Introduction
Cancer has been reported as the leading health risk
worldwide in 2018 (1). In 2017,
there were 1,688,780 cancer cases in the USA. Additionally, 600,920
patients died from cancer in 2017 (1). In the past three decades, an
improvement in the 5-year survival rate has been reported. However,
cancer remains the second leading cause of cancer-associated
mortality in USA (1). The ideal
cancer treatment modality should not only kill local tumor cells
with limited or no damage to surrounding normal tissue, but also
induce metastatic tumors to regress, preventing tumor recurrences
(2). Laser immunotherapy has the
potential to be an ideal cancer treatment modality (3).
Laser immunotherapy was established and applied the
first time in 1997 (4). It is a
convenient, minimally invasive cancer therapy strategy that damages
targeted tumors by hyperthermia and subsequently elicits a
personalized tumor-specific immune response (4). Laser immunotherapy consists of a
combination of photothermal therapy and immunotherapy. Photothermal
therapy, which has also been referred to as a photothermal
interaction, is an important part of laser immunotherapy. The
photothermal effect primarily focuses on noninvasive near-infrared
(NIR) light, which delivers energy to the targeted tumor tissues. A
photosensitizer absorbs the energy in the tumor tissue, causing an
elevation in temperature in the targeted tissue (4). The elevated temperature kills local
tumor cells, subsequently releasing tumor antigens to activate the
immune system (5).
Indocyanine green (ICG) is a water-soluble
tricarbocyanine dye that serves as a photosensitizer (6). The ICG solution is injected into the
center of neoplastic tissues prior to irradiation, which
subsequently increases the temperature of the tissue and results in
selective destruction, leaving the surrounding tissue relatively
undamaged. The heat energy transferred from the laser energy
produces a strong photothermal interaction (7).
Laser immunotherapy has attained a promising
treatment effect in animal experiments and clinical trials
(6,8). In animal experiments, the combination
of laser immunotherapy and surgery significantly extended the
survival time of EMT6 tumor bearing BALB/c mice, which rejected
successfully the following two consecutive challenges with EMT6
cells (4). In a clinical trial,
patients were reported to respond well to laser immunotherapy
during their treatment of metastatic breast cancer (7). It was reported in the aforementioned
study that laser immunotherapy exhibited a promising effect on the
objective response rate, in addition to the clinical beneficial
response rate of these patients (7).
These promising effects were primarily due to the photothermal
therapy that selectively targeted and directly eradicated the tumor
cells (9). Additionally, it has been
reported that tumor cells damaged by high temperatures can activate
an immune response by releasing tumor cell debris and byproducts
(10).
However, the thermal effects and immune effects
induced by thermotherapy require further investigation, as
different temperatures produce different thermal and immune
effects. If the temperature is insufficient, the tumor cells are
not destroyed, whereas if the temperature is too high, the surface
tissue may be damaged and impede further infiltration of laser
energy (11). The temperature
increase in the tumor cells is primarily determined by the laser
power density, ICG concentration and laser irradiation time
(12). A number of studies
demonstrated that the thermal effect is induced by the combination
of laser and ICG (13–15); however, the characteristics of
temperature changes during photothermal therapy have not been
extensively investigated.
The present study aimed to investigate the
absorption spectrum of ICG and subsequently examine the
characteristics of temperature changes during the exposure of ICG
to a laser in water solution and tumor-bearing mice. The present
study also aimed to observe the morphological changes of tumor
tissues following thermal therapy.
Materials and methods
ICG
ICG, obtained from Akorn Inc. (Buffalo Grove, IL),
is a tricarbocyanine dye and was used as a photosensitizer in this
study. The ultraviolet-visible near infrared absorbance spectra of
ICG was recorded by a Perkin Elmer Lambda 750
ultraviolet-visible-NIR spectrophotometer (PerkinElmer, Inc.,
Waltham, MA, USA). A total of 5 different dilutions of ICG,
including 10, 5, 2.5, 1.25, and 0.3725 µg/ml, were used for
detecting the absorption spectrum.
Tumor cell line
The breast tumor cell line 4T1 (mouse mammary gland,
ATCCCRL-2534 4T1) was cultured in Roswell Park Memorial Institute
1640 (RPMI 1640) medium (Invitrogen, Carlsbad, CA) with 10% fetal
bovine serum (AppliChem GmbH, Darmstadt, Germany), 100 U/ml
penicillin and 100 U/ml streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C with 5% CO2 for 48 h. The
cells were harvested and prepared in this medium (1×106
cells/100 µl) for injection.
Animal model
Female BALB/c mice (6–8 weeks; 15–25 g) were used in
the present experiment. A total of 12 mice were kept in specific
pathogen-free animal facilities with controlled temperature and
humidity under conventional conditions at a temperature of 22±2°C,
a relative humidity of 55±10% and a 12 h dark/light illumination
cycle. Water and food were available ad libitum. They were
fed standard diet chow pellets and water ad libitum. The mice were
purchased from Harlan Sprague Dawley Co. (Indianapolis, IN, USA).
The mice were housed and performed in Biophotonics Research
Laboratory Center for Interdisciplinary Biomedical Education and
Research University of Central Oklahoma (Edmond, OK, USA). The mice
were anesthetized with a gas mixture of isoflurane (2%) and oxygen
(2 l/min) prior to laser irradiation. Following the completion of
laser irradiation, the mice were allowed to recover for 30 min. All
animal experiments were approved by the Institutional Animal Care
and Use Committee of Biophotonics Research Laboratory Center for
Interdisciplinary Biomedical Education and Research University of
Central Oklahoma (OK, USA) and were in accordance with National
Institutes of Health guidelines (16). The hair of the BALB/c mice was
removed and the mice were subcutaneously injected with
1×106 4T1 cells suspended in 100 µl of PBS. Tumors grew
predictably in all mice and reached a size of 5–10 mm in diameter
between 8 to 10 days following injection. Tumor growth was assessed
2 times a week from inoculation of tumor cells to mortality. The
orthogonal tumor dimensions (a and b) were measured with a Vernier
caliper. The tumor volume was calculated according to the formula
V=ab2/2, where ‘a’ is the maximum diameter of the tumor
and ‘b’ is the smallest diameter of the tumor (17). The tumor bearing mice were ready for
the treatment when the tumors reached a volume of 100–250
mm3. Mice were monitored carefully throughout the study
and were preemptively euthanized by cervical dislocation when they
became moribund.
Treatment parameters and procedures of
laser thermotherapy in solution
A Laser with a wavelength of 805 nm (ImmunoPhotonics
Inc., Columbia, MO, USA) was used in this study. The laser energy
was delivered by an optical fiber. There is a control device that
is incorporated into the handle at the end of the optical fiber
that can be adjusted to deliver various power densities.
In the solution experiment, solutions with different
concentrations of ICG, including 10, 5, 2.5, 1.25 and 0.3725 µg/ml,
were irradiated by NIR laser diode at a wavelength 805 nm with 1
W/cm2 output. The detailed parameters of the ICG
solution are included in Table I.
Aforementioned solution (~1 ml) were prepared in conical tubes and
irradiated with the 805 nm laser for 120 sec. The surface
temperature of the solution was monitored by an infrared
thermometer 2017 (FLIR® Systems, Inc, Wilsonville, OR,
USA). It was used to detect the temperature at the irradiation time
points of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120
sec.
 | Table I.Different concentrations of ICG in
solution experiment. |
Table I.
Different concentrations of ICG in
solution experiment.
| Group | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| ICG concentration
(mg/ml) | 0.0187 | 0.00935 | 0.00468 | 0.00234 | 0.00117 | 0 |
Treatment parameters and procedures of
laser thermotherapy in tumor-bearing mice
In the animal experiment, various parameters of
power density and ICG concentrations were used to treat the 4T1
tumor-bearing BALB/c mice. The detailed parameters of the different
components for the treatment of tumor bearing mice are presented in
Table II. Prior to the laser
treatment, the 4T1 tumor bearing mice were anesthetized, and the
hair overlying the tumor in each mouse was clipped. In Groups 1
(laser power density: 1 W/cm2), 3 (laser power density:
0.8 W/cm2) and 4 (laser power density: 0.6
W/cm2), the parameters of ICG solution are the same and
a total of 200 µl of ICG solution was injected into the center of
the tumors on the backs of the mice, while in Group 2, 200 of µl
RPMI-1640 medium was used. Laser irradiation was administered
following the ICG solution injection. The different parameters of
laser energy were delivered to the tumor sites by optical fibers.
An infrared thermometer was used to measure the temperature at the
irradiation time points of 0, 20, 40, 60, 120, 180, 240, 300, 360,
420, 480, 540 and 600 sec. The tumor bearing mice were irradiated
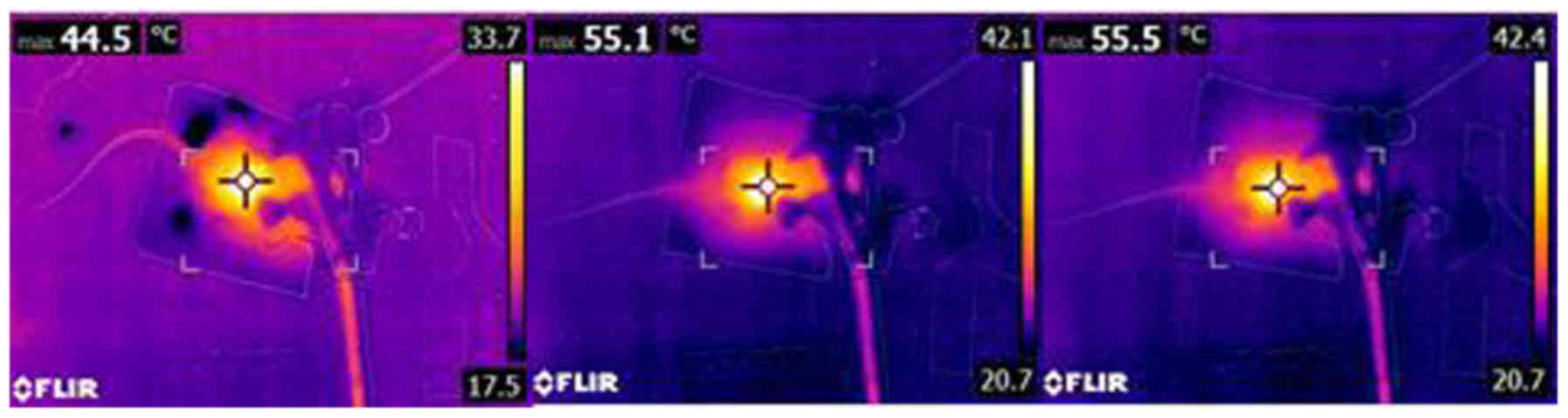
for 10 min. Temperature measurement images of the tumor bearing
mice by forward-looking thermal cameras (FLIR® Systems,
Inc, Wilsonville, OR, USA) at different time points during laser
irradiation are shown in Fig. 1.
 | Table II.Detailed parameters of different
components in photothermal therapy for the treatment of
tumor-bearing mice. |
Table II.
Detailed parameters of different
components in photothermal therapy for the treatment of
tumor-bearing mice.
| Group | Laser power density
(W/cm2) | ICG concentration
(mg/ml) |
|---|
| 1 | 1 | 0.10 |
| 2 | 1 | 0 |
| 3 | 0.8 | 0.10 |
| 4 | 0.6 | 0.10 |
Morphological observation of tumor
tissue after laser irradiation
Tumor tissues of the mice from Group 1 were removed
on the 1st or 7th day following photothermal therapy. The mice in
other groups were sacrificed by spine dislocation. Tissues (4 mm)
were fixed in 10% buffered formalin 24 h at 4°C and embedded in
paraffin. Formalin-fixed paraffin-embedded samples were incubated
with paraffin Stretcher (Sakura Finetek Japan, Tokyo, Japan) at
50°C overnight, and subsequently stained using Hematoxylin 7211 and
Eosin-Y Alcoholic kit (Thermo Fisher Scientific, Inc.) for 10 and 5
min, respectively, at room temperature, as previously described
(18). Morphological changes of the
tumor tissues were observed using an electronic light microscope
(magnification, ×10 and ×40; Olympus, Tokyo, Japan).
Statistical analysis
All data were derived from at least three
independent experiments and are presented as the mean ± standard
error of the mean. The results were evaluated using the
independent-samples Pearson's correlation coefficient was used to
calculate the coefficient of the fitted spectrum. Student' t-test
and one-way analysis of variance (ANOVA) with Dunnett's multiple
comparison post-hoc test. Statistical analysis was performed using
SPSS 20.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
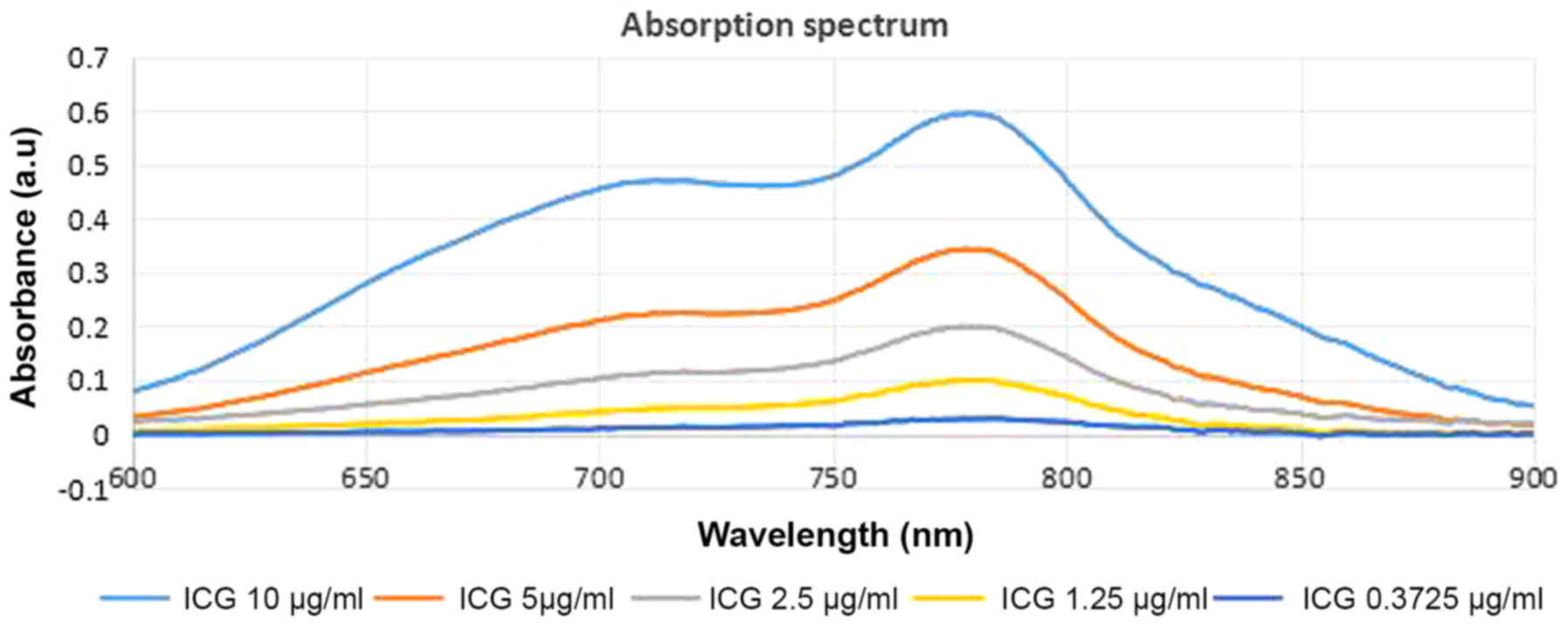
The absorbance spectrum of ICG
The NIR absorption spectrum of different dilutions
of ICG indicated the absorption characteristics of ICG, with an
absorption peak ranging from 750 to 800 nm. The detailed spectrum
is indicated in Fig. 2. The
coefficient of the fitted spectrum curve, which was calculated by
Pearson's correlation coefficient is R2=0.9942. There is
a commercially available 805-nm laser, which was selected for use
in the present study.
Temperature monitoring of water
solutions containing different concentrations of ICG during laser
treatment
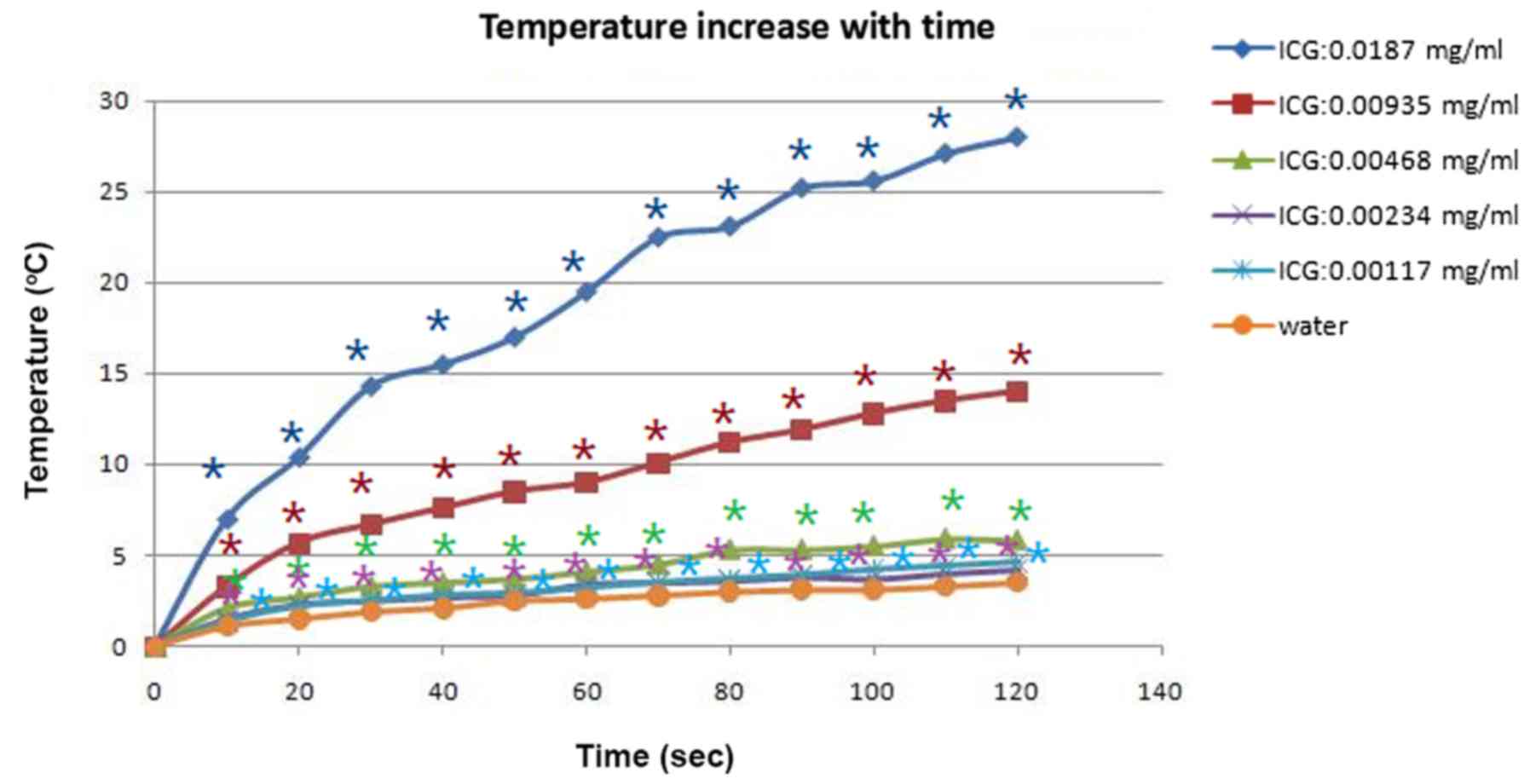
The temperature of water solutions with various
concentrations of ICG solution, during laser treatment under a
power density of 1 W/cm2 output, was tested with an
infrared thermometer. In group 6 (ICG, 0 mg/ml), the temperature of
the water solution without ICG increased 3.5°C after 120 sec of
805-nm laser irradiation. In Groups 1 (ICG 0.0187 mg/ml) and 2 (ICG
0.00935 mg/ml) the temperature increased by 28°C and 14°C,
respectively, after 120 sec of irradiation. In group 3 (ICG 0.00468
mg/ml), group 4 (ICG 0.00234 mg/ml), and group 5 (ICG 0.00117
mg/ml), the temperature rose to 5.8°C, 4.2°C, and 4.6°C,
respectively. The data in the six groups were evaluated using
one-way analysis of variance (ANOVA) with Dunnett's multiple
comparison post-hoc test. The temperatures in group 1–5 were
significantly increased, compared with Group 6 (ICG, 0 mg/ml;
P<0.05). Temperature significantly increased in a ICG
concentration-increasing manner. The results indicated that the
temperature elevation was almost proportional to the concentration
of ICG solution. The characteristics of the temperature variation
are presented in Fig. 3.
Temperature monitoring of
tumor-bearing mice treated by a laser with or without ICG
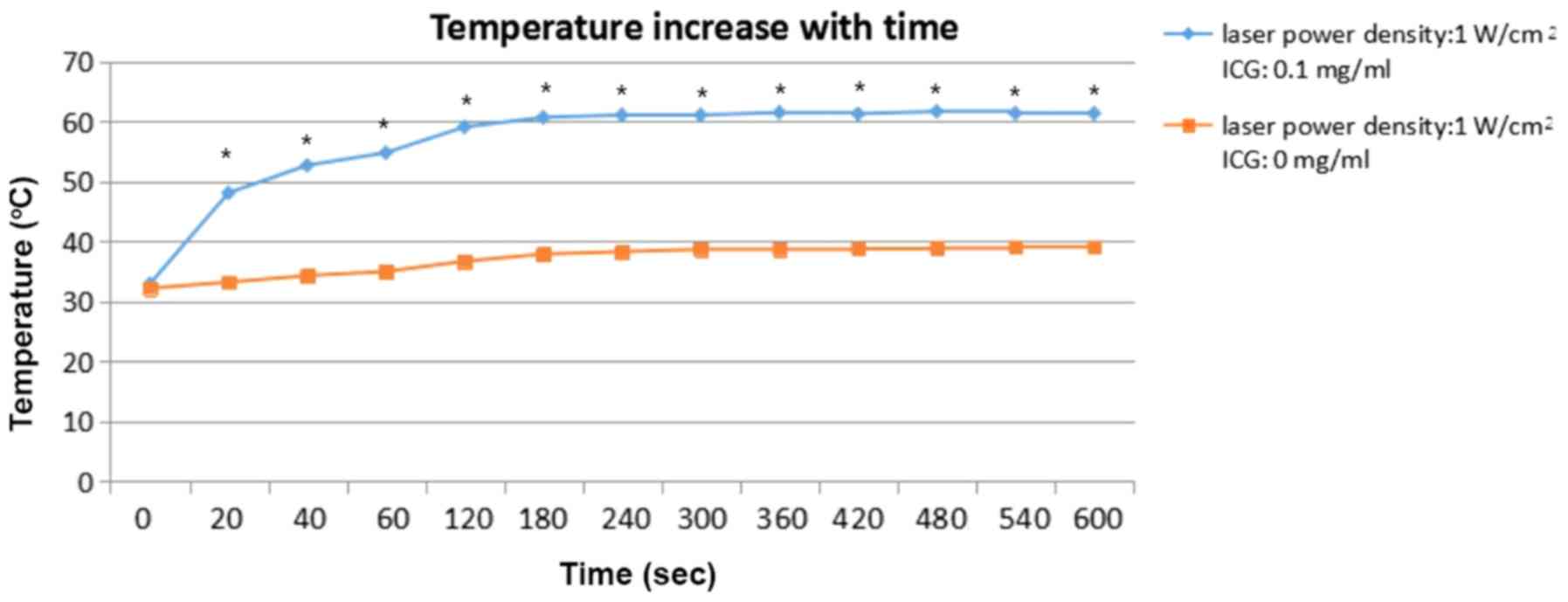
In group 2, the temperature of the tumor tissue was
elevated by 6.9°C at 600 sec after the tumor tissue was treated
with a laser (1 W/cm2) without ICG. In group 1, the
temperature of the tumor tissue was elevated by 28.5°C at 600 sec
when the tumor tissue was treated with a laser (1 W/cm2)
and ICG (0.1 mg/ml). The temperature difference between Group 1 and
Group 2 was ~20°C. The temperature in Group 1 was significantly
increased, compared with the temperature in Group 2. (P<0.05).
The temperatures in Groups 1 and 2 were analyzed using the
independent-samples Student's t-test. The details of the
temperature changes are presented in Fig. 4.
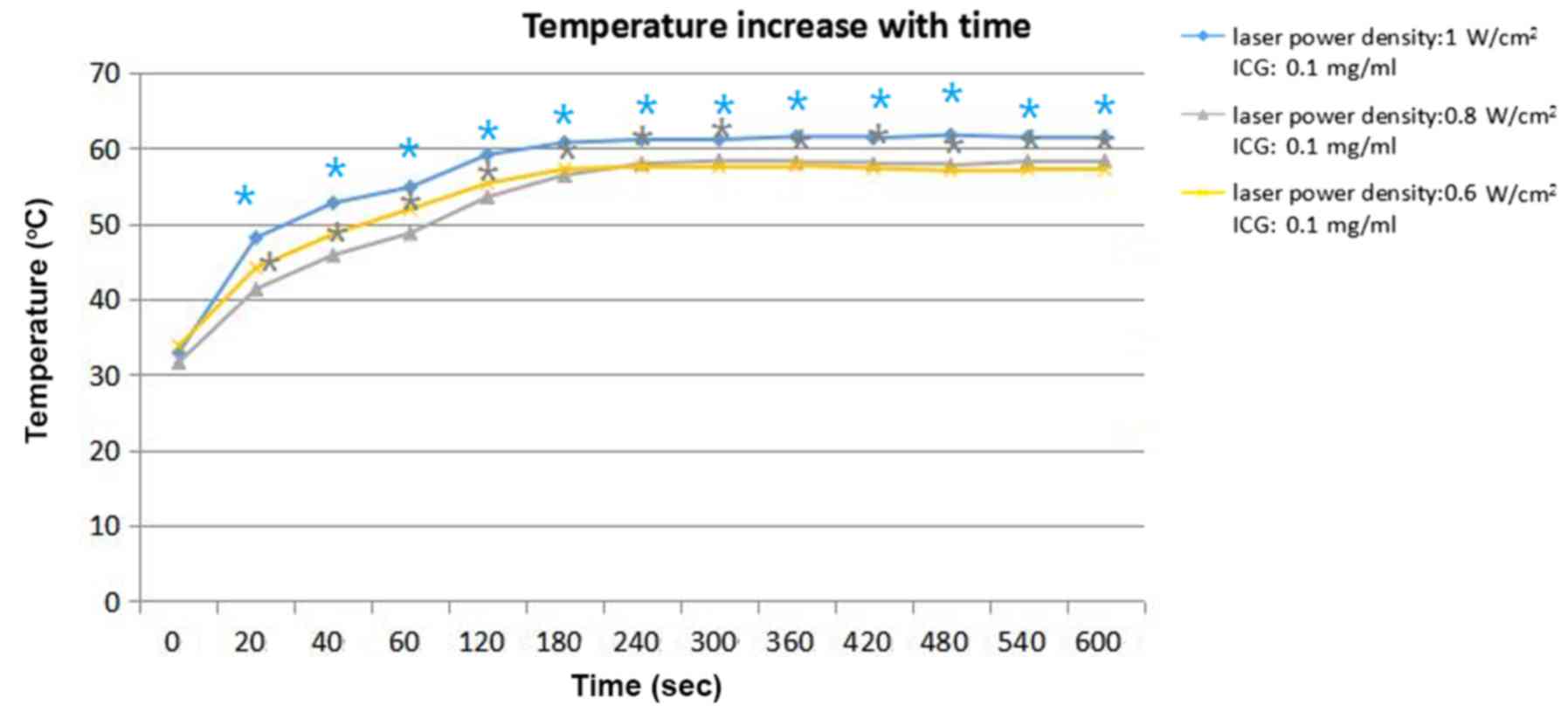
Different parameters of ICG
solution
Temperature of the tumor tissue in group 1 (laser
power density: 1 W/cm2), group 3 (laser power density:
0.8 W/cm2) and group 4 (laser power density: 0.6
W/cm2) increased by 61.4, 58.3, and 57.1°C,
respectively, at 600 sec. The concentration of ICG in these three
groups was 0.1 mg/ml. The temperature elevation data are presented
in Fig. 5. In the experiments in the
tumor-bearing mice, the results (Fig.
5) demonstrated that the proportion of temperature elevation to
laser power density was weak. Group 3 (laser power density: 0.8
W/cm2) and group 4 (laser power density: 0.6
W/cm2) achieved almost the same stable temperature of
~58°C. The stable temperature of Group 1 (laser power density: 1.0
W/cm2) was 8°C higher than the stable temperature of
Group 3 and 4. The statistical analysis of the aforementioned
results was performed by ANOVA with Dunnett's multiple comparison
post-hoc test, indicating that the temperature was significantly
increased with increasing laser power density (P<0.05).
The characteristics of temperature
changes with irradiation time
In solution experiments it was indicated that the
temperature elevated rapidly from 0 to 60 sec of laser irradiation
but only elevated gradually from 100 to 120 sec of laser
irradiation (Fig. 3). In the
experiment with the tumor bearing mice, the temperature range
increased from 30–60°C in the first 4 min, and the temperature
subsequently became stable after 4 min. (Figs. 4 and 5).
Morphological observations
Surface observations indicated that the tumor tissue
from Group 1 (ICG: 0.1 mg/ml; laser power density: 1
W/cm2) did not exhibit any notable changes on the 1st
day after irradiation, while tumor tissue necrosis and scabbing
were observed on the 7th day.
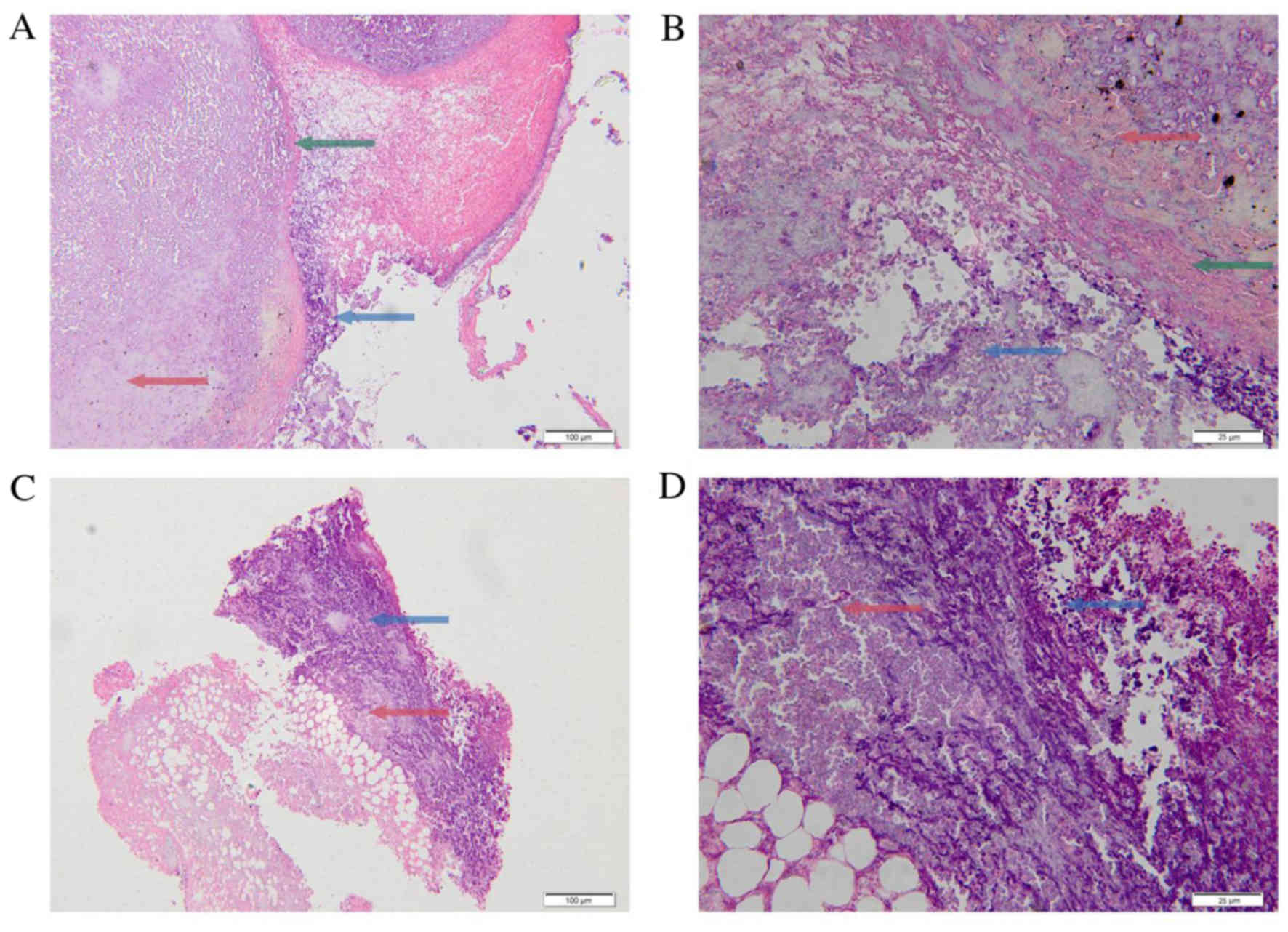
Fig. 6 indicates the
details of the cases with morphological changes in the tumor tissue
with H&E staining from Group 1 (ICG: 0.1 mg/ml; laser power
density: 1 W/cm2) on the 1st day and the 7th day
following laser irradiation. A complete tumor envelope was detected
in the tumor tissue sections from the 1st day following irradiation
(Fig. 6A and B). Additionally, a
small number of tumor cells experienced necrosis and a small number
of inflammatory cells and lymphocytes had infiltrated into the
tumor envelope (Fig. 6A and B). In
the tumor tissue sections from the 7th day following irradiation it
was indicated that the tumor envelopes were almost completed
destroyed and were incomplete in the tumor sections (Fig. 6C and D). Additionally, larger areas
of tumor tissue experienced necrosis on the 7th day, compared with
the 1st day. A large number of inflammatory cells, in particular
lymphocytes, had infiltrated into the tumor tissue (Fig. 6C and D). This appeared to be due to
laser irradiation, which destroyed the tumor cells and induced
tumor tissue necrosis. At the same time, the damaged tumor cells
elicited an immune response and induced the accumulation of
inflammatory cells around the tumor cells. The immune response was
stronger on the 7th day, compared with the 1st day.
Discussion
Photothermal therapy is a minimally invasive and
effective treatment method for cancer (11). It focuses generated heat from
absorbed laser energy to directly destroy targeted tumor tissues
and to indirectly induce a systemic immune response (19). Laser irradiation can the ability to
generate a thermal gradient inside the target tissue, which may
produce different biological responses at different temperatures.
For example, at a temperature of 43–44°C, heat erythema can occur.
At a temperature of 60–100°C, coagulation of protein can occur
(20). When the temperature is
>105°C, carbonization and evaporation of tissue occurs, which is
an undesirable phenomena in the process of photothermal therapy
(11). An extremely high temperature
changes the optical property of the tissue, which makes it
difficult for light to infiltrate into the deeper tissue (21). Considering the importance of
temperature on its biological effects in targeted tissue, the
characteristics of temperature changes during the process of
photothermal therapy should be investigated.
ICG is a water-soluble anionic tricarbocyanine dye
originally used in photography (22). ICG has been approved by the USA Food
and Drug Administration for clinical applications (17). The characteristics of ICG are NIR
absorption and fluorescence, making ICG suitable for bio-imaging
applications (23). ICG can be used
for ophthalmologic angiography, measuring cardiac output, hepatic
functional studies, and guiding biopsies, for example of breast
cancer (24). ICG has also been used
in photodynamic therapy by producing reactive oxygen species to
destroy tumor tissue (25).
Furthermore, ICG can absorb a specific spectrum of light from a
laser and produce a thermal effect subsequently to being injected
into a tumor (24). The combination
of ICG and a laser can produce a selective tumor thermal effect
(7).
In the present study, in the solution experiments,
the temperature elevation was almost proportional to the
concentration of the ICG solution when the same laser power density
was applied. In the tumor-bearing mice expriments, the results
demonstrated that the proportion of temperature elevation to laser
power density was weak. Compared with the concentration of ICG, the
contribution of the laser power density to the temperature
elevation was indicated to be small. Kannadorai and Liu (26) reported that there was almost no
increase in the overall temperature of the tumor during
photothermal therapy as the laser power density was steadily
increased.
When the solution and the tumor-bearing mice were
irradiated by a laser without any ICG there was no notable
elevation in temperature, compared with those treated with a laser
combined with ICG. Results indicated that the temperature
difference between the only-laser group and the combined group
could be up to 20°C. Therefore, the present study demonstrated that
ICG, as a photosensitizer, contributed significantly to temperature
elevation. Wang et al (27)
demonstrated that the combination of ICG and NIR could selectively
destroy the targeted tissue, reaching up to 1.5 cm in depth, with
minimal damage to the overlying surface tissue. Additionally, it
was indicated that by extending irradiation time, the temperature
quickly increased in the first 4 min and subsequently leveled off
after 4 min.
Morphological changes of the tumor tissue
demonstrated that photothermal therapy have the ability to elicit
an immune reaction, in addition to the heat effect. On the 7th day,
the immune reaction was stronger, compared with the 1st day.
Previous studies have demonstrated that different temperatures
achieved in the targeted tissue can elicit different biological
immune responses, including innate immune responses and acquired
immune responses (7,28). Thermal interactions at heat shock
temperatures are not sufficiently high enough to kill tumor cells
directly (29). In heat shock
temperatures ranging from 41 to 43°C however, injured tumor cells
are more sensitive to the immune system, as heat denatures tumor
antigens (30) can modulate immune
cells and cytokines. Cytotoxic temperatures are increased,
comprared with heat-shock temperatures (31). In the cytotoxic temperature range,
>43°C, tumor cells can be directly killed, as numerous tumor
cells disintegrate, therefore, releasing antigens. However, the
optimal time and temperature at which photothermal therapy can
stimulate the strongest immune effect still requires further
research.
The temperature of tumor tissue is not exclusively
dependent on the power density, ICG concentration and irradiation
time. There are numerous other factors that can influence
temperature, particularly in vivo (32). First, energy loss is associated to
blood flow (32). The temperature
distribution in vivo is usually non-uniform, due to tissue
cooling by blood flow (33). The
aforementioned phenomenon remains a challenge to avoid. Secondly,
tissue heterogeneity is another factor that influences temperature
elevation (34). Different types of
tumor tissues have different optical properties, which generate
different temperatures in different tumor tissues or in different
parts of a tumor, with the same laser power. Thirdly, tumor volume
also has an effect on temperature distribution (35) as smaller tumors have a more optimal
temperature distribution, compared with larger tumors (32).
A number of treatment strategies have been reported
decrease heterogeneity; however, to the best of our knowledge,
almost all biological therapeutic interventions have not been able
to overcome the basic neoplastic heterogeneity (34). The efficacy of photothermal therapy
itself for tumor treatment is limited. Combination therapy is the
current trend in cancer treatment. The combination of photothermal
therapy and immunotherapy, called laser immunotherapy, has
exhibited promising effects (28,36).
Laser immunotherapy has significantly prolonged the survival time
of tumor bearing mice that successfully rejected the second
inoculation of tumor cells (4).
Radiotherapy can convert ‘cold’ tumors into ‘hot’ tumors. ‘Cold’
tumors indicate less immunogenicity and are not affected by
immunotherapy, while ‘hot’ tumors are infiltrated with T cells and
therefore are sensitive to immunotherapy (37). Taking the aforementioned into
consideration, laser immunotherapy may be able to guide the immune
system to attack refractory types of cancers and sensitize these
refractory tumors to immune therapy by recruiting antitumor T
cells, as indicated in radiotherapy. Therefore, it is important to
control local temperature elevations within an appropriate range,
where high temperatures can both damage tumor cells and elicit
strong immune responses.
In photothermal therapy, the concentration of
photosensitizer and the laser power density are important
determinates of the temperature elevation. In the present study,
the temperature rise was almost proportional to the concentration
of ICG solution and the laser power density. The concentration of
ICG strongly contributed to the temperature rise compared with the
laser power density. Following the combination of laser with ICG,
temperature was significantly increased in the solution and in
tumor-bearing mice. By extending exposure time, the temperature
rose quickly at the beginning and then stabilized. Results
suggested that photothermal therapy may not only induce tumor
necrosis, but may also induce lymphocyte infiltration. The
characteristics of temperature changes play an important role in
the application of photothermal therapy. Further studies are needed
to investigate the optimal temperature for the generation of an
optimal thermal and immune effect.
Acknowledgements
The authors would like to acknowledge the support
provided by the Biophotonics Research Laboratory Center (OK,
USA).
Funding
This study was partly supported by the National Key
Research and Development Program of China (no. 2018YFB0407200) and
was supported in part by a grant from National Natural Science
Foundation of China (no. 81572953).
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL performed the majority of the experiments and
wrote the manuscript. XL designed the experiment and analyzed the
data. FZ assisted in conducting the experiment and analyzed
experiment data. YX participated in the design of the experiment,
performed experiments and partcipated in drafting of the
manuscript. YY was involved in revising the manuscript and
analyzing the data. BW and YF participated the analysis and
interpretation of data. ND was involved in revising the manuscript
and analyzing the data. All authors have read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee and were in compliance
with National Institutes of Health guidelines. In addition, tumor
burden did not exceed the recommended dimensions and animals were
anesthetized and sacrificed using acceptable method techniques.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu F: Heat-based tumor ablation: Role of
the immune response. Adv Exp Med Biol. 880:131–153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Le H, Wolf RF, Chen VA, Sarkar A,
Nordquist RE, Ferguson H, Liu H and Chen WR: Long-term effect on
EMT6 tumors in mice induced by combination of laser immunotherapy
and surgery. Integr Cancer Ther. 10:368–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen WR, Adams RL, Carubelli R and
Nordquist RE: Laser-photosensitizer assisted immunotherapy: A novel
modality for cancer treatment. Cancer Lett. 115:25–30. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le K, Li X, Figueroa D, Towner RA,
Garteiser P, Saunders D, Smith N, Liu H, Hode T, Nordquist RE and
Chen WR: Assessment of thermal effects of interstitial laser
phototherapy on mammary tumors using proton resonance frequency
method. J Biomed Opt. 16:1280012011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Ferrel GL, Guerra MC, Hode T, Lunn
JA, Adalsteinsson O, Nordquist RE, Liu H and Chen WR: Preliminary
safety and efficacy results of laser immunotherapy for the
treatment of metastatic breast cancer patients. Photochem Photobiol
Sci. 10:817–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bailey CA, Cowan TM, Liu VG, Lemley EC and
Chen WR: Optimization of selective hyperthermia. J Biomed Opt.
9:648–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slovak R, Ludwig JM, Gettinger SN, Herbst
RS and Kim HS: Immuno-thermal ablations-boosting the anticancer
immune response. J Immunother Cancer. 5:782017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vankayala R, Huang YK, Kalluru P, Chiang
CS and Hwang KC: First demonstration of gold nanorods-mediated
photodynamic therapeutic destruction of tumors via near infra-red
light activation. Small. 10:1612–1622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vankayala R, Lin CC, Kalluru P, Chiang CS
and Hwang KC: Gold nanoshells-mediated bimodal photodynamic and
photothermal cancer treatment using ultra-low doses of near
infra-red light. Biomaterials. 35:5527–5538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou X, Tao Y, Pang Y, Li X, Jiang G and
Liu Y: Nanoparticle-based photothermal and photodynamic
immunotherapy for tumor treatment. Int J Cancer. 143:3050–3060.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crochet JJ, Gnyawali SC, Chen Y, Lemley
EC, Wang LV and Chen WR: Temperature distribution in selective
laser-tissue interaction. J Biomed Opt. 11:340312006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Naylor MF, Le H, Nordquist RE,
Teague TK, Howard CA, Murray C and Chen WR: Clinical effects of in
situ photoimmunotherapy on late-stage melanoma patients: A
preliminary study. Cancer Biol Ther. 10:1081–1087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou F, Li X, Naylor MF, Hode T, Nordquist
RE, Alleruzzo L, Raker J, Lam SS, Du N, Shi L, et al: InCVAX-a
novel strategy for treatment of late-stage, metastatic cancers
through photoimmunotherapy induced tumor-specific immunity. Cancer
Lett. 359:169–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor JS, Zeki J, Ikegaki N, Chen LL and
Chiu B: Combined application of Indocyanine green (ICG) and laser
lead to targeted tumor cell destruction. J Pediatr Surg.
53:2475–2479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandberg K and Umans JG; Georgetown
Consensus Conference Work Group, : Recommendations concerning the
new U.S. National Institutes of Health initiative to balance the
sex of cells and animals in preclinical research. FASEB J.
29:1646–1652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou G, Yao Y and Wang G: Inhibitory effect
of quercetin on ACC-M cell xenografts in nude mice in vivo. J Oral
Sci Res. 34:554–557. 2018.
|
|
18
|
Kourea H and Kotoula V: Towards tumor
immunodiagnostics. Ann Transl Med. 4:2632016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang C, Diao S, Wang C, Gong H, Liu T,
Hong G, Shi X and Liu Z: Tumor metastasis inhibition by
imaging-guided photothermal therapy with single-walled carbon
nanotubes. Adv Mater. 26:5646–5652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HG, Mehta K, Cohen P and Guha C:
Hyperthermia on immune regulation: A temperature's story. Cancer
Lett. 271:191–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iizuka MN, Vitkin IA, Kolios MC and Sherar
MD: The effects of dynamic optical properties during interstitial
laser photocoagulation. Phys Med Biol. 45:1335–1357. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fox IJ, Brooker LG, Heseltine DW, Essex HE
and Wood EH: A tricarbocyanine dye for continuous recording of
dilution curves in whole blood independent of variations in blood
oxygen saturation. Proc Staff Meet Mayo Clin. 32:478–484.
1957.PubMed/NCBI
|
|
23
|
Giraudeau C, Moussaron A, Stallivieri A,
Mordon S and Frochot C: Indocyanine green: Photosensitizer or
chromophore? Still a debate. Curr Med Chem. 21:1871–1897. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Min M, Du N, Gu Y, Hode T, Naylor M,
Chen D, Nordquist RE and Chen WR: Chitin, chitosan, and glycated
chitosan regulate immune responses: The novel adjuvants for cancer
vaccine. Clin Dev Immunol. 2013:3870232013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porcu EP, Salis A, Gavini E, Rassu G,
Maestri M and Giunchedi P: Indocyanine green delivery systems for
tumour detection and treatments. Biothchnol Adv. 34:768–789. 2016.
View Article : Google Scholar
|
|
26
|
Kannadorai RK and Liu Q: Optimization in
interstitial plasmonic photothermal therapy for treatment planning.
Med Phys. 40:1033012013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang LV, Nordquist RE and Chen WR: Optimal
beam size for light delivery to absorption-enhanced tumors buried
in biological tissues and effect of multiple-beam delivery: A Monte
Carlo study. Appl Opt. 36:8286–8291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Xu L, Liang C, Xiang J, Peng R and
Liu Z: Immunological responses triggered by photothermal therapy
with carbon nanotubes in combination with anti-CTLA-4 therapy to
inhibit cancer metastasis. Adv Mater. 26:8154–8162. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon TJ, Kim JY, Kim H, Hong C, Lee H, Lee
CK, Lee KH, Hong S and Park SH: Anti-tumor immunostimulatory effect
of heat-killed tumor cells. Exp Mol Med. 40:130–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleinovink JW, Fransen MF, Löwik CW and
Ossendorp F: Photodynamic-immune checkpoint therapy eradicates
local and distant tumors by CD8+ T cells. Cancer Immunol
Res. 5:832–838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X and Chen WR: Laser immunotherapy:
Novel modality to treat cancer through specific antitumor immune
response. Chin J Lasers. 37:2698–2702. 2010. View Article : Google Scholar
|
|
32
|
Dillon C, Roemer R and Payne A: Magnetic
resonance temperature imaging-based quantification of blood
flow-related energy losses. NMR Biomed. 28:840–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ganguly M, Miller S and Mitra K: Model
development and experimental validation for analyzing initial
transients of irradiation of tissues during thermal therapy using
short pulse lasers. Lasers Surg Med. 47:711–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SS, Roche PJ, Giannopoulos PN,
Mitmaker EJ, Tamilia M, Paliouras M and Trifiro MA:
Prostate-specific membrane antigen-directed nanoparticle targeting
for extreme nearfield ablation of prostate cancer cells. Tumour
Biol. 39:10104283176959432017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gnyawali SC, Chen Y, Wu F, Bartels KE,
Wicksted JP, Liu H, Sen CK and Chen WR: Temperature measurement on
tissue surface during laser irradiation. Med Biol Eng Comput.
46:159–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Xu L, Liang C, Wang C, Peng R and
Liu Z: Photothermal therapy with immune-adjuvant nanoparticles
together with checkpoint blockade for effective cancer
immunotherapy. Nat Commun. 7:131932016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Demaria S, Coleman CN and Formenti SC:
Radiotherapy: Changing the game in immunotherapy. Trends Cancer.
2:286–294. 2016. View Article : Google Scholar : PubMed/NCBI
|