Introduction
Ovarian cancer is a malignant tumor that represents
a serious threat to women's health, and has the highest mortality
among all gynecological tumors (1).
Although platinum-based chemotherapy is often effective in reducing
tumor size in ovarian cancer, the majority of cases recur or
metastasize due to the development of drug resistance (2). Drug resistance is a major cause of
post-treatment relapse, metastasis, and even mortality, and thus it
is a primary obstacle to the survival of patients with ovarian
cancer. The development of ovarian cancer multi-resistance is
complex, including abnormal cell proliferation, apoptosis, cell
growth and invasion, epithelial-mesenchymal transition (3), tumor angiogenesis, the prevention of
intracellular drug accumulation (4),
intervention in DNA damage repair (5), and associated signaling pathways
(6).
In recent years, the influence of cytokines present
in the tumor microenvironment on resistance has been widely
reported. Interactions between tumor cell adhesion molecules (CAM)
and the extracellular matrix (ECM) have important effects on tumor
drug resistance (7). Integrins are
members of the adhesion molecules family, and through signaling
cascades initiated by interactions between the extracellular
domains and the matrix, the intracellular domains and various
signaling molecules, these molecules serve important roles in
regulating cell survival, proliferation, adhesion, differentiation
and apoptosis (8). Previous studies
have demonstrated that, when cells undergo malignant
transformation, the integrin configurations on the cell surface
and/or their expression levels also change, ultimately affecting
tumor cell growth, differentiation, apoptosis and adhesion
(8–10). ITGA6 is a member of the integrin
family, that inhibits DNA damage and enhances DNA repair, which
ultimately enhances the drug-resistance of tumor cells (11) such as human acute myeloid leukemia
(12) and breast cancer (13). In the present study, it was
investigated whether ITGA6 is a molecule that potentially controls
multi-drug resistance. Exploring the full range of functions
performed by ITGA6 is an important strategy for addressing the
challenge of drug resistance in ovarian cancer.
Materials and methods
Cell culture
The Human ovarian cancer cell lines SKOV3 and A2780
were cultured generated in our lab (14). The stable cisplatin-resistant cell
lines SKOV3/DDP and A2780/DDP were established from the parental
SKOV3 and A2780 cell lines, respectively, by continuous exposure of
the cells to increasing concentrations of cisplatin and maintenance
in RPMI-1640 media (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Corning, Inc.,
Corning, NY, USA) and 2 µmol/l L-glutamine, at 37°C in a humidified
(95%) atmosphere containing 5% CO2.
Patients and samples
Formalin-fixed, paraffin-embedded tissue specimens
from 54 patients with stage Ic-IV ovarian [International Federation
of Gynecology and Obstetrics (FIGO) Stage] (15) serous adeno carcinoma were collected
from the Department of Gynecologic Oncology, Affiliated Tumor
Hospital of Guangxi Medical University from April 2005 to December
2012. The Ethics Committees of Guangxi Medical University approved
the study protocol, and all patients received an explanation of the
aims of the study and provided signed informed consent. All
patients had undergone cytoreductive surgeries prior to the study
and the diagnosis of ovarian serous adenocarcinoma was confirmed by
two pathologists. Patients received platinum-paclitaxel
chemotherapy for ≥6 cycles following surgery. Classification of the
response to primary chemotherapy was designated as either sensitive
(S, complete remission and relapse >6 months after stopping
chemotherapy) or resistant (R, complete remission and relapse <6
months after stopping chemotherapy). The clinical data for the S
(n=29) and R groups (n=25) are listed in Table I.
 | Table I.Association of ITGA6 with
clinic-pathological parameters in 54 cases of ovarian cancer
tissues. |
Table I.
Association of ITGA6 with
clinic-pathological parameters in 54 cases of ovarian cancer
tissues.
| Characteristics | ITGA6
positive/negative | P-value |
|---|
| Total no. | 24/30 |
|
| Age (years) |
| 0.808 |
|
<50 | 12/16 |
|
| ≥50 | 12/14 |
|
| Stage |
| 0.695 |
| I/II | 5/5 |
|
|
III/IV | 19/25 |
|
| Histotype |
| 0.325 |
|
Serous | 12/11 |
|
|
Other | 12/19 |
|
| Grade |
| 0.594 |
| G1-2 | 8/8 |
|
| G3 | 16/22 |
|
| Serum ca125
(U/ml) |
| 0.901 |
|
<400 | 10/12 |
|
| ≥400 | 14/18 |
|
| Primary surgery |
| 0.300 |
|
Optimal | 11/18 |
|
|
Suboptimal | 13/12 |
|
| Lymph node
metastasis |
| 0.614 |
| Yes | 16/8 |
|
| No | 8/12 |
|
| Chemosensitivity |
| 0.033a |
|
Sensitive | 9/20 |
|
|
Resistant | 15/10 |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted using an RNeasy® Mini
Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's
protocol. First-strand cDNA was synthesized using 1 µg total RNA
using a Transcriptor First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Primer
sequences were generated according to ITGA6 gene cDNA sequences in
Genbank. The ITGA6 gene-specific primers were as following:
forward: 5′-CAGTGGAGCCGTGGTTTTG-3′, and reverse:
5′-CCACCGCCACATCATAGCC-3′ (product length 113 bp). GAPDH was used
as control, the primer sequences are forward
5′-GTCAAGGCTGAGAACGGGA-3′, and reverse 5′-AAATGAGCCCCAGCCTTCTC-3′
(product length, 225 bp). RT-qPCR was completed with a One Step
SYBR PrimeScript Plus RT-PCR kit (Takara Bio, Inc., Otsu, Japan)
and a total volume of 20 µl, using an ABI 7500 Real-Time PCR System
(Thermo Fisher Scientific, Inc.). The conditions were as follows:
95°C for 10 min and 40 cycles of two-step PCR (95°C for 30 sec and
60°C for 30 sec). Average fold changes were calculated according to
differences in the quantification cycles (Cq) between pairs of
samples to be compared. The 2−ΔΔCq method (16) was used for data quantification.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections (4
µm) were deparaffinized in xylene and rehydrated through a graded
ethanol series, followed by antigen retrieval endogenous
peroxidase. The primary antibody included a mouse monoclonal
antibody against human ITGA6 (Abcam, Cambridge, UK; cat. no.
ab181551; 1:150; 4°C, incubated overnight), secondary antibody
(cat. no. KIT-5001; MaxVision™ HRP-Polymer anti-Mouse IHC kit; 1:1;
37°C, incubated for 60 min) was purchased from Fuzhou Maixin
Biotech Co, Ltd, Fuzhou, China. Negative controls were generated by
substituting the primary antibody with PBS. All slides were
evaluated independently by two pathologists. A total of 5
microscopic fields (Olympus Corporation, Tokyo, Japan IX71/IX81,
confocal, natural light ×400) per slide were selected for the
evaluation of ITGA6 staining. Any section that demonstrated a
detectable membranous and/or cytoplasmic positivity was defined as
positive. The intensity of immunostaining was graded as follows: 0,
weak; 1+, moderate; 2+, strong; and 3+, very strong. The area of
positive cancer cells (%) in each microscopic field was categorized
as follows: 1+, 0–10%; 2+, 11–50%; 3+, 51–75%; and 4+, 75–100%. The
score for each section was calculated by multiplying the scores for
both the staining intensity and the area of positive cells. Scores
of 0–3 were designated as ‘low expression’; scores of 4–12 were
designated as ‘high expression’.
A Kaplan-Meier plotter data portal (kmplot.com/analysis/index.php?p=service&cancer=ovar)
was used to access 1,583 ovarian carcinoma cases. The ITGA6 mRNA
expression data in the Kaplan-Meier plotter database were divided
by a median into high and low expression groups. The associations
between ITGA6 mRNA expression and the overall survival (OS) and the
progression-free survival (PFS) were analyzed. Text mining
performed by Coremine Medical software (www.coremine.com) was used to elucidate the
associations of ITGA6 with drug resistance in ovarian cancer.
Statistical analysis
All data were analyzed using SPSS v.19.0 for Windows
statistical software package (IBM Corp., Armonk, NY, USA). The
RT-qPCR data are presented as the mean ± standard deviation, or as
the median, and were analyzed with the Student's t-test and a
chi-squared test. Associated factors of drug resistance were
analyzed using the multivariable logistic regression method.
Survival curves were constructed and compared using the
Kaplan-Meier method and the log-rank test, and a Cox proportional
hazards model analysis was performed to study the influence of
various factors on the survival time of patients with ovarian
epithelial carcinoma. P<0.05 was considered to indicate a
statistically significant difference.
Results
ITGA6 is associated with drug
resistance in ovarian cancer
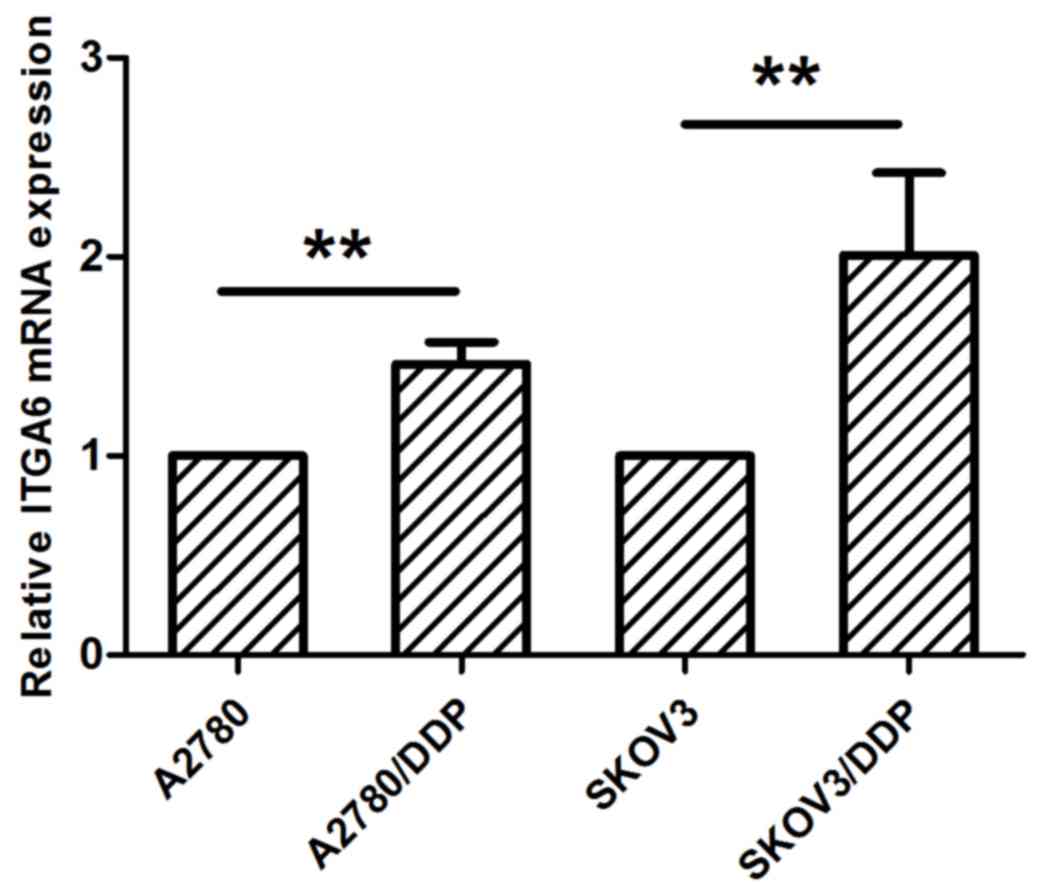
RT-qPCR analysis indicated that the expression of
ITGA6 is upregulated in SKOV3/DDP2 and A2780/DDP cells, in
comparison with the parental cell expression profiles (P<0.05;
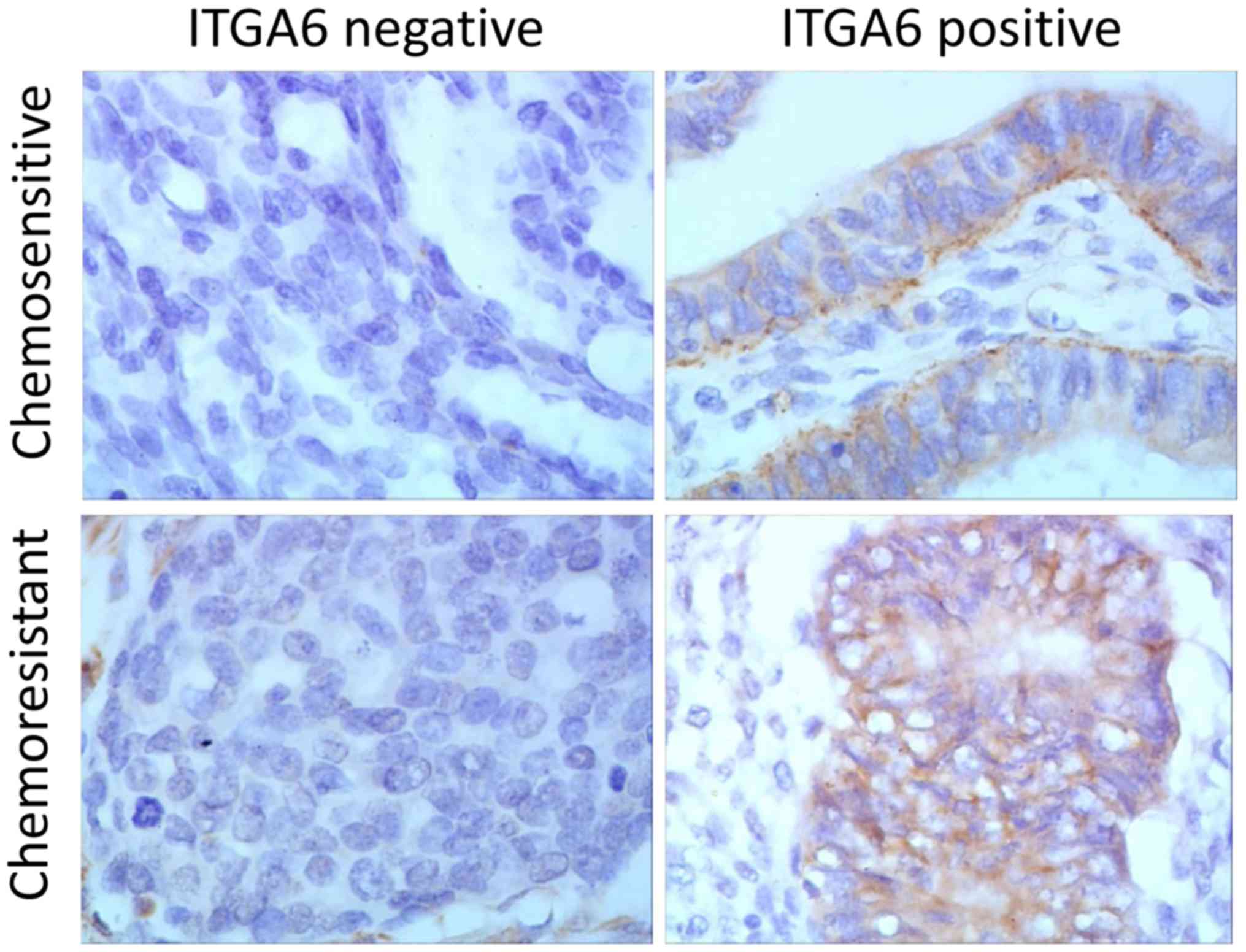
Fig. 1). Immunohistochemistry
analysis, performed on a total of 54 ovarian cancer tissues (25
drug resistant and 29 drug sensitive tissues), indicated that ITGA6
was predominantly located in the cell membrane (Fig. 2). The percentage of chemo-resistant
cases expressing ITGA6 was 60.0% (15/25), but the expression of
ITGA6 in chemo-sensitive tissues was 31.0% (9/29) (P<0.05),
indicating that the expression of ITGA6 was increased in
chemo-resistant tissues (Table
I).
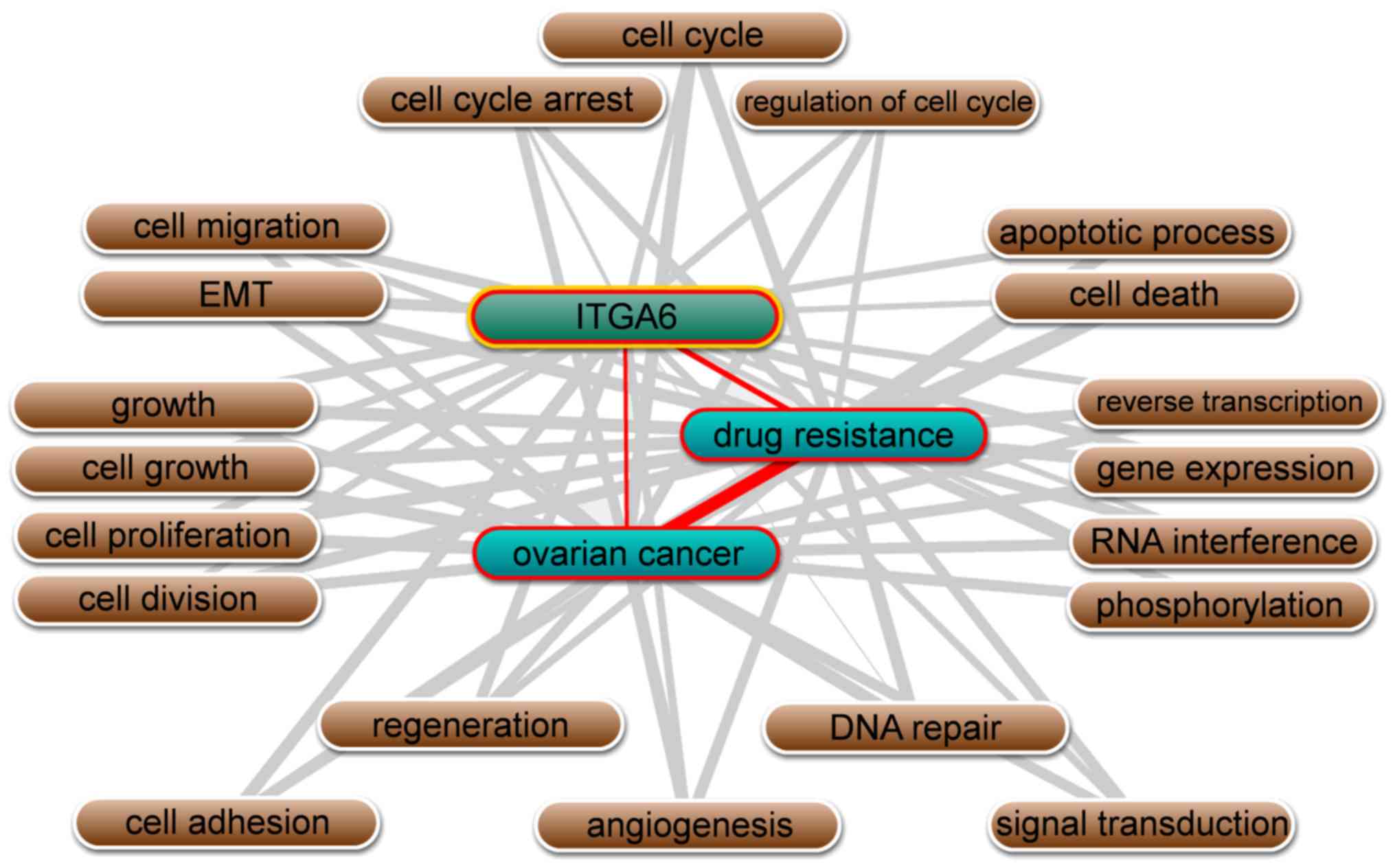
Besides, text mining performed by Coremine Medical
software using the gene names and ‘ovarian neoplasms’, and ‘drug
resistance’ as keywords in co-occurrence analysis, it was
identified that ITGA6 is significantly associated with ovarian
cancer and drug resistance, in addition to being involved in
numerous biological processes, including cell adhesion, cell-matrix
adhesion, epithelial-mesenchymal transition (EMT), apoptotic
processes and cell proliferation (P<0.01; Fig. 3).
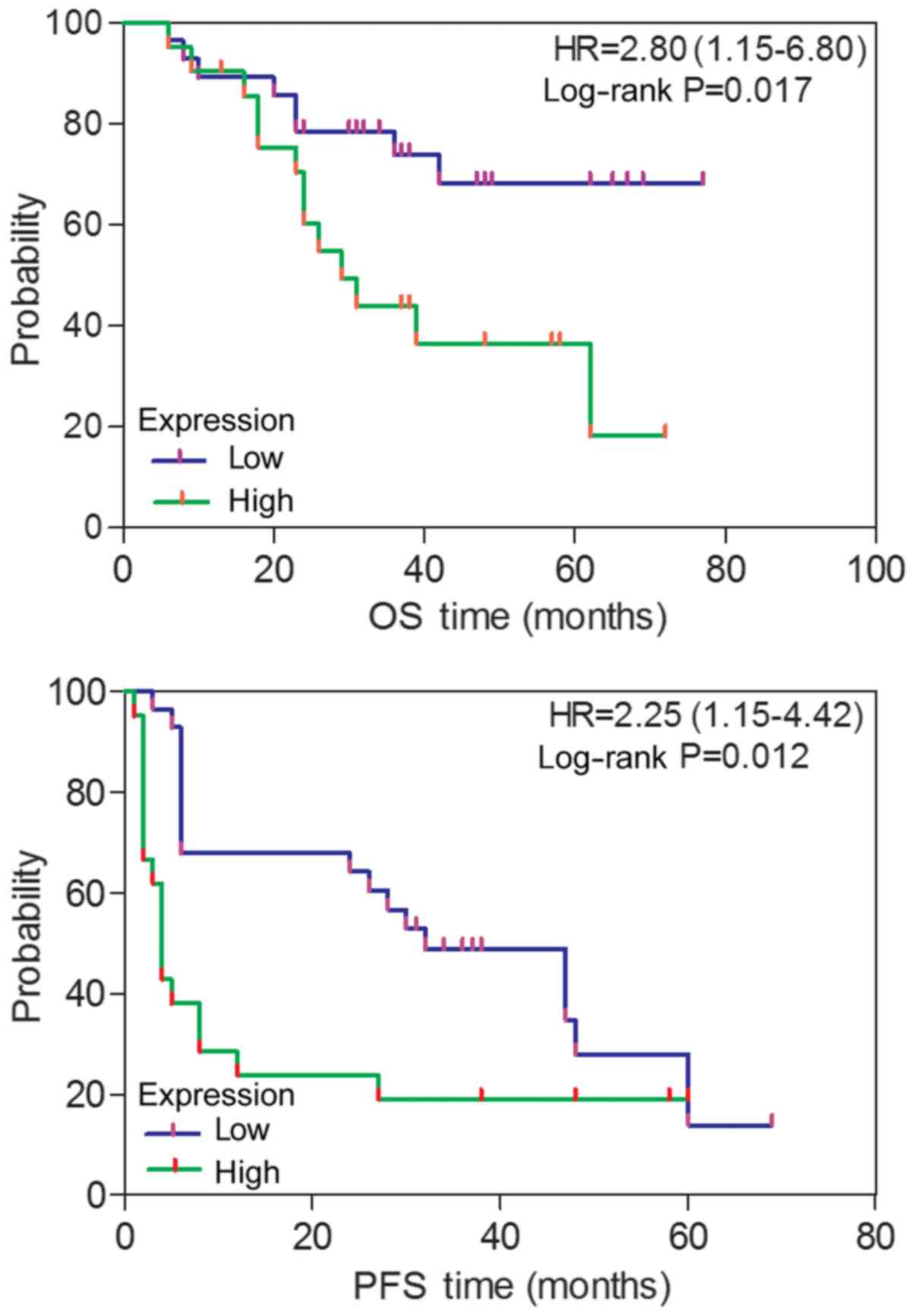
High expression levels of ITGA6
correlate with the survival of patients with ovarian cancer
Associations between ITGA6 and various clinical
factors were analyzed, based on protein expression evaluated
through immunohistochemistry. As depicted in Fig. 4, patients with ovarian cancer and
high ITGA6 expression exhibited a poorer PFS (P=0.012) and an OS
(P=0.017), compared with patients exhibiting low ITGA6 expression,
as determined by Kaplan-Meier survival curves. This was further
confirmed using the univariate Cox regression analysis method
(HR=2.25, 95% CI, 1.15–4.42 for PFS; HR=2.80, 95% CI, 1.15–6.80 for
OS). However, the associations of ITGA6 with age, FIGO stage,
histological type, grade, primary surgery type, serum CA125 levels
and lymph node metastasis were not statistically significant
(P≥0.05; Table I).
The clinical importance of ITGA6 in ovarian cancer
was further investigated using Kaplan-Meier analysis, with a cohort
comprising 1,583 ovarian carcinoma cases. Consistent with the
results obtained from the 54 patients with ovarian cancer, high
expression of ITGA6 in 1,583 patients with ovarian cancer from the
Kaplan-Meier cohort was significantly associated with poorer OS
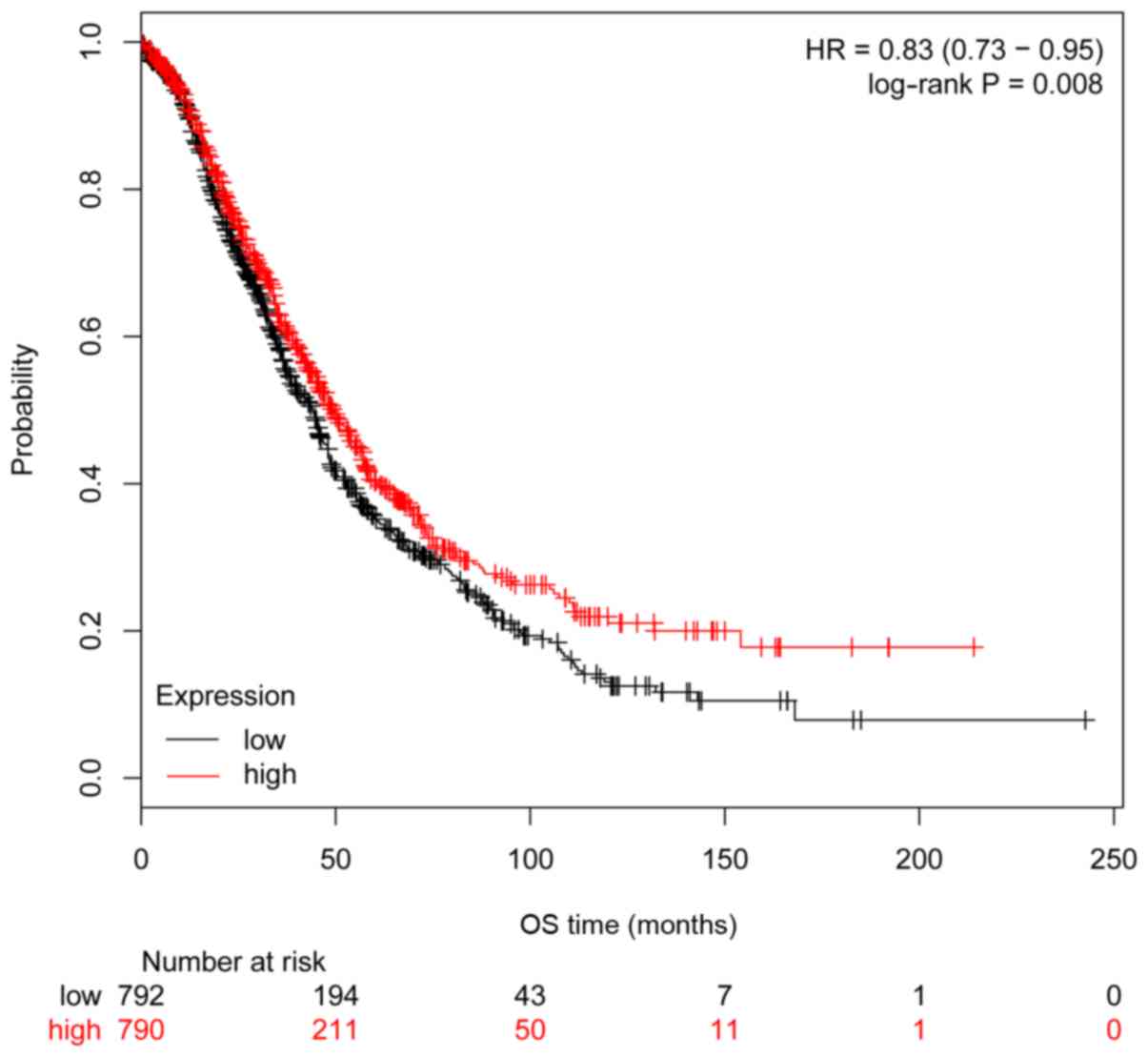
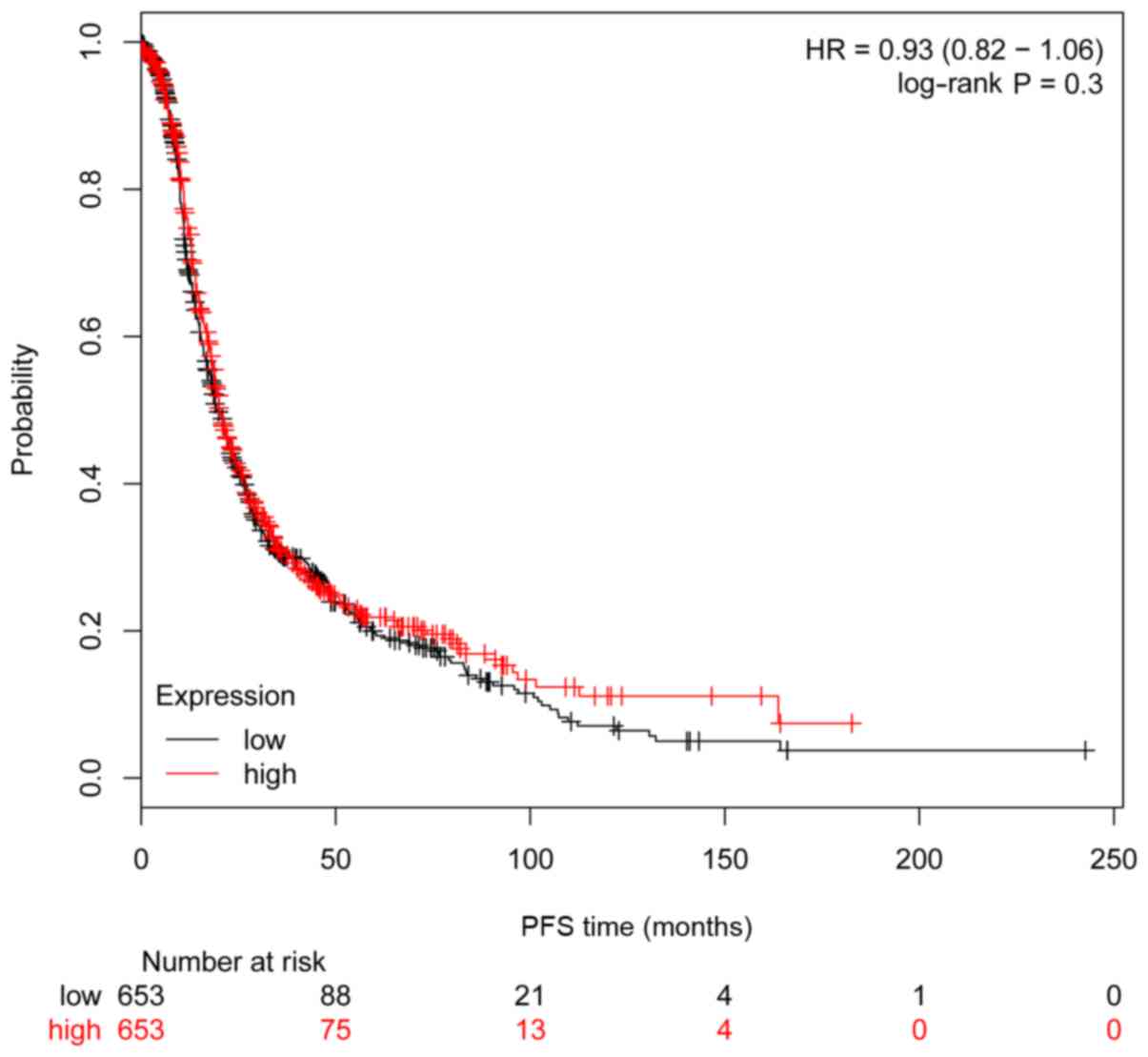
(P=0.008, HR=0.83 95% CI, 0.73–0.95; Fig. 5), but not significantly associated
with PFS (P=0.30, HR=0.93 95% CI, 0.82–1.06; Fig. 6).
Discussion
Ovarian cancer is a type of malignancy with the
highest mortality rate among all gynecological tumors (1). Paclitaxel plus platinum-based
chemotherapy is the current standard treatment strategy for ovarian
cancer, with cisplatin as the preferred agent (2). Although platinum-based chemotherapy is
often effective in reducing tumor size in ovarian cancer, the
majority of cases present with recurrence or metastasis due to the
development of drug resistance (17). Therefore, cisplatin resistance
remains a serious obstacle that greatly affects patient survival.
One of the causes of tumor recurrence is the frequent acquisition
of drug resistant phenotypes during long-term chemotherapy. Thus,
the identification of potential multi-drug resistance genes and
their functions may be one effective strategy to meet the
challenges of drug-resistant ovarian cancer.
A previous study demonstrated that the combination
of ovarian cancer cells and ECM can increase the susceptibility of
cancer cells to chemotherapy drugs; this mechanism was designated
cell adhesion-mediated drug resistance (CAM-DR), and is principally
mediated by adhesion molecules (18).
Integrins are an important family among cell surface
adhesion molecules. They are transmembrane proteins comprised of
two glycoprotein α and β subunits, that may also serve as receptors
for various ECM components (8,19).
Previous studies have reported that integrins can regulate cell
growth, differentiation and metastasis through transmembrane signal
transduction pathways (17). The
growth and metastasis of tumor cells are closely associated with
drug resistance, and metastatic tumor cells are more likely to
become drug resistant (9,20,21).
The association between integrins and drug
resistance has attracted global attention. Prior studies have
demonstrated that integrins are highly expressed in drug-resistant
tumor cells, including esophageal cancer (17), myeloid leukemia (12) and colon cancer (18). It was demonstrated that ITGA6 is
significantly dysregulated in drug resistant ovarian cancer cells
and tissues (22), and that it is
overexpressed in ovarian cancer SKOV3/DDP2 cells, compared with in
SKOV3 cells; these expression trends were the same as those
observed in A2780/DDP and A2780 cells. Immunohistochemical analysis
of a series of ovarian cancer cases demonstrated that ITGA6
expression is closely associated with ovarian cancer drug
resistance. Another study reported that, when the ECM is combined
with integrins, it can activate the downstream Akt2 signaling
pathway, resulting in the activation of various downstream factors
involved in regulating cell function and promoting survival
(9). The signaling pathway linking
integrins and the ECM is critical to cell homeostasis and survival.
The lack of cell adhesion leads to disordered integrin signaling
pathways (including the PI3K/AKT, MEK/ERK, FAK and NF-κB pathways),
ultimately inducing cell apoptosis and drug-resistance (9,23). ITGA6
not only mediates interactions with the ECM, but it also drives
intracellular signaling events of the tumor microenvironment and
inside the tumor cell, promoting the metastasis and infiltration
(13). Bioinformatics and text
mining data indicated that ITGA6 is associated with drug resistance
in ovarian cancer, which is consistent with the results obtained by
the present study. This involves numerous biological processes,
including cell adhesion, EMT, cell-matrix adhesion, apoptotic
processes and cell proliferation. Thus, ITGA6 was selected for
further studies into whether there are potential important genes
associated with the regulation of drug resistance in ovarian
cancer, as the available data indicated that the ECM and the
integrin family are critical to the development of ovarian cancer
cell resistance.
Associations between ITGA6 and cancer prognosis have
been poorly investigated previously, with only a limited number of
studies conducted and all reporting that its high expression was
associated with a significantly poorer OS in patients with
colorectal (24) and prostate cancer
(25). Yamakawa et al
(12) demonstrated that ITGA6 is
associated with drug resistance through increasing cell adhesion,
resulting in a poor prognosis for patients with acute myeloid
leukemia. According to the Kaplan-Meier analysis data used in the
present study, patients with ovarian cancer with high ITGA6
expression exhibited a significantly poorer OS. Additionally, 59
patients with ovarian cancer and high ITGA6 expression, who were
enrolled in the present study, exhibited significantly a poorer PFS
and OS.
Taken together, these studies indicated that ITGA6
expression is closely associated with multi-drug resistance in
ovarian cancer. Therefore, we hypothesized that the mechanism
underlying the involvement of ITGA6 in ovarian epithelial carcinoma
drug-resistance may mediating CAM-DR.
In conclusion, based on bioinformatics analysis and
molecular biology data, the present study demonstrated that ITGA6
may function as a regulatory gene in ovarian cancer cells,
participating in the development of multi-drug resistance, and may
be a prognostic risk factor for patients with OS. The investigation
of IGTA6 expression in the present study may be involved in the
regulation of drug resistance and prognosis in ovarian cancer
cells; however, further studies are required to confirm the present
findings.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant. nos. 81572579, 81302283 and
81560424), the Ministry of Education Special Fund for the Doctoral
Program in Colleges and Universities (grant. no. 20124503110003),
the Guangxi Science and Technology Development Program (grant. no.
14124004-1-24), and the Key Laboratory of High-Incidence Tumor
Prevention and Treatment, Guangxi Medical University (grant. no.
GK2015-TKF01).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
LW, FY and LL made substantial contributions to the
conception or design of the work; CC had substantial contributions
to the analysis of data for the present study; LL gave thier final
approval of the version to be published.
Ethics and consent to participate
The ethics committees of Guangxi Medical University
(Nanning, China) approved the study. All patients received an
explanation of the aims of the study and provided written informed
consent.
Consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Groeneweg JW, Foster R, Growdon WB,
Verheijen RH and Rueda BR: Notch signaling in serous ovarian
cancer. J Ovarian Res. 7:952014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kathawala RJ, Gupta P, Ashby CR Jr and
Chen ZS: The modulation of ABC transporter-mediated multidrug
resistance in cancer: A review of the past decade. Drug Resist
Updat. 18:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuorkey MJ: Curcumin a potent cancer
preventive agent: Mechanisms of cancer cell killing. Interv Med
Appl Sci. 6:139–146. 2014.PubMed/NCBI
|
|
6
|
Han X, DU F, Jiang L, Zhu Y, Chen Z, Liu
Y, Hong T, Wang T, Mao Y, Wu X, et al: A2780 human ovarian cancer
cells with acquired paclitaxel resistance display cancer stem cell
properties. Oncol Lett. 6:1295–1298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan L, Wang C, Lin B, Liu J, Liu D, Hou R,
Wang Y, Gao L, Zhang S and Iwamori M: Lewis y enhances CAM-DR in
ovarian cancer cells by activating the FAK signaling pathway and
upregulating Bcl-2/Bcl-XL expression. Biochimie. 113:17–25. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Marco R, Tolomelli A, Juaristi E and
Gentilucci L: Integrin ligands with α/β-hybrid peptide structure:
Design, bioactivity, and conformational aspects. Med Res Rev.
36:389–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blandin AF, Renner G, Lehmann M,
Lelong-Rebel I, Martin S and Dontenwill M: β1 Integrins as
therapeutic targets to disrupt hallmarks of cancer. Front
Pharmacol. 6:2792015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burkhalter RJ, Symowicz J, Hudson LG,
Gottardi CJ and Stack MS: Integrin regulation of beta-catenin
signaling in ovarian carcinoma. J Biol Chem. 286:23467–23475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elliott T and Sethi T: Integrins and
extracellular matrix: A novel mechanism of multidrug resistance.
Expert Rev Anticancer Ther. 2:449–459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamakawa N, Kaneda K, Saito Y, Ichihara E
and Morishita K: The increased expression of integrin α6 (ITGA6)
enhances drug resistance in EVI1(high) leukemia. PLoS One.
7:e307062012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brooks DL, Schwab LP, Krutilina R, Parke
DN, Sethuraman A, Hoogewijs D, Schörg A, Gotwald L, Fan M, Wenger
RH and Seagroves TN: ITGA6 is directly regulated by
hypoxia-inducible factors and enriches for cancer stem cell
activity and invasion in metastatic breast cancer models. Mol
Cancer. 15:262016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin F, Liu L, Liu X, Li G, Zheng L, Li D,
Wang Q, Zhang W and Li L: Downregulation of tumor suppressor gene
ribonuclease T2 and gametogenetin binding protein 2 is associated
with drug resistance in ovarian cancer. Oncol Rep. 32:362–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prat J: FIGO staging for uterine sarcomas.
Int J Gynaecol Obstet. 104:177–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: Latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia Y, Zeng ZZ, Markwart SM, Rockwood KF,
Ignatoski KM, Ethier SP and Livant DL: Integrin fibronectin
receptors in matrix metalloproteinase-1-dependent invasion by
breast cancer and mammary epithelial cells. Cancer Res.
64:8674–8681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dransfield I, Cabanas C, Barrett J and
Hogg N: Interaction of leukocyte integrins with ligand is necessary
but not sufficient for function. J Cell Biol. 116:1527–1535. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janouskova H, Ray AM, Noulet F,
Lelong-Rebel I, Choulier L, Schaffner F, Lehmann M, Martin S,
Teisinger J and Dontenwill M: Activation of p53 pathway by
Nutlin-3a inhibits the expression of the therapeutic target alpha5
integrin in colon cancer cells. Cancer Lett. 336:307–318. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toquet C, Colson A, Jarry A, Bezieau S,
Volteau C, Boisseau P, Merlin D, Laboisse CL and Mosnier JF: ADAM15
to α5β1 integrin switch in colon carcinoma cells: A late event in
cancer progression associated with tumor dedifferentiation and poor
prognosis. Int J Cancer. 130:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maubant S, Cruet-Hennequart S, Poulain L,
Carreiras F, Sichel F, Luis J, Staedel C and Gauduchon P: Altered
adhesion properties and alphav integrin expression in a
cisplatin-resistant human ovarian carcinoma cell line. Int J
Cancer. 97:186–194. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HI, Huang H, Cheepala S, Huang S and
Chung J: Curcumin inhibition of integrin (alpha6beta4)-dependent
breast cancer cell motility and invasion. Cancer Prev Res (Phila).
1:385–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linhares MM, Affonso RJ Jr, Viana Lde S,
Silva SR, Denadai MV, de Toledo SR and Matos D: Genetic and
immunohistochemical expression of integrins ITGAV, ITGA6, and ITGA3
As prognostic factor for colorectal cancer: Models for global and
disease-free survival. PLoS One. 10:e01443332015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marthick JR and Dickinson JL: Emerging
putative biomarkers: The role of alpha 2 and 6 integrins in
susceptibility, treatment, and prognosis. Prostate Cancer.
2012:2987322012. View Article : Google Scholar : PubMed/NCBI
|