 plus irinotecan and radiation in patients with locally
advanced rectal cancer: Clinical feasibility and response rate. Int
J Radiat Oncol Biol Phys. 79:677–683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsusaka S, Ishihara S, Kondo K, Horie H,
Uehara K, Oguchi M, Murofushi K, Ueno M, Mizunuma N, Shimbo T, et
al: A multicenter phase II study of preoperative chemoradiotherapy
with S-1 plus oxaliplatin for locally advanced rectal cancer
(SHOGUN trial). Radiother Oncol. 116:209–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marques RP, Duarte GS, Sterrantino C, Pais
HL, Quintela A, Martins AP and Costa J: Triplet (FOLFOXIRI) versus
doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line
treatment of metastatic colorectal cancer: A systematic review and
meta-analysis. Crit Rev Oncol Hematol. 118:54–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardiman KM, Ulintz PJ, Kuick RD, Hovelson
DH, Gates CM, Bhasi A, Rodrigues Grant A, Liu J, Cani AK, Greenson
JK, et al: Intra-tumor genetic heterogeneity in rectal cancer. Lab
Invest. 96:4–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panczyk M: Pharmacogenetics research on
chemotherapy resistance in colorectal cancer over the last 20
years. World J Gastroenterol. 20:9775–9827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenhalgh TA, Dearman C and Sharma RA:
Combination of novel agents with radiotherapy to treat rectal
cancer. Clin Oncol (R Coll Radiol). 28:116–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lawrence TS, Blackstock AW and McGinn C:
The mechanism of action of radiosensitization of conventional
chemotherapeutic agents. Semin Radiat Oncol. 13:13–21. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hill EJ, Nicolay NH, Middleton MR and
Sharma RA: Oxaliplatin as a radiosensitiser for upper and lower
gastrointestinal tract malignancies: What have we learned from a
decade of translational research? Crit Rev Oncol Hematol.
83:353–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu AX and Willett CG: Chemotherapeutic
and biologic agents as radiosensitizers in rectal cancer. Semin
Radiat Oncol. 13:454–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leal F, Ferreira FP and Sasse AD:
FOLFOXIRI regimen for metastatic colorectal cancer: A systematic
review and meta-analysis. Clin Colorectal Cancer. 16:405–409.e2.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung M, Shin SJ, Koom WS, Jung I, Keum KC,
Hur H, Min BS, Baik SH, Kim NK, Kim H, et al: A randomized phase 2
study of neoadjuvant chemoradiaton therapy with
5-fluorouracil/leucovorin or irinotecan/S-1 in patients with
locally advanced rectal cancer. Int J Radiat Oncol Biol Phys.
93:1015–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong YS, Kim DY, Lim SB, Choi HS, Jeong
SY, Jeong JY, Sohn DK, Kim DH, Chang HJ, Park JG and Jung KH:
Preoperative chemoradiation with irinotecan and capecitabine in
patients with locally advanced resectable rectal cancer: Long-term
results of a Phase II study. Int J Radiat Oncol Biol Phys.
79:1171–1178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sato T, Hayakawa K, Tomita N, Noda M,
Kamikonya N, Watanabe T, Kato D, Sakai Y, Hiraoka M, Shimada M, et
al: A multicenter phase I study of preoperative chemoradiotherapy
with S-1 and irinotecan for locally advanced lower rectal cancer
(SAMRAI-1). Radiother Oncol. 120:222–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishihara S, Matsusaka S, Kondo K, Horie H,
Uehara K, Oguchi M, Murofushi K, Ueno M, Mizunuma N, Shinbo T, et
al: A phase I dose escalation study of oxaliplatin plus oral S-1
and pelvic radiation in patients with locally advanced rectal
cancer (SHOGUN trial). Radiat Oncol. 10:242015. View Article : Google Scholar : PubMed/NCBI
|