Introduction
Lung cancer typically has a poor prognosis, as
metastases are often present at the time of diagnosis (1). Distal metastases are more commonly
found in the adrenal glands, liver, bone, brain and kidney;
metastases located along the gastrointestinal tract are very rare
and account for a small number of patients (2,3). More
specifically, such metastatic lesions are more commonly encountered
in the advanced stages of the disease and are consequently
associated with unfavorable disease prognosis. With regards to
associated symptomatology, when the metastatic disease involves the
upper gastrointestinal tract, bleeding is the most common symptom,
whilst when the small intestine is involved, the most typical
manifestation is intestinal obstruction, with or without
perforation (4). Herein, a rare case
of a surgical patient with acute abdomen due to small bowel
perforation and multiple intestinal metastases from lung carcinoma
is reported.
Case report
An 81-year-old woman was admitted to the emergency
department of our institution due to fatigue and malaise. Upon
admission the patient was hemodynamically stable. The patient's
history of atrial fibrillation was significant and a Coumadin
analog was administered. Additionally, the patient had a history of
a locally advanced non-small cell carcinoma of the right lung
(stage IIIB); her lesion was considered unresectable at the time
and the patient received cisplatin and etoposide combined with
radiotherapy, showing a significant response. Approximately 1.5
years later, she was diagnosed with a metastatic lesion of the left
lung during follow-up. Chemotherapy was restarted, but the patient
was lost to follow-up in the next year. The patient reported mild
abdominal discomfort, which had started 2 weeks prior to the
current presentation. Upon admission, she presented with severe
anemia (Hct 18.5%), leucopenia (WBC 2,330 K/µl), thrombocytopenia
(PLT 55,000 K/µl), and a protracted INR of 6.41. Chest X-ray showed
diffuse pulmonary infiltrations and lobular pneumonia at the right
lower lobe. The abdominal ultrasound showed multiple focal lesions
in both hepatic lobes, with features of metastasis. At 2 days
post-admission, the patient presented with diffuse abdominal pain
combined with fever (38.1°C), and surgical consultation was
demanded. At physical examination the patient had diffuse abdominal
tenderness and guarding, rebound tenderness, abdominal distention
and obstipation. Her blood test revealed Hct 22.1%, WBC 20,000
K/µl, PLT 47,000 K/µl, whereas the biochemical tests were within
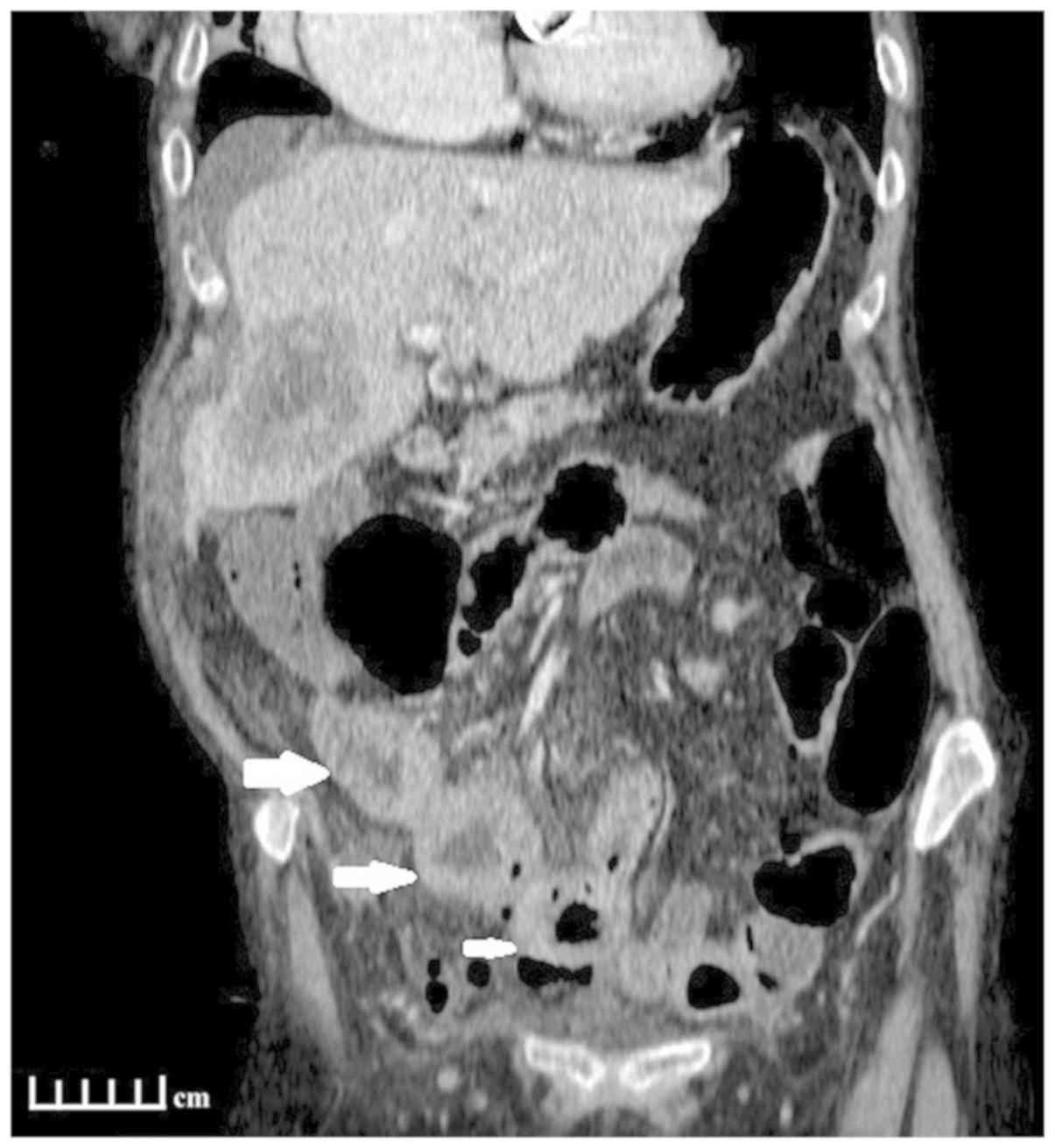
normal limits. An abdominal CT revealed wall thickening at the
level of the mid-sigmoid and the presence of free air in the
peritoneal cavity, likely due to perforation of the
gastrointestinal tract. Several round lesions were infiltrating the
wall of the small bowel, 2–3 cm in diameter, and large metastatic
lesions were also revealed within the liver parenchyma. A moderate
quantity of ascitic fluid, and multiple enlarged lymph nodes within
the mesentery were also identified (Fig.
1). The patient was urgently led to surgery for an exploratory
laparotomy. Upon entering the peritoneal cavity, diffuse feculent
peritonitis, due to perforation of the jejunum 1 m from the
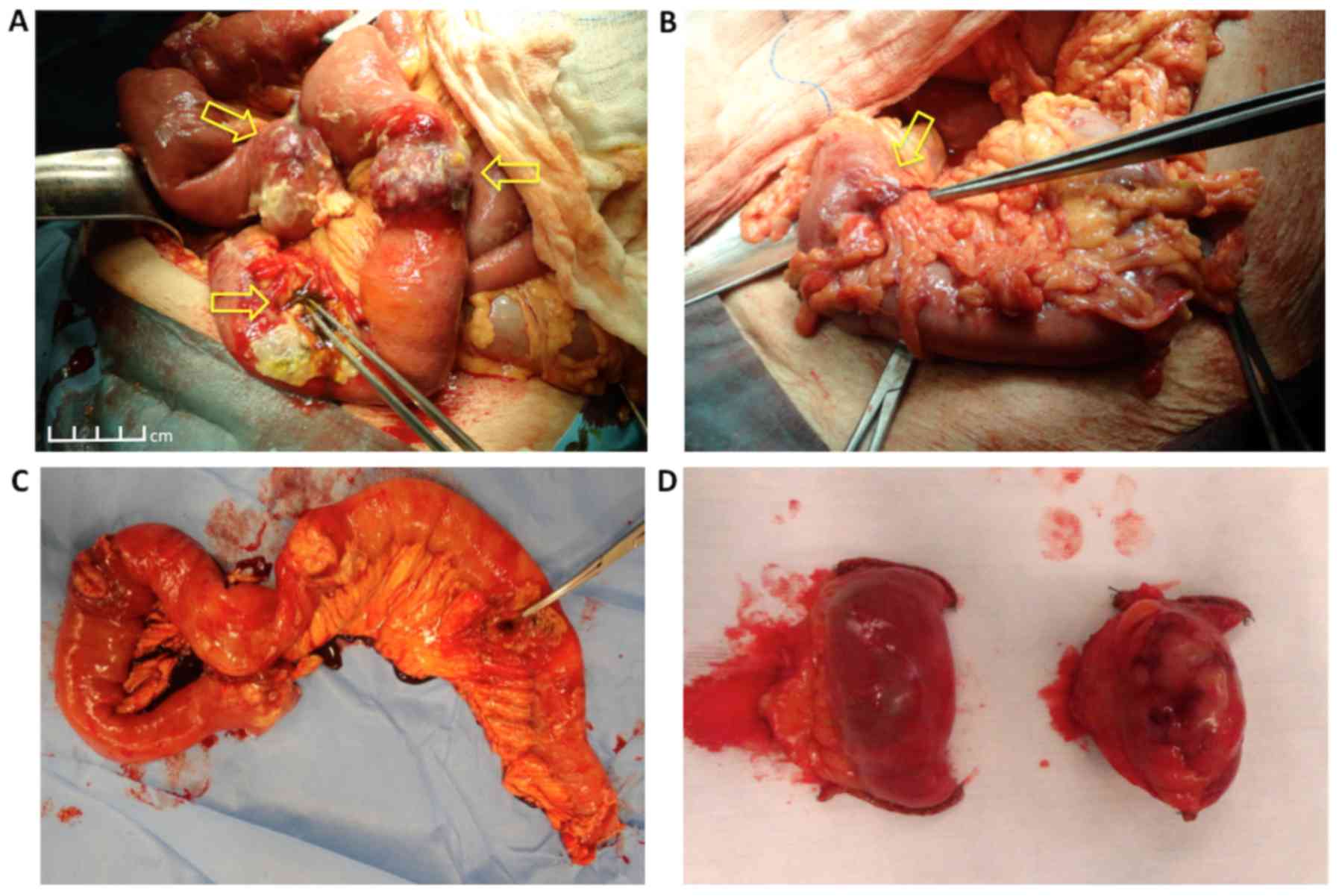
ligament of Treitz, was found. In addition, six distinct tumors of
the jejunum close to the perforation site were found, as well as
two more similar lesions caudally at the ileum (Fig. 2A). The tumors were round, hard and
protruding from the bowel wall, with dimensions <4 cm. A
separate stenotic tumor at the wall of the mid-sigmoid was
identified, which caused moderate dilation of the proximal
intestine (Fig. 2B). Enterectomy,
including the perforation site and the proximal six tumors, was
first performed, followed by limited enterectomies for the distal
two tumors (Fig. 2C and D). The
continuity of the gastrointestinal tract was reinstated with
side-to-side anastomoses of the small intestine using a linear
stapler. For the sigmoid tumor, a Hartmann procedure with limited
resection of the mid-sigmoid was performed. The patient remained in
a severe septic condition following surgery and succumbed the next
day.
The resected specimens consisted of three segments
of small bowel and a segment of large bowel measuring 85, 6.4, 4.7
and 9.2 cm in length, respectively. Macroscopic examination of the
resected bowel revealed six tumors with a maximum diameter from 2.4
to 4.9 cm; the jejunal segment exhibited a point of serosal
involvement and perforation. Additional findings included two
tumors measuring 3.7 and 3.5 cm, found in the other two segments of
the small bowel. Upon examination of the large bowel, an
intraluminal polypoid tumor measuring 1.1 cm with a mass of 3.3 cm
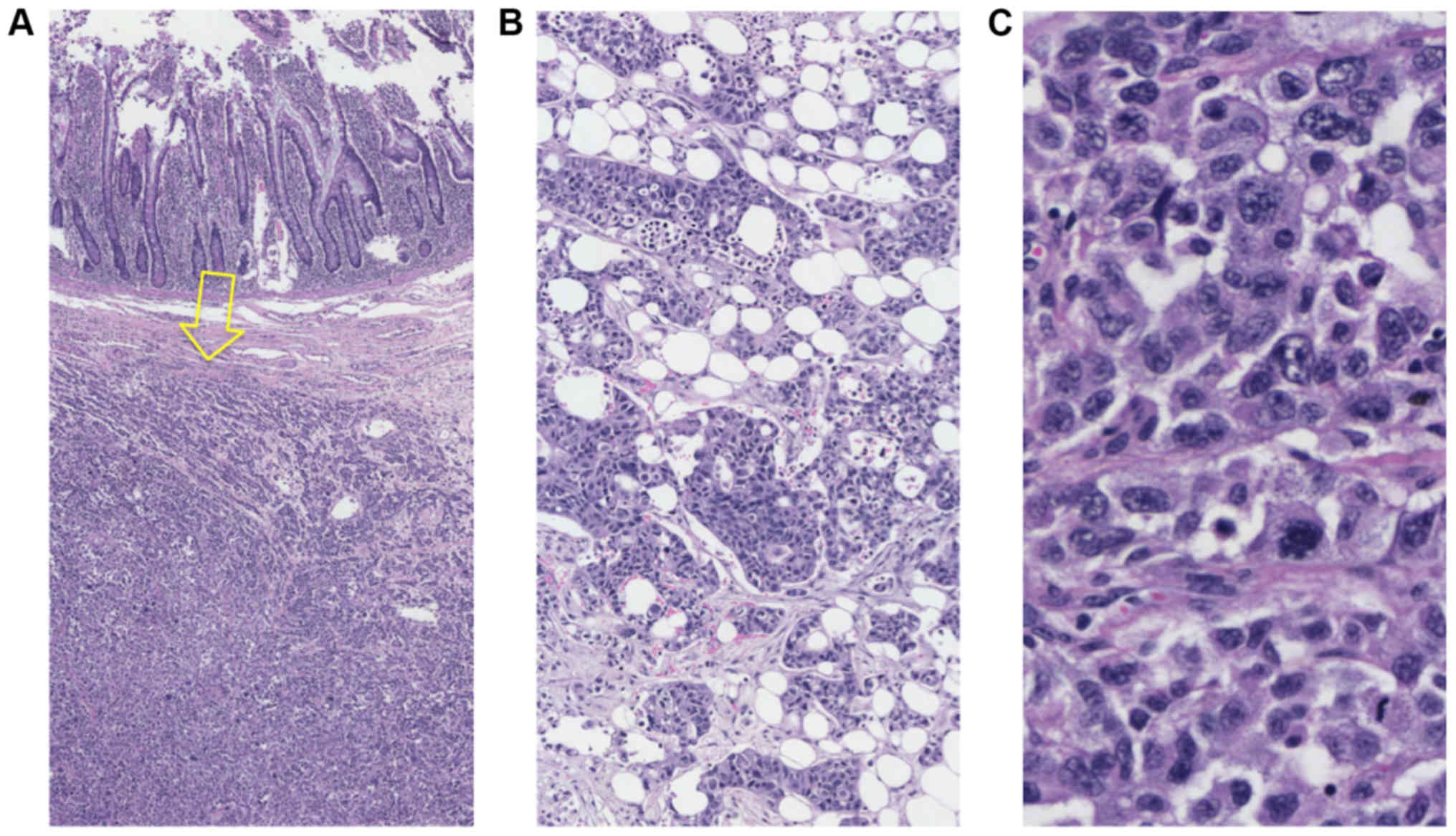
in the pericolic fat was noted. On microscopic examination,
hematoxylin and eosin (H&E)-stained sections from the tumors
showed features of a poorly differentiated neoplasm (Fig. 3) in the submucosa. By contrast, the
intraluminal large bowel tumor was an adenoma with low-grade
dysplasia. The possibility of numerous metastases was the primary
consideration. The differential diagnosis was that of multiple
intestinal neoplasms, either in the setting of familial adenomatous
polyposis or associated with Lynch syndrome. Molecular analysis
revealed mutation of p.Gly12Val (c.35 G>T) in KRAS
proto-oncogene, GTPase (KRAS), with no mutations in NRAS
proto-oncogene, GTPase (NRAS) or B-Raf proto-oncogene,
serine/threonine kinase (BRAF). Immunohistochemical (IHC) testing
for Mismatch Repair (MMR) proteins showed intact nuclear
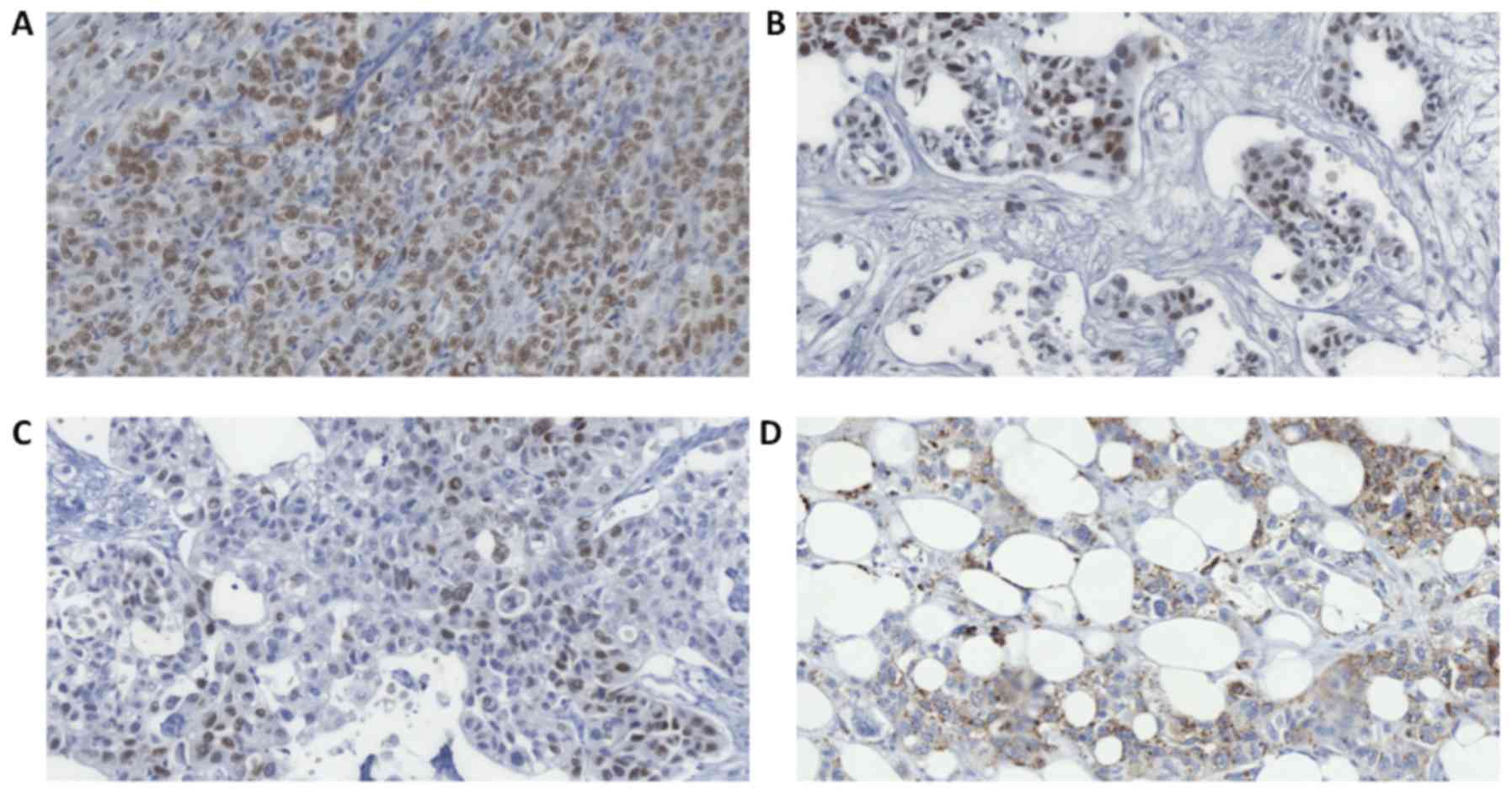
expression. Further IHC analysis performed on sections of a small
bowel mass showed strong immunoreactivity for cytokeratin (CK)7, CK
8/18 and, particularly, thyroid transcription factor 1 (TTF1;
Fig. 4A and B). Analysis of the
pericolic fat mass additionally showed focal immunoreactivity for
polyclonal carcinoembryonic antigen (pCEA), p63 (Fig. 4C) and CK5/6. Both tumors were
negative for chromogranin and synaptophysin. Histochemical analysis
with Alcian blue did not detect mucin. Based on this profile, a
diagnosis of metastatic lung carcinoma-likely adenocarcinoma or
adenosquamous carcinoma-was rendered. Finally, Napsin A staining
was performed, which was also positive (Fig. 4D).
Discussion
Metastases to the gastrointestinal tract are rather
rare in patients with lung cancer and are more commonly encountered
in the advanced stages of the disease. The spread to the
gastrointestinal tract occurs via the hematogenous and lymphatic
routes. When the metastatic disease involves the upper
gastrointestinal tract, the most common symptom is bleeding, but
when it involves the small intestine, the most typical
manifestation is intestinal obstruction, with or without
perforation (4). Historically,
Oschner and Debakey in 1942 reported gastrointestinal involvement
in 4.3% of 3,047 autopsies (5).
Further, Yang et al (6)
estimated the incidence of symptomatic gastrointestinal secondaries
from primary lung cancer at 1.77%. Other reports have shown that
the prevalence of these tumors at autopsy is much higher, ranging
from 4.7 to 14% (7,8). Such higher rates are attributed to the
fact that most patients with small bowel metastases often present
with non-specific symptoms. McNeill et al (2) reviewed 431 patients with diagnosed lung
cancer and demonstrated that small bowel metastases were present in
10.7% of these patients at autopsy. All patients with small bowel
metastases had at least one more metastatic site, with an average
of 4.8 sites (2). In a published
series of 423 autopsies, in which 58 patients presented with
gastrointestinal tract secondary lesions from primary lung cancer,
the most frequently encountered histological types were squamous
cell (33%), large cell (29%) and oat cell (19%) (7,9).
Perforation of the small intestine is an extremely
rare and serious complication in lung cancer patients, occurring at
the advanced stages of the disease. Morgan et al (10) reported the first case in 1961, in a
patient with advanced lung carcinoma receiving cyclophosphamide.
Garwood et al (11) performed
a Medline search to identify all cases of gastrointestinal tract
perforations attributed to metastatic lung cancer reported in the
literature. Data were collected from the medical literature between
1960 and 2005. They identified 98 cases of perforated lung cancer
metastasis to the small intestine. Perforations occurred most often
in the jejunum (53%) followed by the ileum (28%), whereas combined
jejunum-ileum lesions accounted for 4% of perforations. Other
causes of small bowel perforation included adenocarcinoma (23.7%),
squamous cell carcinoma (22.7%), large cell carcinoma (20.6%), and
small cell carcinoma (19.6%) (11).
The tumor replaces all or part of the bowel wall,
resulting in various presentations: Bulky tumors causing lumen
obstruction, necrotic tumors that often perforate, ulcerative
lesions causing bleeding, or extensive mucosal surface involvement
leading to malabsorption (12).
McNeill et al (2) showed that
when the primary cancer is in the lungs, metastases at the small
bowel are more likely to perforate, as the metastatic tumors
typically undergo necrosis prior to becoming large enough to cause
intestinal obstruction (2).
Αt endoscopy, lung cancer metastases to the
intestinal wall have no specific features, and usually appear as
diffuse involvement of the intestinal mucosa, multiple nodules
with/without mucosal ulceration, or as a solitary ‘volcano-like’
tumor of the intestine (4,13). There has been a debate regarding the
most common lung cancer histotype that metastasizes in the
gastrointestinal tract. Some authors have suggested squamous cell
carcinoma as the most common histological type (4,14),
whereas other reports have revealed that poorly differentiated
pulmonary adenocarcinomas and large cell undifferentiated
carcinomas have a specific predilection for the digestive tract
(4,7,15).
Studies from Italy (4) and Taiwan
(6) have suggested that 0.5–1.7% of
patients with primary lung cancer develop gastrointestinal tract
metastasis. The cell type in Taiwan was squamous cell carcinoma
(3/6) in the majority of cases, whereas large cell carcinoma
(10/18) was the dominant type in Italy. Therefore, the histological
type predominantly associated with gastrointestinal metastasis
remains unclear.
Gray et al (16) reported a case of small bowel
perforation in a patient with non-small cell lung cancer following
treatment with bevacizumab (16).
Although several reports followed, the pathogenesis of perforation
is not yet clear. Leidich and Rudolf (17) reported that perforations occur once
the bowel wall is replaced by tumor cells, which is followed by
cellular necrosis (17). However,
chemotherapy also plays an important role in the development of
bowel perforation. In this regard, Owen and Chasen (18) reported a case of intestinal
perforation in a patient with an extrapulmonary large cell
carcinoma of the small bowel, who received platinum-based
chemotherapy. Chemotherapy may have induced rapid tumor necrosis,
resulting in the loss of bowel wall integrity (18). Patients with small bowel perforation
due to metastatic lung cancer typically present with signs of acute
abdomen. Management consists of patient resuscitation,
stabilization with fluids and electrolytes, followed by exploratory
laparotomy. At surgery, resection of the affected part of the
bowel, including the site of perforation, should be performed, with
or without prophylactic stoma formation (17,19).
Meticulous exploration and irrigation of the peritoneal cavity
should also be performed on an emergency basis and is the only way
to revert ongoing sepsis. Despite the poor prognosis of these
patients, surgery may provide acceptable palliation and lead to
satisfactory survival (19,20). Indeed, Nagashima et al
(21) reviewed 48 cases operated on
for intestinal perforation due to metastatic lung cancer, and
reported a median postoperative survival time of 48 days. They also
concluded that patient performance status may be an important
prognostic factor (21).
Pathological diagnosis with the use of
immunohistochemical stains is the only reliable way to
differentiate a primary small bowel malignancy from a metastatic
lesion deriving from the lung (22).
Primary lung carcinomas usually exhibit a
CK7+/CK20− immunophenotype as opposed to the
usual CK7−/CK20+ pattern of intestinal
adenocarcinomas (4). However,
primary rectal or small bowel adenocarcinomas may also be
CK7+/CK20− (8). Thus, TTF-1 and or Napsin A positivity
is critical in establishing a primary lung cancer origin. TTF-1 is
highly specific for adenocarcinomas of pulmonary origin exhibiting
a positive predictive value of >90% (4,19).
Finally, according to the most recent review of the
literature, surgical resection of the involved portion of the small
bowel with primary end-to-end anastomosis may be the optimal
treatment strategy, particularly in cases with obstruction,
hemorrhage and/or perforation (23).
Notably, following successful resection of such lesions and despite
the presence of additional metastatic lesions besides the
intestinal tract, survival for >1 year has been reported.
However, in the vast majority of these patients, survival outcomes
are poor (24).
In conclusion, metastatic lesions from lung
carcinoma in the small bowel wall are a rare finding predisposing
to bowel perforation. Perforation peritonitis associated with
multiple intestinal metastases carries a high mortality rate, even
with prompt surgical therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
EPM and AM contributed to the conception and design
of the study. ARG, VD, DT, DP, FAF, AT, DS and NM contributed to
the acquisition, analysis and interpretation of data. EPM, DS and
NM drafted the manuscript and/or revised it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
sister of the patient, who was the next of kin for this patient,
for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu W, Zhou W, QL WL, Ma YD and Xu YY:
Gastrointestinal hemorrhage due to ileal metastasis from primary
lung cancer. World J Gastroenterol. 21:3435–3440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossi G, Marchioni A, Romagnani E,
Bertolini F, Longo L, Cavazza A and Barbieri F: Primary lung cancer
presenting with gastrointestinal tract involvement:
Clinicopathologic and immunohistochemical features in a series of
18 consecutive cases. J Thorac Oncol. 2:115–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oschner A and Debakey M: Significance of
metastasis in primary carcinoma of the lungs: report of two cases
with unusual sites of metastasis. J Thorac Surg. 11:357–387.
1942.
|
|
6
|
Yang CJ, Hwang JJ, Kang WY, Chong LW, Wang
TH, Sheu CC, Tsai JR and Huang MS: Gastro-intestinal metastases of
primary lung carcinoma: Clinical presentations and outcome. Lung
Cancer. 54:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antler AS, Ough Y, Pitchumoni CS, Davidian
M and Thelmo W: Gastrointestinal metastases from malignant tumors
of the lung. Cancer. 49:170–172. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimoto A, Kasahara E and Kawashima A:
gastrointestinal metastases from primary lung cancer. Eur J Cancer.
42:3157–3160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuen JS, Chow PK and Ahmed Q: Metastatic
lung cancer causing bowel perforations: Spontaneous or
chemotherapy-related? ANZ J Surg. 72:245–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgan MW, Sigel B and Wolcott MW:
Perforation of a metastatic carcinoma of the jejunum after cancer
chemotherapy. Surgery. 49:687–689. 1961.PubMed/NCBI
|
|
11
|
Garwood RA, Sawyer MD, Ledesma EJ, Foley E
and Claridge JA: A case and review of bowel perforation secondary
to metastatic lung cancer. Am Surg. 71:110–116. 2005.PubMed/NCBI
|
|
12
|
Sujith NS, Sima RR, Thimmappa A and
Govindaraj S: An unusual presentation of metastatic lung cancer.
Int Surg J. 3:1700–1704. 2016. View Article : Google Scholar
|
|
13
|
Hsu CC, Chen JJ and Changchien CS:
Endoscopic features of metastatic tumors in the upper
gastrointestinal tract. Endoscopy. 28:249–253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berger A, Cellier C, Daniel C, Kron C,
Riquet M, Barbier JP, Cugnenc PH and Landi B: Small bowel
metastases from primary carcinoma of the lung: Clinical findings
and outcome. Am J Gastroenterol. 94:1884–1887. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edwards R and Royle G: Metastatic
carcinoma causing haematemesis. Br Med J. 2:5981975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gray J, Murren J, Sharma A, Kelley S,
Detterbeck F and Bepler G: Perforated viscus in a patient with
non-small cell lung cancer receiving bevacizumab. J Thorac Oncol.
2:571–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leidich RB and Rudolf LE: Small bowel
perforation secondary to metastatic lung carcinoma. Ann Surg.
193:67–69. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Owen S and Chasen M: Chemotherapy-induced
small bowel perforation in a patient with extrapulmonary small-cell
carcinoma of the small bowel. Curr Oncol. 15:298–301.
2008.PubMed/NCBI
|
|
19
|
Goh BK, Yeo AW, Koong HN, Ooi LL and Wong
WK: Laparotomy for acute complications of gastrointestinal
metastases from lung cancer: Is it a worthwhile or futile effort?
Surg Today. 37:370–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salemis NS, Nikou E, Liatsos C, Gakis C,
Karagkiouzis G and Gourgiotis S: Small bowel perforation secondary
to metastatic non-small cell lung cancer. A rare entity with a
dismal prognosis. J Gastrointest Cancer. 43:391–395. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagashima Y, Okamoto H, Narita Y, Hida N,
Naoki K, Kunikane H and Watanabe K: Perforation of the small
intestine caused by metastasis from primary lung cancer: Report of
two cases and the discussion of 48 cases published in the Japanese
literature. Nihon Kokyuki Gakkai Zasshi. 45:430–435. 2007.(In
Japanese). PubMed/NCBI
|
|
22
|
Gonzalez-Tallon AI, Vasquez-Guerrero J and
Garcia-Mayor MA: Colonic metastases from lung carcinoma: A case
report and review of the literature. Gastroenterology Res. 6:29–33.
2013.PubMed/NCBI
|
|
23
|
Di JZ, Peng J and Wang ZG: Prevalence,
clinicopathological characteristics, treatment, and prognosis of
intestinal metastasis of primary lung cancer: A comprehensive
review. Surg Oncol. 23:72–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yildirim M, Tasli F, Bayam ME and Postaci
H: A rare cause of small bowel transection: Metastatic lung cancer.
Med Princ Pract. 19:232–234. 2010. View Article : Google Scholar : PubMed/NCBI
|