Introduction
Cancer of the prostate, a gland in the male genital
system, is a growing concern in the field of global epidemiology.
Statistics for the USA demonstrate that ~20% of males will be
diagnosed with prostate cancer during their lifetime (1). Prostate cancer contributes
significantly to the mortality of men and ranks as the fifth most
common cause of cancer-associated mortality among men in the USA
(1). Every year, >1,000,000 cases
are diagnosed and >300,000 individuals succumb to the disease
worldwide (2). Therefore, there is
an urgent requirement for alternative therapeutic strategies to
improve prostate cancer prognosis.
Although improvements have been made in the field of
prostate cancer therapy, therapy failure and low survival rates
persist among patients. This can be attributed to metastasis, drug
resistance and high rates of recurrence (3,4). One of
the main causes for these phenomena is the persistence of prostate
cancer-initiating cells (CICs) (5–7).
Therefore, the elimination of prostate CICs may contribute
significantly towards treatment strategies for prostate cancer.
Cluster of differentiation (CD) 44, a multi-functional protein
associated with cell adhesion and signaling, is one of the major
markers for prostate CICs (8,9).
Patrawala et al (9)
demonstrated that compared with CD44− prostate cancer
cells, CD44+ cells are more aggressive, as is reflected
by their higher clonogenicity, tumorigenicity and metastatic
ability. Furthermore, certain intrinsic characteristics of
progenitor cells have been identified in CD44+ prostate
CICs; this includes increased expression of a group of stemness
genes, including β-catenin and octamer-binding transcription factor
3/4 (9). Therefore, the eradication
of CD44+ prostate CICs may enhance therapeutic efficacy
in the treatment of prostate cancer.
Salinomycin, an antibiotic isolated from
Streptomyces albus, is a therapeutic drug with potent
activity against CICs in various types of cancer (10–13). The
mechanisms of action of this drug include the inhibition of the Wnt
pathway and the induction of apoptosis (12). A number of studies have reported that
salinomycin can exert potent anticancer effects in prostate cancer
cells via these mechanisms (14–16).
However, to the best of our knowledge, only one study has confirmed
the superior therapeutic effects of salinomycin against prostate
CICs (13). Therefore, more data is
required to support claims that salinomycin exhibits high
therapeutic efficacy against prostate CICs.
Despite its promise as an anticancer agent, the
aqueous solubility of salinomycin is poor, resulting in low
bioavailability and poor therapeutic efficacy in vivo
(10,11,17). A
possible solution to this problem involves nanoparticle-based
strategies. Nanoparticles have been demonstrated to markedly
improve the solubility and therapeutic index of poorly soluble
drugs by their controlled and targeted delivery (6,7). With
this in mind, numerous studies have developed salinomycin-loaded
nanoparticles to facilitate the preclinical investigation of this
drug as a cancer therapeutic strategy (10,11,17).
Lipid-polymer hybrid nanoparticles consisting of
biodegradable polymers and lipids represent superior candidate drug
delivery systems, as they combine the advantages of liposomes and
polymer nanoparticles (18,19). Liposomes are characterized by
superior biocompatibility and are attractive due to the ease with
which modifications can be made to their component hydrophilic
polymer, polyethylene glycol (PEG), or their targeting molecules,
including antibodies, peptides and aptamers (20). The advantages of polymer
nanoparticles, including poly(lactide-co-glycolide acid) (PLGA),
which is the most commonly used, include controlled and sustained
release, high drug loading capacity and superior stability
(21,22). Therefore, the advantages of
lipid-polymer hybrid nanoparticles include superior
biocompatibility, ease of modification, controlled and sustained
release, stability and high drug loading capacity (18,19).
There is currently considerable interest in
antibody-targeted nanoparticles as a strategy to promote
chemotherapeutic efficiency by ensuring targeted delivery of
therapeutic drugs, and this approach has been demonstrated to be
successful in the treatment of several types of cancer (23,24).
Since CD44 is a marker for prostate CICs, it may be possible to use
the CD44 antibody to promote the targeted delivery of
salinomycin-loaded nanoparticles to CICs.
In order to accomplish this, the current study
generated salinomycin-encapsulated lipid-PLGA nanoparticles linked
with CD44 antibodies (SM-LPN-CD44). The characteristics of
SM-LPN-CD44 were then investigated to evaluate its targeting
ability and its therapeutic effect against prostate CICs.
Materials and methods
Reagents and cell culture
PLGA (50:50 molar ratio between lactide and
glycolide; 40–75 kDa), polyvinyl alcohol (PVA; 30–70 kDa),
2-iminothiolane, salinomycin and organic reagents were all
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
lipids, including
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide
(PEG)-2000](DSPE-PEG-Mal),
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein)
(PECF), phosphatidylcholine and cholesterol were obtained from
Avanti Polar Lipids (Alabaster, AL, USA). R&D Systems, Inc.,
(Minneapolis, MN, USA) provided the rat anti-human CD44 Alexa
Fluor® 488-conjugated antibody (cat. no. FAB6127G) and
recombinant rat anti-human CD44 monoclonal antibody (cat. no.
MAB6127). The CD44 Fab' from the recombinant rat anti-human CD44
monoclonal antibody was isolated using a protocol described in a
previous study (16). The Pierce
bicinchoninic acid (BCA) protein assay kit, RPMI 1640 medium,
Dulbecco's modified Eagle's medium (DMEM), DMEM/Ham's F-12
(DMEM/F12) medium, fetal bovine serum (FBS), B27, epidermal growth
factor (EGF), basic fibroblast growth factor (bFGF) and
insulin-transferrin-selenium (ITS) were purchased from Thermo
Fisher Scientific Inc. (Waltham, MA, USA). The CD44 MicroBead kit
was obtained from Miltenyi Biotec GmbH (Bergisch Gladbach,
Germany).
The DU145 cell line, a human prostate cancer cell
line derived from a metastatic site, and the 22RV1 cell line, a
human prostate carcinoma epithelial cell line, were obtained from
the American Type Culture Collection (Manassas, VA, USA). The
cultures were maintained in DMEM supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a humidified
incubator with 5% CO2.
CD44 expression in prostate cancer
cell lines
Flow cytometry was performed to analyze the
expression of CD44 in the two prostate cancer cell lines. The
cultured cells were dissociated into a single cell suspension,
which was then incubated with rat anti-human CD44 Alexa
Fluor® 488-conjugated antibody diluted in 1% fetal
bovine serum (1:500 dilution; FBS used as a blocking reagent,
Thermo Fisher Scientific, Inc.) at 1 µg/ml for 30 min at 4°C.
Subsequently, the cells were washed with PBS to remove unconjugated
antibody and resuspended in PBS. The FACSCalibur flow cytometer
(Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used
to determine the proportion of positively stained cells. Expression
was analyzed using FlowJo, version 10 (FlowJo LLC, Ashland, OR,
USA).
Magnetic cell sorting-based separation
of CD44+ cells
The separation of CD44+ cells was
performed using the CD44 MicroBead kit according to the
manufacturer's protocol. The final proportion of positively stained
cells was determined by flow cytometry.
Evaluation of the tumorsphere
formation ability of cells
The formation of tumorspheres when single cells are
suspended in serum-free medium indicates the self-renewal ability
of CICs (25–27). Briefly, prostate cancer cells were
suspended in stem cell medium in Corning® ultra-low
adherent 6-well dishes (Corning Inc., Corning, NY, USA) at a
density of 5×103 cells/well. The composition of the stem
cell medium was as follows: DMEM/F12 supplemented with B27 (1×),
ITS (1×), EGF (20 ng/ml) and bFGF (20 ng/ml). The cells were
cultured for 7 days, following which the number of tumorspheres was
counted using light microscopy (magnification, ×50). The
tumorsphere formation rate of the untreated group was used as a
control, in which the rate was defined as 100%. To obtain a second
passage, the first passage tumorspheres were washed with PBS,
disaggregated using cell dissociation reagent (StemPro®
Accutase®; Thermo Fisher Scientific, Inc.) and
propagated in stem cell medium for 7 days.
In vivo investigation of
tumorigenicity
The tumorigenicity of prostate cancer cells in
vivo was studied in BALB/c nude mice (4–5 weeks old; male; ~20
g; 24 mice were used, 6 mice/group) purchased from the Shanghai
Experimental Animal Center (Shanghai, China). The mice were
acclimated for ~7 days in a pathogen-free environment. Animals were
housed in separate cages (3–4 animals per cage) maintained under a
controlled atmosphere (humidity of 50±7% and a temperature of
21±1°C) and with a 12:12 h light/dark cycle. The mice were allowed
free access to food and water. All animal procedures were approved
by the Animal Administrative Committee of the Naval Medical
University (Shanghai, China) and performed in accordance with their
guidelines. Briefly, varying numbers of CD44+ or
CD44− prostate cancer cells (range,
2×103−1×106 cells) were isolated from the
cell lines using the aforementioned magnetic cell-sorting method.
The collected cells were mixed with BD Matrigel™ (Becton, Dickinson
and Company) and the mixture was injected subcutaneously into the
right flank of the mice. Subsequent tumor formation was observed
and recorded for a period of 15 weeks. Mice were sacrificed if the
tumor size exceeded 1,500 mm3.
Preparation of lipid-PLGA hybrid
nanoparticles
Lipid-PLGA hybrid nanoparticles were generated using
the emulsion-solvent evaporation-based procedure. In brief, 0.5 mg
salinomycin and 5 mg PLGA were completely dissolved in acetone to
form the oil phase. The oil solution was injected into 2% PVA
solution, followed by homogenization. The mini-emulsion was then
poured into a 0.2% PVA solution and mixed rapidly for 6 h to remove
any remaining acetone by evaporation. The nanoparticles were
recovered by ultracentrifugation (80,000 × g) at 25°C for 30 min.
At the same time, a lipid film composed of phosphatidylcholine
(Avanti Polar Lipids), DSPE-PEG-Mal and cholesterol (57:3:40 molar
ratio) was formed in a round-bottomed flask upon using a vacuum
rotary evaporator. Once the lipid film was formed, the recovered
nanoparticles were added to hydrate it. A hand-held extruder
(Avanti Polar Lipids) with 200-nm membranes was used to extrude the
lipid-polymer suspension in order to create small and homogeneous
nanoparticles. The resultant lipid-polymer nanoparticles were
washed with distilled water by ultracentrifugation in
Amicon® Ultra-4 centrifugal filter devices (nominal
molecular weight limit, 100,000; EMD Millipore, Billerica, MA,
USA). Furthermore, CD44 Fab' was thiolated by the addition of
2-iminothiolane (molar ratio of 1:100) (16). Thiolated CD44 Fab' was incubated with
the nanoparticles (molar ratio of 1:10) for 6 h at room temperature
in order to conjugate thiolated antibodies with the nanoparticles.
The Amicon centrifugal filters were used to remove unconjugated
Fab'. Non-targeted nanoparticles were developed using a similar
protocol, but with the exclusion of Fab'. Blank nanoparticles were
developed using a similar protocol, but with the exclusion of
salinomycin and Fab'. The fluorescent PECF-labeled nanoparticles
were constructed using a similar protocol except that 0.1% PECF was
included as part of the lipid film composition. The following
abbreviations are used to describe the nanoparticles used in the
present study: SM-LPN, salinomycin-encapsulated lipid-PLGA
nanoparticles; SM-LPN-CD44, salinomycin-encapsulated lipid-PLGA
nanoparticles linked with CD44 antibodies; and LPN-CD44, blank
lipid-PLGA nanoparticles linked with CD44 antibodies.
Conjugation efficacy of antibodies
with nanoparticles
Ultrafiltration of the nanoparticles was performed
to evaluate the conjugation efficacy of antibodies with
nanoparticles. Briefly, the antibodies were incubated with the
nanoparticles and the mixture was centrifuged in Amicon centrifugal
filters (molecular weight cut-off value, 100 kDa) to exclude
unconjugated antibodies. The unconjugated antibodies removed were
measured using the Pierce BCA protein assay kit. The efficacy of
conjugation between antibodies and the fabricated nanoparticles
were evaluated using the following equation:
(Mt-Mu)/Mt, where Mt
denotes the total mass of added antibodies and MU
represents the mass of unconjugated antibodies.
Size, ζ potential, morphology and drug
loading capacity of lipid-PLGA nanoparticles
A lipid-PLGA solution, prepared by diluting 100 µl
nanoparticles in 1.9 ml distilled water, was run through a
Zetasizer Nano ZS90 (Malvern Instruments, Ltd., Malvern, UK) to
evaluate the nanoparticle size and ζ potential. The salinomycin
encapsulation efficiency and loading capacity of the lipid-PLGA
nanoparticles was determined by reverse-phase high-performance
liquid chromatography (HPLC) using the universal reverse phase
Diamonsil® C-18 column (size of packing carriers, 5 µm;
length × width, 250×4.5 mm; Dikma Technologies Inc., Lake Forest,
CA, USA). Briefly, 1 ml dichloromethane was added to 2 mg
lyophilized nanoparticles to dissolve them. The dichloromethane was
removed completely by evaporation via vacuum drying, and methanol
was added to dissolve the residual nanoparticles by thorough
vortexing. The analysis was performed using the L-2000 HPLC system
(Hitachi, Ltd., Tokyo, Japan). The mobile phase used was
water/tetrahydrofuran/acetonitrile/phosphoric acid (v/v/v/v,
10/4/86/0.01) and the flow rate was 1 ml/min. The detection
wavelength for salinomycin was set at 210 nm. Finally, a PECF
calibration curve was used to evaluate the drug loading capacity of
the labeled nanoparticles.
Salinomycin release profile of
encapsulated lipid-PLGA nanoparticles
Lipid-PLGA nanoparticles (0.5 mg/ml) were suspended
in PBS or PBS supplemented with 10% FBS in a centrifuge tube.
Subsequently, the nanoparticles were placed on an orbital shaker
moving at 100 rpm at a temperature of 37°C. At multiple designated
time points (0.5, 1, 2, 3, 4, 6, 8, 12, 24, 48, 72 and 148 h)
during the 150 h drug release period, the tubes were centrifuged
(12,000 × g for 30 min) and the supernatant was evaluated by
reverse-HPLC, as aforementioned. The cumulative salinomcyin release
rate of the nanoparticles was calculated using the following
formula: (Mi/Mt) × 100, where Mi
is the mass of cumulative released salinomycin and Mt is
the total mass of salinomycin used to encapsulate the
nanoparticles.
In vitro targeting of fluorescent
nanoparticles to prostate cancer cells
Flow cytometry was used to study the in vitro
targeting of fluorescent nanoparticles as described previously
(10). Briefly, prostate cancer
cells were seeded in a 12-well cell culture plate at a density of
5×105 cells per well, and incubated overnight at 37°C.
The old medium was replaced with fresh medium in which PECF-loaded
nanoparticles (1 µg/ml PECF) were dissolved. The cells were
incubated for a further 2 h. The cells were then washed three times
with PBS to remove unbound nanoparticles and trypsinized. The cells
were subsequently resuspended in PBS and analyzed using the
FACSCalibur flow cytometer.
Evaluation of cytotoxicity of
nanoparticles towards prostate cancer cell lines
The cytotoxicity of nanoparticles against the
prostate cancer cell lines was evaluated with the CCK-8 assay kit
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocol (10). Briefly, the cells were washed,
trypsinized, seeded in 96-well cell culture plates at a density of
3×103 cells/well and cultured overnight. Spent medium
was discarded and replaced with fresh medium containing free
salinomycin or salinomycin PLGA-lipid nanoparticles at various
concentrations (0.01, 0.04, 0.12, 0.37, 1.11, 3.33, 10.00, 30.00,
90.00 and 270.00 µg/ml). Following incubation for 72 h at 37°C, the
medium was replaced with fresh medium containing no drug. The
viability of the treated cells was determined by performing a CCK-8
assay using a microplate reader. The data was analyzed using
GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA,
USA) to calculate the final half maximal inhibitory concentration
(IC50) values.
Evaluation of the impact of
nanoparticles on the proportion of CICs in cultured cells
Tumorsphere formation and the proportion of
CD44+ cells were taken as parameters for evaluation of
the impact of the generated nanoparticles on the proportion of CICs
in the cultured cells. In brief, cells were washed, trypsinized and
seeded at a density of 5×104 cells/well in 12-well cell
culture plates. Following incubation overnight, the cells were
washed with PBS and treated with fresh medium containing dissolved
nanoparticles (an amount equivalent to 5 µg/ml salinomycin).
Following 24 h of incubation, the spent medium was aspirated and
fresh medium with no nanoparticles was added. Following a further
72 h incubation, the cells were washed, trypsinized and propagated
to evaluate the formation of tumorspheres. Alternatively, flow
cytometry was performed to measure the percentage of
CD44+ cells from trypsinized cells.
Statistical analysis
The data were analyzed using SPSS (version 13; SPSS,
Inc., Chicago, IL, USA). Two groups were statistically compared
using the Student's non-paired t-test. Three or more groups were
compared using one-way analysis of variance followed by the
Student-Newman-Keuls or Dunnett's post hoc tests. P<0.05 was
considered to indicate a statistically significant difference. All
data are expressed as the mean ± standard deviation.
Results
Characteristics of generated
lipid-PLGA hybrid nanoparticles
The lipid-PLGA nanoparticles were generated by
following three simple steps. Firstly, the PLGA nanoparticle core
was prepared by the emulsion-solvent evaporation procedure.
Secondly, the core was coated with the lipid shell by lipid-film
based hydration. Next, the thiolated antibodies were linked to the
nanoparticles by a reaction between sulfhydryl and maleimide
groups. The size, ζ potential and drug loading capacity of the
nanoparticles are presented in Table
I. SM-LPN, the unconjugated nanoparticles, had a size of 125.6
nm. Nanoparticles conjugated with antibodies were larger;
SM-LPN-CD44 and LPN-CD44 had a size of 139.9 and 31.1 nm,
respectively. The ζ potential of all the nanoparticles was negative
and ~-15 mV. The drug loading capacity of all nanoparticles was ~8%
and they all exhibited an encapsulation efficiency of ~75%. The
conjugation efficiency of antibodies on SM-LPN-CD44 was ~12%. As
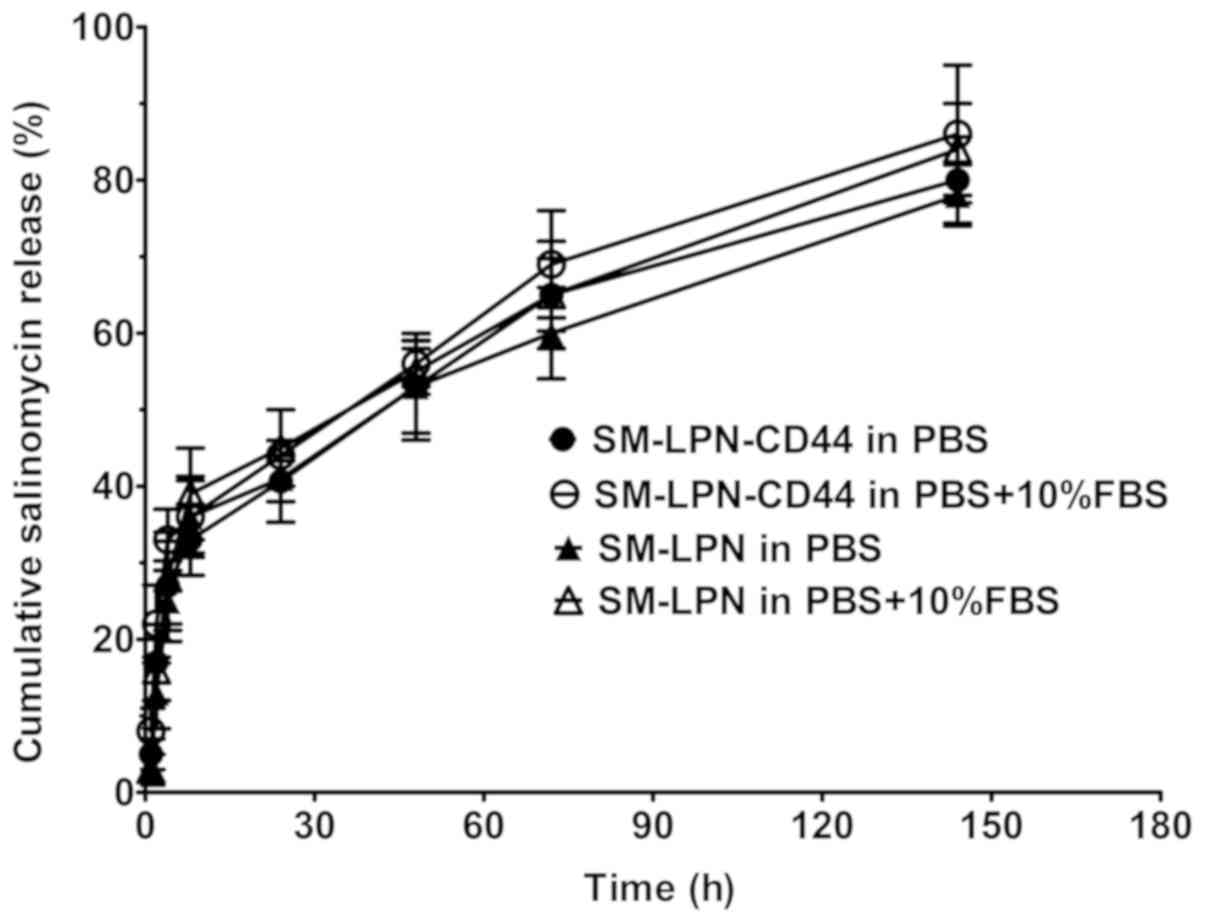
demonstrated in Fig. 1, the
salinomycin release assay indicated that all the nanoparticles
exhibited a burst release, with ~45% of salinomycin released within
the first 24 h. Following 120 h, the cumulative salinomycin
released gradually reached 80%, suggesting that all the
nanoparticles exhibit a sustained drug release for 120 h following
the initial 24 h period. The salinomycin release profile of the
nanoparticles in PBS was not significantly different from their
release profile in PBS supplemented with 10% FBS (P>0.05).
 | Table I.Examination characteristics of
nanoparticles. |
Table I.
Examination characteristics of
nanoparticles.
| Nanoparticle | Size, nm | ζ potential,
mv | PDI | Drug loading,
% | EE, % |
|---|
| SM-LPN | 125.6±15.1 | −13.4±5.9 | 0.13±0.05 | 8.1±3.7 | 76.3±9.1 |
| SM-LPN-CD44 | 139.9±18.5 | −17.3±4.4 | 0.17±0.06 | 8.9±2.6 | 74.2±8.7 |
| LPN-CD44 | 131.1±16.7 | −15.8±6.1 | 0.14±0.07 | – |
|
CD44+ prostate cancer cells
exhibit properties of CICs
CD44 MicroBead Kit-based cell sorting was used to
isolate CD44+ cells from prostate cancer cells. A high
percentage (>98%) of CD44+ cells were obtained from
the original cell mixture, in which the percentage of
CD44+ cells was 20–35% (data not shown). A tumorsphere
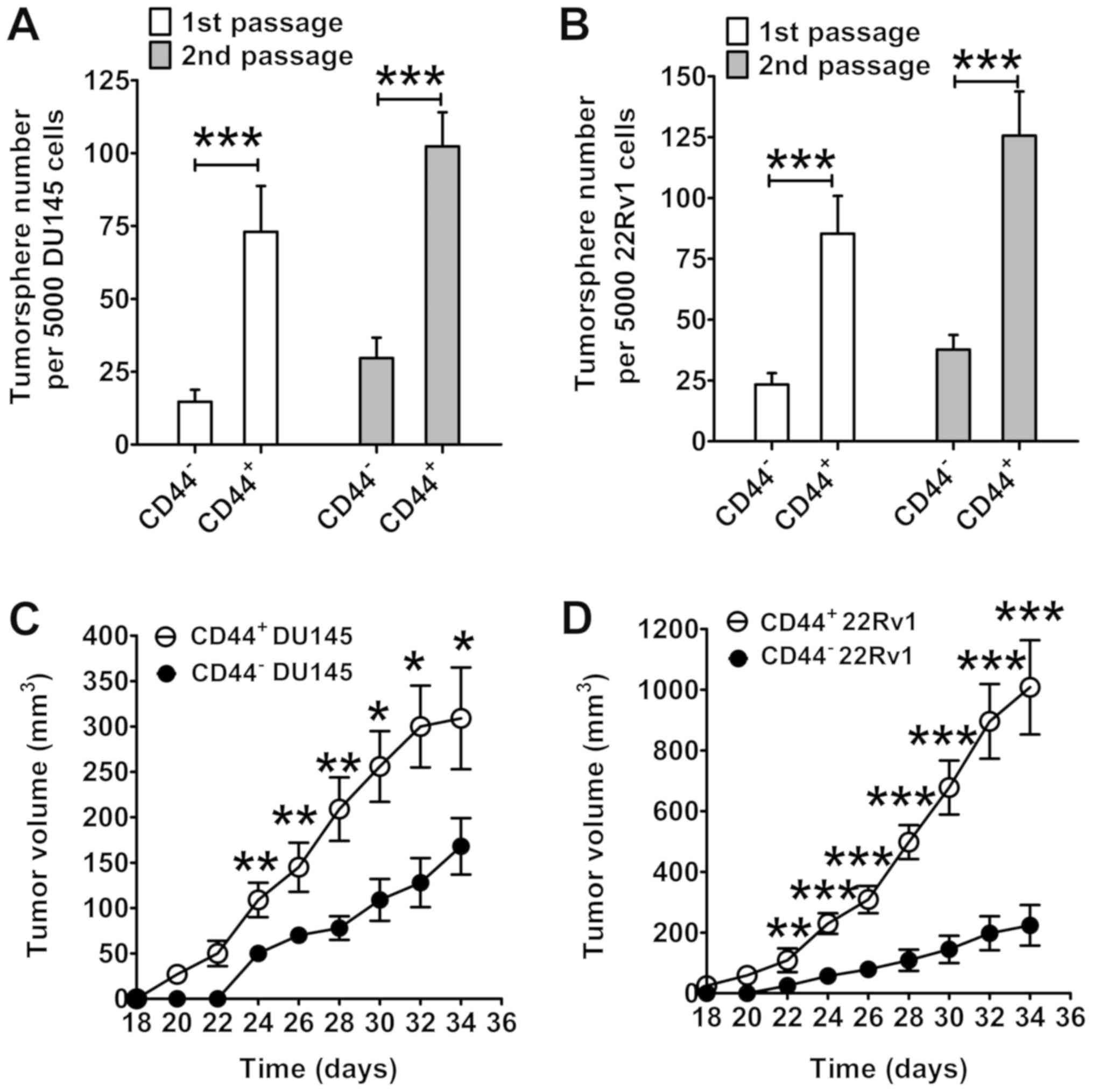
formation assay was used to identify CICs. As presented in Fig. 2A, CD44+ DU145 cells
generated a significantly higher number of tumorspheres compared
with the CD44− DU145 cells (first passage, P<0.001;
second passage, P<0.001). Similar results were obtained in 22RV1
cells (first passage, P<0.001; second passage, P<0.001;
Fig. 2B). Furthermore,
CD44+ cells demonstrated an enhanced capacity for
promoting prostate cancer formation in nude mice compared with
CD44− cells (Fig. 2C and
D). Compared with tumors formed from CD44− cells,
the mean volume of CD44+ cell-initiated tumors was
significantly higher from day 24 onward for DU145 cells, and from
day 22 onward for 22RV1 cells (P<0.05). At the endpoint (day
34), the mean volume of CD44+ DU145 cell-initiated
tumors was 309 mm3, which was significantly larger than
the mean volume of CD44− DU145 cell-initiated tumors
(168 mm3) (P<0.001; Fig.
2C). Fig. 2D presents similar
results in 22RV1 cells. On day 34, the mean volume of tumors
initiated by CD44+ 22RV1 cells was 1,008 mm3,
which was significantly larger than the mean volume of tumors
initiated by CD44− 22RV1 cells (224 mm3;
P<0.001).
Subsequently, the tumorigenicity of various numbers
of prostate cancer cells introduced into mice was evaluated
(Table II). Notably, a 100%
incidence rate of tumors (9/9) was identified in mice injected with
CD44+ DU145 cells at a concentration ≥1×104
cells. By contrast, only a 55% incidence rate of tumors (5/9) was
observed in mice injected with 1×106 CD44−
DU145 cells, indicating that CD44+ DU145 cells exhibit a
significantly greater tumorigenic potential compared with
CD44− DU145 cells. Similarly, CD44+ 22RV1
cells demonstrated significantly greater tumorigenic potential
compared with CD44− 22RV1 cells. A 100% incidence rate
of tumors (9/9) was identified in mice injected with
CD44+ 22RV1 cells at a concentration of
≥1×104 cells. By contrast, only a 78% incidence rate of
tumors (7/9) was observed in mice injected with 1×106
CD44− 22RV1 cells, indicating that CD44+
22RV1 cells exhibit significantly greater tumorigenic potential
compared with CD44− 22RV1 cells. Based on the
aforementioned results, it may be concluded that the tumorigenicity
of CD44+ prostate cancer cells was significantly higher
compared with that of CD44− prostate cancer cells,
indicating that CD44+ cells possess the features of
prostate CICs.
 | Table II.In vivo tumorigenicity of
prostate cancer cells in mice. |
Table II.
In vivo tumorigenicity of
prostate cancer cells in mice.
|
| Number of
cells |
|---|
|
|
|
|---|
| Cell type |
1×106 |
1×105 |
5×104 |
1×104 |
5×103 |
2×103 |
|---|
| CD44−
DU145 | 5/9 | 3/9 | 1/9 | 0/9 | 0/9 | 0/9 |
| CD44+
DU145 | 9/9 | 9/9 | 9/9 | 9/9 | 8/9 | 5/9 |
| CD44−
22RV1 | 7/9 | 6/9 | 3/9 | 1/9 | 0/9 | 0/9 |
| CD44+
22RV1 | 9/9 | 9/9 | 9/9 | 9/9 | 7/9 | 4/9 |
Fluorescently labeled CD44
antibody-conjugated nanoparticles demonstrate highly specific
targeting to CD44+ prostate cancer cells in vitro
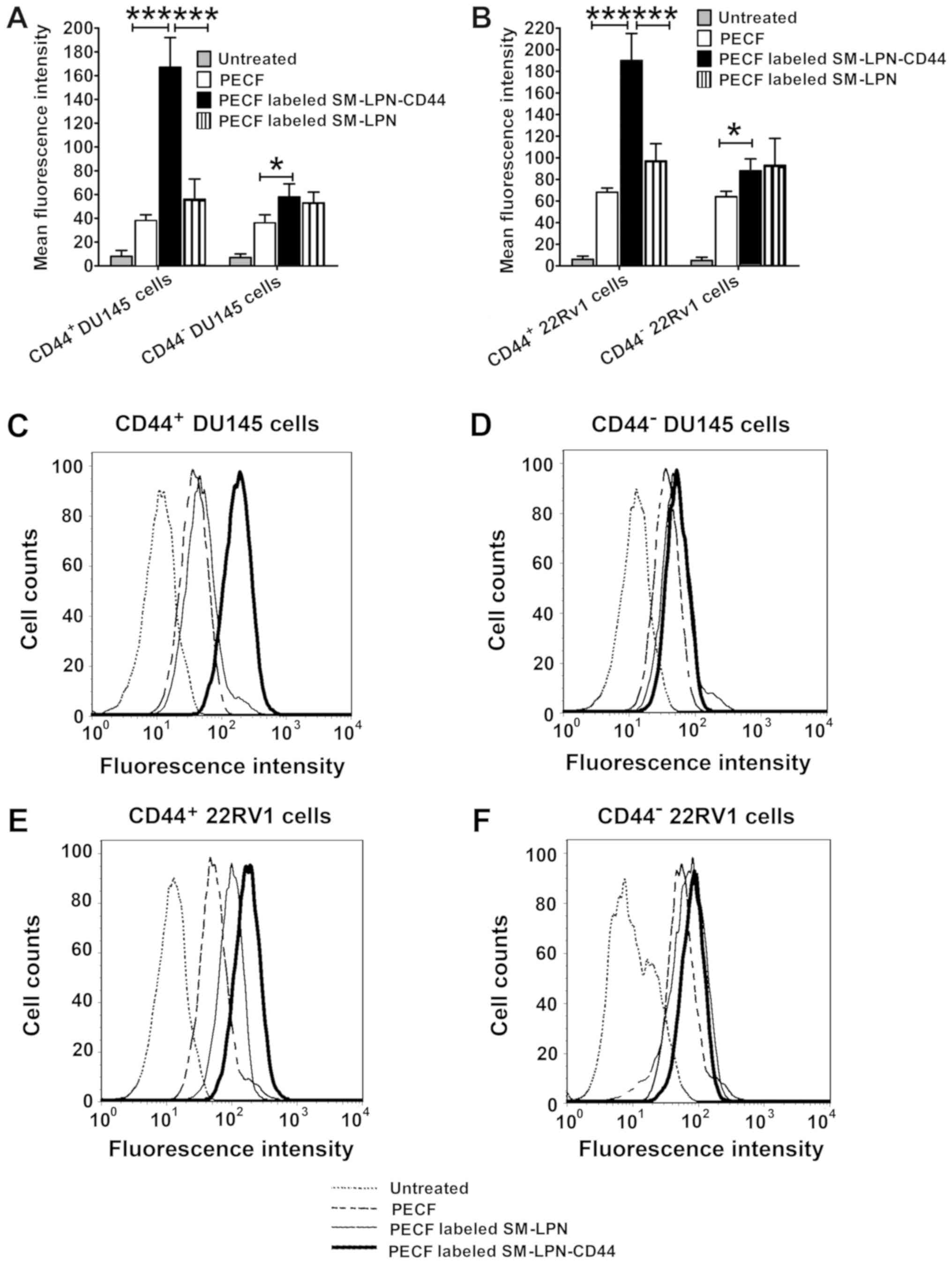
As a common green fluorescent tracer, PECF was used
to evaluate the in vitro targeting of fluorescent
nanoparticles to cancer cells (Fig.
3). As demonstrated in Fig. 3A, C
and D, the uptake of PECF-labeled SM-LPN-CD44 in
CD44+ DU145 cells was significantly higher compared with
that of PECF labeled SM-LPN and free PECF (P<0.001).
PECF-labeled SM-LPN-CD44 demonstrated a similar uptake rate to that
of PECF-labeled SM-LPN in CD44− DU145 cells, but
exhibited a significantly higher uptake rate compared with free
PECF (P<0.05). In 22RV1 cells, similar results were obtained
(Fig. 3B, E and F). PECF-labeled
SM-LPN-CD44 exhibited a significantly greater uptake rate compared
with PECF-labeled SM-LPN and free PECF in CD44+ 22RV1
cells (P<0.001), whereas it demonstrated a similar uptake rate
compared with PECF-labeled SM-LPN in CD44− 22RV1
cells.
Nanoparticles demonstrate no
significant cytotoxicity, whereas salinomycin exhibits
dose-dependent cytotoxicity against prostate cancer cells
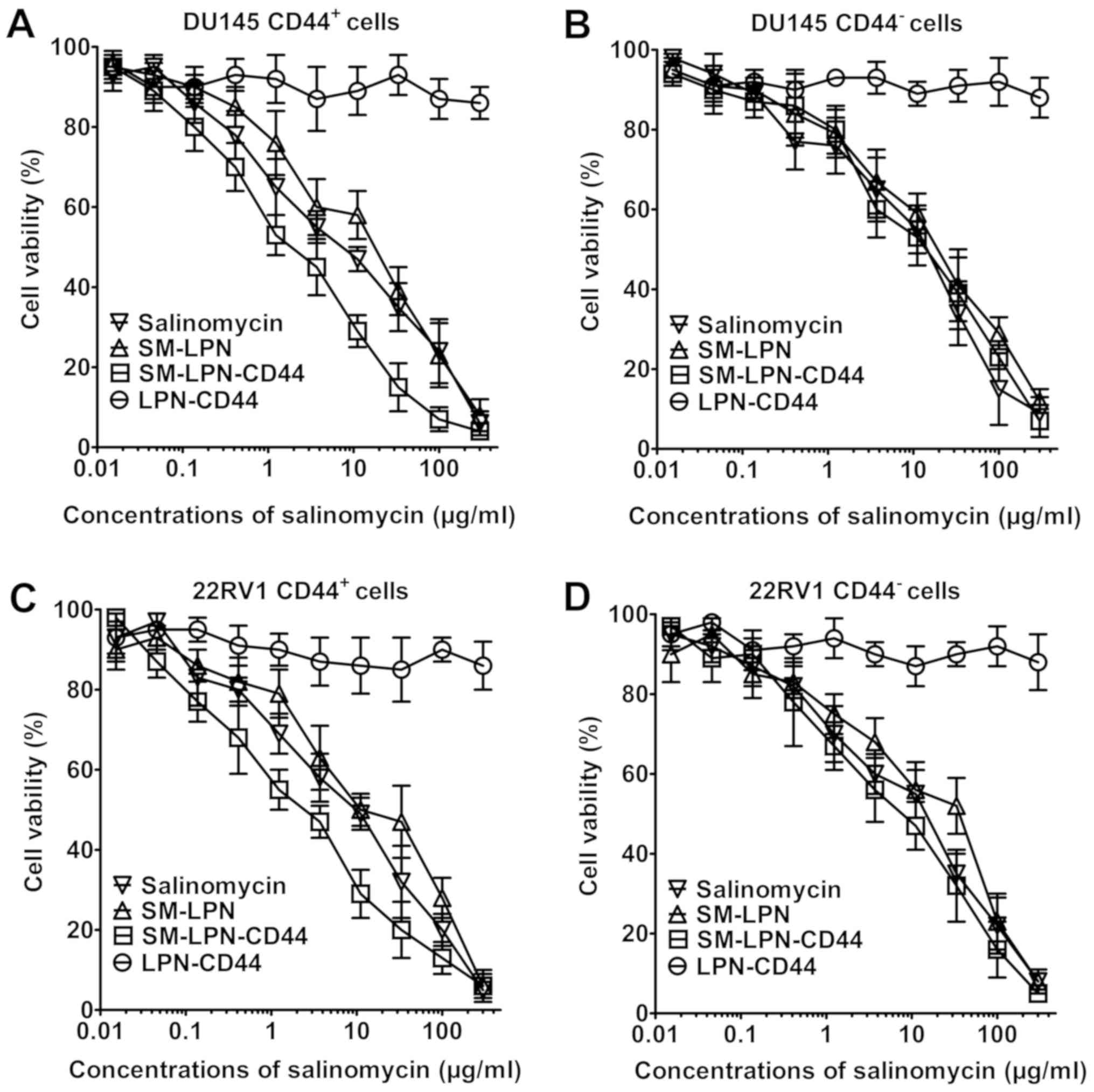
As demonstrated in Fig.
4, LPN-CD44 exhibited no significant cytotoxicity against
prostate cancer cells. By contrast, dose-dependent cytotoxicity was
induced by salinomycin, SMP-LPN and SM-LPN-CD44, as can be observed
from their respective inverse sigmoid dose-dependent curves. The
IC50 values of the drugs are presented in Table III. In CD44+ DU145
cells, SM-LPN demonstrated cytotoxicity similar to that of free
salinomycin (IC50, 8.6 vs. 5.3 µg/ml, respectively).
Compared with SM-LPN and salinomycin, SM-LPN-CD44 exhibited
significantly increased cytotoxicity (IC50, 1.4 µg/ml;
P<0.05). By contrast, no significant difference was identified
in the IC50 values for SM-LPN-CD44 (16.3 µg/ml), SM-LNP
(18.1 µg/ml) and salinomycin (15.0 µg/ml) in CD44− DU145
cells. Similar results were observed in CD44+ 22RV1
cells, with SM-LPN-CD44 cytotoxicity (2.4 µg/ml) revealed to be
significantly higher compared with that of SM-LNP (10.9 µg/ml) and
salinomycin (7.7 µg/ml) (P<0.05). By contrast, no significant
differences were observed in the cytotoxicity of SM-LPN-CD44 (20.8
µg/ml) compared with SM-LPN (23.4 µg/ml) and salinomycin (17.7
µg/ml) in CD44− 22RV1 cells. It was concluded that
SM-LPN-CD44 demonstrates preferential cytotoxicity against
CD44+ prostate cancer cells.
 | Table III.IC50 values of salinomycin
and nanoparticles in prostate cancer cells at 72 h. |
Table III.
IC50 values of salinomycin
and nanoparticles in prostate cancer cells at 72 h.
|
| IC50,
µg/ml |
|---|
|
|
|
|---|
| Treatment | CD44+
DU145 | CD44−
DU145 | CD44+
22RV1 | CD44−
22RV1 |
|---|
| Salinomycin | 5.3±2.3 | 15.9±7.6 | 7.7±5.3 | 17.7±5.4 |
| SM-LPN | 8.6±4.9 | 18.1±7.3 | 10.9±5.7 | 23.4±6.5 |
| SM-LPN-CD44 | 1.4±1.3 | 19.3±6.8 | 2.4±1.6 | 20.8±6.9 |
| LPN-CD44 | >300.0 | >300.0 | >300.0 | >300.0 |
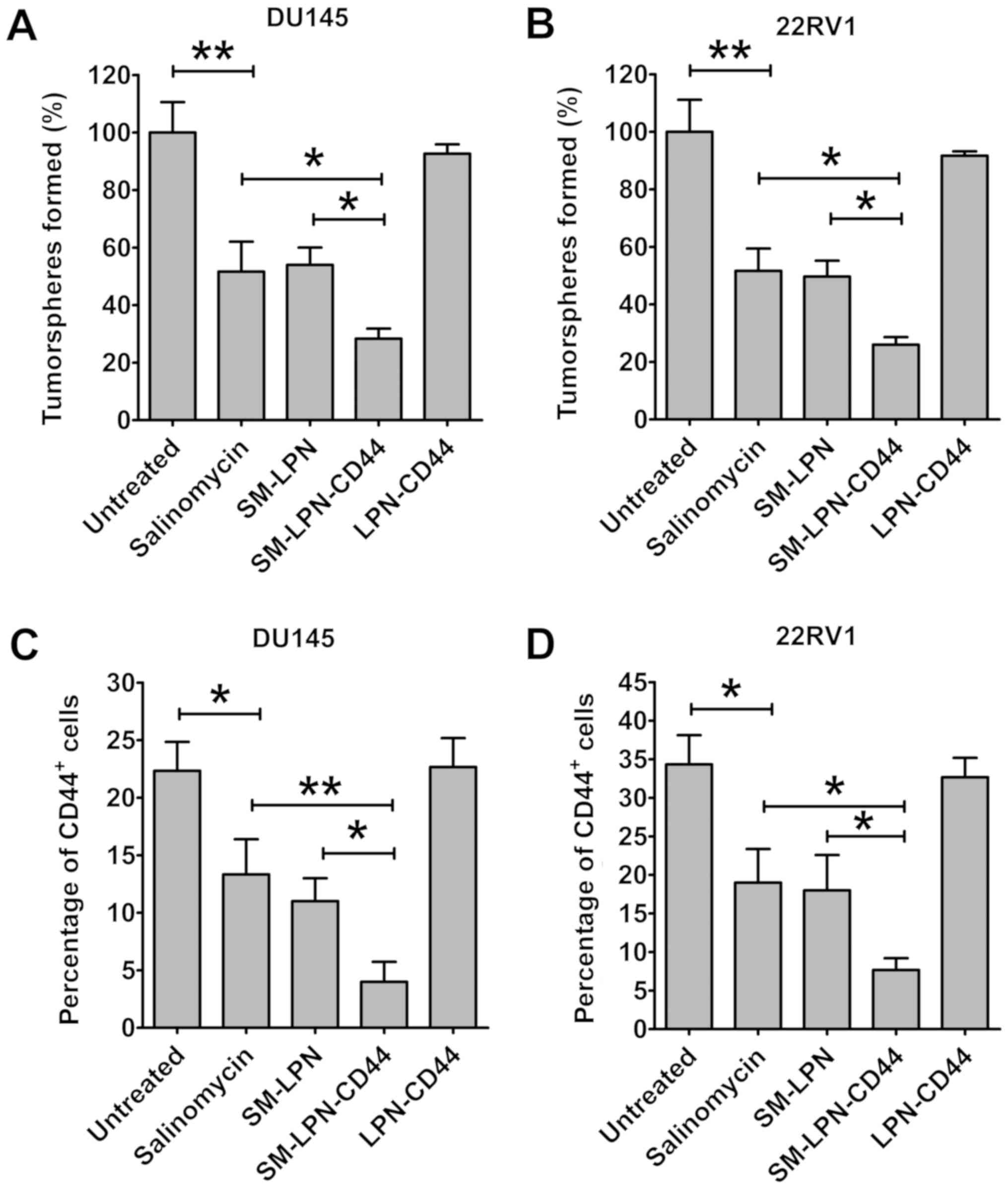
A proportion of CICs is reduced in
prostate cancer cell cultures treated with SM-LPN-CD44
Fig. 5 demonstrates
the impact of nanoparticles on the proportion of CICs in cultured
prostate cancer cells. In DU145 cells, treatment with salinomycin
significantly reduced the number of tumorspheres propagated from
passaged cells (P<0.01; Fig. 5A).
The inhibitory capacity of SM-LPN was similar to that of
salinomycin. However, the number of tumorspheres observed following
SM-LPN-CD44 treatment was further reduced when compared with the
number following salinomycin or SM-LPN treatment (P<0.05).
Treatment with LPN-CD44 exhibited no significant effect on the
number of tumorspheres. Furthermore, in 22RV1 cells, SM-LPN-CD44
was the most effective inhibitor of tumorsphere formation, whereas
treatment with LPN-CD44 exhibited no significant effect (Fig. 5B).
Consistent with the aforementioned results, it was
identified that the percentage of CD44+ DU145 cells was
significantly reduced following salinomycin treatment (P<0.05;
Fig. 5C). Salinomycin and SM-LPN
each demonstrated a degree of inhibition that was similar to their
inhibition of tumorsphere propagation. Furthermore, the percentage
of CD44+ DU145 cells following treatment with
SM-LPN-CD44 was the lowest among all experimental groups. Similar
results were obtained in 22RV1 cells (Fig. 5D). Therefore, SM-LPN-CD44 was
confirmed to exhibit the highest efficiency in inhibiting the
formation of tumorspheres and reducing the percentage of
CD44+ cells in prostate cancer cells.
Discussion
Since CICs are considered the seed cells for
prostate cancer, their eradication may assist in achieving improved
results in cancer therapy. CD44 antigen is one of the most
important markers for CICs, therefore, the present study
constructed salinomycin-encapsulated lipid-PLGA nanoparticles
linked with CD44 antibodies, referred to as SM-LPN-CD44. It was
identified that SM-LPN-CD44 treatment resulted insignificantly
improved potency against CICs compared with free salinomycin
treatment or administration of non-targeted nanoparticles.
Unlike biodegradable organic nanoparticles,
inorganic nanoparticles cannot be degraded and may therefore cause
damage to humans (28,29). These safety considerations
significantly limit the potential clinical uses of inorganic
nanoparticles (28). By contrast,
biodegradable organic nanoparticles provide more promise for
clinical application due to their improved safety (28,29). In
the present study, the constituent components of SM-LPN-CD44 were
PLGA, phosphatidylcholine and cholesterol, all of which are
FDA-approved (29). With respect to
salinomycin, its therapeutic effects and its potential side effects
have been examined in a pilot clinical trial involving patients
with cancer, and the results demonstrated that salinomycin
administered to the patients exhibited therapeutically beneficial
effects and caused no severe side effects (12). In the present study, the results of
the CCK-8 assay revealed that blank lipid-PLGA nanoparticles linked
with CD44 antibodies were highly biocompatible with prostate cancer
cells. Therefore, preliminary safety data from the current study
have demonstrated that nanoparticles represent a safe drug delivery
system.
Although salinomycin has been demonstrated to exert
potent activity against various types of cancer (10–13), to
the best of our knowledge, no previous studies have investigated
its therapeutic efficacy against CD44+ prostate CICs.
The present study revealed that salinomycin preferentially killed
CD44+ prostate cancer cells. By performing a
cytotoxicity assay, it was identified that the IC50
value of SM-LPN-CD44 in CD44+ prostate cancer cells was
significantly lower compared with that in CD44− cells.
Using a tumorsphere formation assay, salinomycin was revealed to
inhibit the number of tumorspheres propagated in DU145 and 22RV1
passages. Consistent with these findings, it was also demonstrated
that the percentage of CD44+ cells in DU145 and 22RV1
cell cultures decreased significantly following salinomycin
treatment. To the best of our knowledge, this is the first report
that demonstrates the potency of salinomycin against
CD44+ prostate CICs.
Antibody-conjugated nanoparticles represent a
promising strategy for the treatment of various cancer types, as
they can significantly enhance the therapeutic efficacy of
chemotherapy drugs (23). Notably,
three antibody-conjugated nanoparticles loaded with doxorubicin or
docetaxel have previously been successfully translated into
early-phase clinical trials (30–32). The
results from these trials support the safety and efficacy of
antibody-conjugated nanoparticles (30–32). The
selection of CD44 antibodies is critical to ensure the specific
targeting of the generated nanoparticles to prostate CICs. From the
experimental studies it was revealed that in CD44+
prostate CICs, SM-LPN-CD44 demonstrated significantly increased
targeting compared with SM-LPN, resulting in increased CIC-specific
cytotoxic effects and improved inhibition of tumorsphere formation.
However, in CD44− prostate cancer cells, the
cytotoxicity and tumorsphere suppression induced by SM-LPN-CD44
were not significantly greater compared with those induced by
SM-LPN. These data firmly demonstrate that SM-LPN-CD44 exhibits
improved therapeutic efficacy against prostate CICs, with the
linked CD44 antibodies promoting targeting of the nanoparticles to
prostate CICs. To the best of our knowledge, the current study is
the first to report targeted drug delivery via nanoparticles to
prostate CICs through the use of the CD44 antibody.
Since CD44 is a stem cell marker for CICs and
hematopoietic stem cells (33), it
could be argued that the targeting of SM-LPN-CD44 to CD44 may have
potential risks in terms of damage to normal hematopoietic stem
cells. However, even in light of this concern, SM-LPN-CD44 is
believed to be a promising candidate for further preclinical
development for a number of reasons. Firstly, since hematopoietic
stem cells and CICs share self-renewal pathways, numerous agents
targeting CICs run the risk of damaging hematopoietic stem cells
(25). For example, the γ-secretase
inhibitors, which have gained attention due to their potential to
inhibit Notch signaling, may inhibit CICs and normal stem cells
(25). Therefore, a number of
CIC-targeting strategies possess the risk of destroying normal
hematopoietic stem cells and the problem is not exclusive to our
proposed nanoparticles. Furthermore, hematopoietic stem cells
exhibit strong regenerative ability and the donation of
hematopoietic stem cells can be safely accomplished with the aid of
healthy donors (26,27). Therefore, loss of healthy
hematopoietic stem cells can easily be treated. Finally, following
optimization of the dosing regimen for agents targeting CICs, it is
possible that normal stem cells may be found to recover following
treatment with a dose that would irreversibly damage the targeted
CICs (34).
In conclusion, the present study was the first to
report the anticancer activity of salinomycin against prostate
CICs, and the first report describing targeted drug delivery via
nanoparticles to prostate CICs, using the CD44 antibody.
SM-LPN-CD44 nanoparticles were confirmed to selectively target
CD44+ prostate CICs. Therefore, these nanoparticles may
represent a novel approach towards the treatment of prostate CICs.
Since prostate CICs serve a crucial role in drug resistance and
metastasis of prostate cancer, patients with a poor prognosis may
benefit greatly from the type of targeted therapy proposed and
developed in the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL contributed to the design of the study and wrote
the manuscript. JW performed the experiments. JS analyzed the data.
All authors have read and approved this manuscript.
Ethics approval and consent to
participate
The animal experimental protocols were approved by
the Animal Administrative Committee of the Naval Medical University
(Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pollock PA, Ludgate A and Wassersug RJ: In
2124, half of all men can count on developing prostate cancer. Curr
Oncol. 22:10–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. JAMA. 317:2532–2542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sartor O and de Bono JS: Metastatic
prostate cancer. N Engl J Med. 378:645–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leão R, Domingos C, Figueiredo A, Hamilton
R, Tabori U and Castelo-Branco P: Cancer stem cells in prostate
cancer: Implications for targeted therapy. Urol Int. 99:125–136.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao J, Li W, Guo Y and Feng SS:
Nanomedicine strategies for sustained, controlled and targeted
treatment of cancer stem cells. Nanomedicine (Lond). 11:3261–3282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Feng SS and Guo Y: Nanomedicine for
treatment of cancer stem cells. Nanomedicine (Lond). 9:181–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maitland NJ and Collins AT: Prostate
cancer stem cells: A new target for therapy. J Clin Oncol.
26:2862–2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong Z, Chen D, Xie F, Liu J, Zhang H, Zou
H, Yu Y, Chen Y, Sun Z, Wang X, et al: Codelivery of salinomycin
and doxorubicin using nanoliposomes for targeting both liver cancer
cells and cancer stem cells. Nanomedicine (Lond). 11:2565–2579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie F, Zhang S, Liu J, Gong Z, Yang K,
Zhang H, Lu Y, Zou H, Yu Y, Chen Y, et al: Codelivery of
salinomycin and chloroquine by liposomes enables synergistic
antitumor activity in vitro. Nanomedicine (Lond). 11:1831–1846.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naujokat C and Steinhart R: Salinomycin as
a drug for targeting human cancer stem cells. J Biomed Biotechnol.
2012:9506582012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Liu L, Li F, Wu T, Jiang H, Jiang
X, Du X and Wang Y: Salinomycin exerts anticancer effects on PC-3
cells and PC-3-derived cancer stem cells in vitro and in vivo.
Biomed Res Int. 2017:41016532017.PubMed/NCBI
|
|
14
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18(pii): E10882017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanrahan K, O'Neill A, Prencipe M, Bugler
J, Murphy L, Fabre A, Puhr M, Culig Z, Murphy K and Watson RW: The
role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in
mediating docetaxel-resistant prostate cancer. Mol Oncol.
11:251–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mirkheshti N, Park S, Jiang S, Cropper J,
Werner SL, Song CS and Chatterjee B: Dual targeting of androgen
receptor and mTORC1 by salinomycin in prostate cancer. Oncotarget.
7:62240–62254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Xie F, Wen X, Chen H, Zhang H, Liu
J, Zhang H, Zou H, Yu Y, Chen Y, et al: Therapeutic PEG-ceramide
nanomicelles synergize with salinomycin to target both liver cancer
cells and cancer stem cells. Nanomedicine (Lond). 12:1025–1042.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao J, Xia Y, Chen H, Yu Y, Song J, Li W,
Qian W, Wang H, Dai J and Guo Y: Polymer-lipid hybrid nanoparticles
conjugated with anti-EGF receptor antibody for targeted drug
delivery to hepatocellular carcinoma. Nanomedicine (Lond).
9:279–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong HL, Rauth AM, Bendayan R, Manias JL,
Ramaswamy M, Liu Z, Erhan SZ and Wu XY: A new polymer-lipid hybrid
nanoparticle system increases cytotoxicity of doxorubicin against
multidrug-resistant human breast cancer cells. Pharm Res.
23:1574–1585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Chen H, Song H, Su X, Niu F, Li W,
Li B, Dai J, Wang H and Guo Y: Antibody-targeted immunoliposomes
for cancer treatment. Mini Rev Med Chem. 13:2026–2035. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo X, Zhu X, Gao J, Liu D, Dong C and Jin
X: PLGA nanoparticles with CD133 aptamers for targeted delivery and
sustained release of propranolol to hemangioma. Nanomedicine
(Lond). 12:2611–2624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kapoor DN, Bhatia A, Kaur R, Sharma R,
Kaur G and Dhawan S: PLGA: A unique polymer for drug delivery. Ther
Deliv. 6:41–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao J, Feng SS and Guo Y: Antibody
engineering promotes nanomedicine for cancer treatment.
Nanomedicine (Lond). 5:1141–1145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Wu Z, Pan G, Ni J, Xie F, Jiang B,
Wei L, Gao J and Zhou W: Enhanced doxorubicin delivery to
hepatocellular carcinoma cells via CD147 antibody-conjugated
immunoliposomes. Nanomedicine. 16:1949–1961. 2018. View Article : Google Scholar
|
|
25
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SH, Wang TF and Yang KL:
Hematopoietic stem cell donation. Int J Hematol. 97:446–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Billen A, Madrigal JA and Shaw BE: A
review of the haematopoietic stem cell donation experience: Is
there room for improvement? Bone Marrow Transplant. 49:729–736.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Auffan M, Rose J, Bottero JY, Lowry GV,
Jolivet JP and Wiesner MR: Towards a definition of inorganic
nanoparticles from an environmental, health and safety perspective.
Nat Nanotechnol. 4:634–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cushing BL, Kolesnichenko VL and O'Connor
CJ: Recent advances in the liquid-phase syntheses of inorganic
nanoparticles. Chem Rev. 104:3893–3946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mamot C, Ritschard R, Wicki A, Stehle G,
Dieterle T, Bubendorf L, Hilker C, Deuster S, Herrmann R and
Rochlitz C: Tolerability, safety, pharmacokinetics, and efficacy of
doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid
tumours: A phase 1 dose-escalation study. Lancet Oncol.
13:1234–1241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miller K, Cortes J, Hurvitz SA, Krop IE,
Tripathy D, Verma S, Riahi K, Reynolds JG, Wickham TJ, Molnar I and
Yardley DA: HERMIONE: A randomized Phase 2 trial of MM-302 plus
trastuzumab versus chemotherapy of physician's choice plus
trastuzumab in patients with previously treated,
anthracycline-naïve, HER2-positive, locally advanced/metastatic
breast cancer. BMC Cancer. 16:3522016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hrkach J, Von Hoff D, Mukkaram Ali M,
Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M,
Horhota A, et al: Preclinical development and clinical translation
of a PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128ra392012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao H, Heazlewood SY, Williams B, Cardozo
D, Nigro J, Oteiza A and Nilsson SK: The role of CD44 in fetal and
adult hematopoietic stem cell regulation. Haematologica. 101:26–37.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cullion K, Draheim KM, Hermance N, Tammam
J, Sharma VM, Ware C, Nikov G, Krishnamoorthy V, Majumder PK and
Kelliher MA: Targeting the Notch1 and mTOR pathways in a mouse
T-ALL model. Blood. 113:6172–6181. 2009. View Article : Google Scholar : PubMed/NCBI
|