Introduction
Bufalin (BF) is a cardiotonic steroid isolated from
the Chinese toad venom, Chansu, a galenical preparation of the
dried white venom of Chinese Bufo gargarizans (Asiatic toad)
(1,2), with a molecular formula of
C24H3404 and a relative molecular
weight of 386.5 g/mol. As an active compound extracted from a
Chinese traditional medicine, BF exerts various biological effects,
including pain relief, myocardial contraction stimulation, blood
pressure stimulation, anti-inflammatory and antineoplastic
activities (3–5). Since 2010, BF has received increased
attention due to its anticancer effects on a wide range of cancer
types (e.g., lung, liver, prostate, gastric, colon and gastric
cancer) (6–12). This compound could mediate cell cycle
arrest, cell growth inhibition, apoptosis and the expression of
genes associated with the malignant phenotype in human cancer cells
(13). In addition, BF can be used
safely for an extended period without marked side effects (12,13).
Furthermore, transformed cells could be more susceptible to the
effects of BF than normal cells (13). All of these factors have prompted the
present review to analyze the potential of BF in anticancer
treatments. However, the precise molecular mechanisms by which BF
induces tumor suppression remain unclear. Microarray analysis
revealed possible target-related proteins and genes of BF in cancer
cells (11). A proteomic-based study
was performed by Xie et al (11), and following BF treatment, 24
differentially expressed proteins were identified using a
comparative proteomics approach. The study found that the
downregulation of heat shock protein 27 (Hsp27) could serve a
critical role in BF-induced apoptosis in osteosarcoma cells.
Subsequently, Zhang et al (14) explored target-related proteins using
two quantitative proteomic methods (isobaric tags for relative and
absolute quantitation-based and label-free proteomic analysis) in
lung cancer. These two proteomic methods were complementary, and
suggested that oxidative stress and regulation of relevant gene
expression were significantly involved in the effects of BF, while
the fibronectin-associated pathway was found to be important.
Bioinformatics analysis revealed that the fibronectin-associated
pathway is the most distinct pathway in the signal network of BF,
and BF-induced protein expression changes, including decreased
expression of fibronectin, increased expression of paxillin,
calpain 2 and cell division control protein 42 homolog, have been
further confirmed in the fibronectin-associated pathway using
immunoblotting (14). In addition,
the genetic mechanisms underlying BF-induced DNA damage and
apoptosis in lung cancer cells have been further elucidated
(15). Wu et al (15) demonstrated that numerous genes
associated with cell cycle regulation, apoptosis and DNA repair are
significantly altered following BF treatment. Analysis of these
gene alterations by Wu et al (15) and Zhang et al (14) could aid the elucidation of the
mechanism underlying the cytotoxicity of BF at the genetic level
and potentially offer various biomarkers for the treatment and
diagnosis of lung cancer. Certainly, further studies are required
to improve the understanding of how BF suppresses cancerous cells
and does not affect normal cells. Furthermore, combinations of BF
with cytotoxic agents, differentiation-inducing agents and even
gene therapy may represent potential novel therapeutic strategies
for cancer treatment. The present review aims to evaluate the
anticancer properties of BF from the perspective of emerging
treatment options for cancer patients.
BF could exert antitumor activity by
inducing the apoptosis of various human cancer cells
Inducing apoptosis in target cells could be a key
mechanism for the majority of anticancer therapies. BF is a
cardiotonic steroid that has the potential to induce cancer cell
apoptosis (12). Cell apoptosis was
induced in human non-small cell lung cancer A549 cells following
treatment with BF, while suppression of cell proliferation occurred
in a time- and dose-dependent manner, and induced cell cycle arrest
at the G1 phase was found (6). Li et al (7) identified that BF exerts antitumor
effects by triggering apoptosis and inducing cell cycle arrest in
pancreatic cancer cells. Notably, in pancreatic cancer cells, BF
could also promote the growth inhibition effect of gemcitabine
(7). For the first time, Jiang et
al (16) indicated that BF could
be a potential therapy for treating gallbladder cancer. The study
demonstrated that BF induces cell cycle arrest and apoptosis in
gallbladder carcinoma cells. Treatment of human bladder carcinoma
T24 cells with BF had a significant growth inhibition effect
(P<0.05), compared with vehicle treatment. This effect is likely
attributable to the prominent arrest of cancer cells in the
G2/M phase of the cell cycle and the apoptosis
stimulated by BF (17), as evidenced
by formation of apoptotic bodies, chromatin condensation and cell
accumulation in the sub-G1 phase. In breast cancer, BF
greatly sensitized estrogen receptor (ER) α-positive MCF-7 and
ERα-negative MDA-MB-231 human breast cancer cells to tumor necrosis
factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced
apoptosis (18), compared with
vehicle treatment. Notably, BF increases TRAIL-induced apoptosis
from 2.0±0.5 to 30.1±1.2% in MCF-7 cells, and from 6.9±1.8 to
41.5±1.4% in MDA-MB-231 cells, which indicated that MCF-7 cells are
more sensitive to BF than MDA-MB-231 cells. The enhanced apoptotic
effects of the TRAIL/BF combination were also associated with the
augmentation of caspase activation (18). In leukemia, BF could exert strong
differentiation-inducing activity in three human leukemia-derived
cell lines (myeloblastic ML1, human promyelocytic HL60 and
monoblastic U937) at a concentration of 10 nM. However, treatment
of human leukemia K562 cells with other cardiotonic steroids,
including digitoxigenin, cinobufagin and ouabain, at the same
concentration only had a weak or no effect on these cells (19,20).
These findings indicate that BF may have potential in human
myelogenous leukemia differentiation therapy (19–21).
Notably, Jing et al (22)
demonstrated that in normal polymorphonuclear and mononuclear
cells, apoptotic cell death was not induced by BF, indicating that
the anticancer effects of BF may be cell-type specific. In gastric
cancer, BF could inhibit the proliferation of gastric cancer MGC803
cells by inducing apoptosis, and the phosphatidylinositol
3-kinase/protein kinase B (Akt) pathway may serve a key role in
this process (23). The
antiproliferative and apoptosis-inducing mechanisms of BF in
prostate cancer cells have also been investigated (24,25). In
hepatocellular carcinoma, BF exhibited significant anticancer
effects on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice and could promote the
apoptosis of transplanted tumor cells with no marked toxicity
(26). In our previous study
investigating glioma (27), it was
demonstrated that BF inhibits glioma growth by promoting
proteasomal degradation of the sodium/potassium-adenosine
5′-triphosphatase α-1 subunit (ATP1A1), and ATP1A1 deficiency could
inhibit the proliferation of glioma via promotion of apoptosis.
Notably, BF was revealed to penetrate the blood-brain barrier (BBB)
(27). Rats were intraperitoneally
injected with BF and cerebrospinal fluid (CSF) was collected from
the cerebello-medullary cistern by penetrating the foramen magnum.
The content of BF in the CSF was detected using liquid
chromatography/mass spectrometry assays to prove that BF could
traverse the BBB (27). Therefore,
we hypothesize that BF can enter the brain via the BBB and thus
exert a more powerful effect on tumors in the central nervous
system.
BF could exert anticancer effects by
triggering autophagic cancer cell death
Apoptosis, necrosis and autophagic cell death are
the three major morphological processes responsible for cell death
(28,29). Autophagy is an important cellular
catabolic process that maintains homeostasis by degrading
dysfunctional cellular organelles and excessive proteins in living
cells (30). The cytoplasms and
double smooth membranes (phagophores) of numerous types of
organelles, including the endoplasmic reticulum, peroxisomes and
mitochondria, can form autophagosomes. The autophagosome then fuses
with the lysosome to form autophagolysosomes, finally leading to
degradation of the captured proteins or organelles by lysosomal
enzymes (31–33). Promoting autophagic cell death is an
important strategy for the chemotherapeutic treatment of cancer
(34–36). Xie et al (10) demonstrated that BF could induce
autophagic cell death via c-Jun N-terminal kinase activation and
the generation of reactive oxygen species in human colon cancer
HT29 cells. Tsai et al (37)
also found that in SK-HEP-1 cells, BF induced autophagic cell death
and cell cycle arrest via the Akt/mechanistic target of rapamycin
signaling pathway. These findings indicate that BF may be a
possible treatment of human hepatocellular carcinoma. Notably, Shen
et al (38) explored the
potential of BF in glioma for the first time. The study found that
preventing autophagy promoted apoptosis and increased the induction
of ER stress-associated proteins, indicating that autophagy could
exert a cytoprotective effect on cell death and ER stress induced
by BF. This result indicates that BF could inhibit glioma cell
growth and induce interplay between autophagy and apoptosis via ER
stress, and more importantly, provides a molecular basis for
developing BF into a drug candidate for glioma treatment. The
amount of research on the association between autophagy and cancer
has increased rapidly. Cancer cells may utilize autophagy to
enhance their survival in the hostile tumor microenvironment with
an altered metabolism, indicating that suppression of autophagy is
required from therapeutic cancer treatment strategies (39). Indeed, the interaction between
autophagy and apoptosis, which depends on the use of
chemotherapeutic drugs, as well as the type of cancer cell, could
significantly impact the fate of human cancer cells.
BF could exert anticancer effects via its
anti-inflammatory activity
Chansu has been used in the treatment of
inflammatory diseases in China for thousands of years (40); however, little is known about the
anti-inflammatory mechanisms of BF. Nuclear factor-κB (NF-κB) is a
central regulator of the inflammatory process and could regulate a
group of proinflammatory mediators, including inducible nitric
oxide synthase, cyclooxygenase-2, interleukin (IL)-1β, TNF and IL-6
(41). In 2011, Ye et al
(42) suggested that BF suppresses
the nuclear translocation of NF-κB in response to TNF in human
kidney tissue cells. BF was also shown to modulate NF-κB activity
in human osteosarcomas in a previous study (11). NF-κB signaling could be a potential
therapeutic target of BF for the pathogenesis of inflammation and
cancer. Wen et al (43)
demonstrated that compared with vehicle treatment, BF exerts a
strong anti-inflammatory effect on carrageenan-induced paw edema in
rats, which is a commonly used model for the investigation of
inflammation. The study demonstrated that BF significantly
(P<0.05) suppressed the activation of NF-κB in vivo by
maintaining IκBα levels and inhibiting the nuclear translocation of
NF-κB p65. Furthermore, the downstream NF-κB proinflammatory
mediators, cyclooxygenase-2, inducible nitric oxide synthase,
TNF-α, IL-6 and IL-1β were also suppressed. Therefore, BF could
possess strong in vivo anti-inflammatory activity, thus
serving a role in reducing NF-κB activation and the inhibition of
downstream proinflammatory mediators. Inflammatory pathways have
been targeted in attempts to treat cancer (44–46), and
the association between cancer and inflammation has returned to the
forefront of clinical oncology. These findings support BF as a
potential novel therapeutic agent for alleviating inflammation, and
more importantly, for treating various cancer types.
Promising BF derivatives may have even
greater anticancer activity
While research into BF has revealed its potential in
the treatment of different human cancer types, further
investigation is required prior to its use as a cancer treatment.
BF has a narrow therapeutic window, extremely low water solubility,
unsatisfactory bioavailability and severe adverse effects,
including high cardiac toxicity, which limits its clinical
applications (47,48). Since the purification and total
synthesis of BF, various methods have been used to improve its
activity and expand its therapeutic potential in different
biological systems by modifying its structure (49,50).
More importantly, efforts should be directed towards identifying
additional BF derivatives that exert stronger anticancer effects
and have a lower toxicity level compared with the natural compound
to promote the development of novel anticancer agents from cardiac
steroids, including BF.
Notably, Liu et al (51) synthesized a novel polymeric prodrug
of BF, poly (ethylene glycol)-based polymeric prodrug of BF
(PEGS-BF). The water solubility and stability of PEGS-BF were
improved without loss of its anticancer activity compared with BF.
In addition, in vitro and in vivo experiments showed
that PEGS-BF exerted anticancer effects comparable to those of
unbound BF. The reported improved stability, water solubility and
controlled drug release features of this polymeric prodrug provide
strong arguments for its use in potential clinical
applications.
BF211 is a BF derivative with a stronger cytotoxic
activity than BF in cancer cells (52,53). In
nude immunodeficient Balb/c-nu-nu mice inoculated with A549 cells,
BF211 also exhibited significantly (P<0.05) stronger suppressive
effects on tumor growth compared with BF (53). Notably, as the acute toxicity of
BF211 was lower compared with that of BF [lethal dose 50
(LD50) value of BF211 was 14.75 mg/kg for male mice and
18.21 mg/kg for female mice, whereas LD50 value of BF in
mice was ~2.2 mg/kg], BF211 could be used at higher concentrations
for cancer treatment, indicating that BF211 could have a wider
therapeutic window. In summary, studies by Liu et al
(53) demonstrated that BF211 is a
potential novel anticancer compound with a relatively lower
toxicity compared with BF as aforementioned (BF211 LD50
value could be >6 times higher than BF LD50 value),
and this may be due to its specific binding characteristics for
sodium-potassium adenosine triphosphatase. Furthermore, Sun et
al (54) attempted to identify
target-related proteins of BF211 in A549 cells to further elucidate
the mechanism underlying its anticancer effects. Their findings
suggested that BF211 may impact multiple cellular functions,
including translation, transcription and protein synthesis. In
addition, a previous study (54)
also explored the effect of BF211 on regulating proteasome
activities and revealed only moderate inhibitory effects; thus,
there is currently no direct evidence that the anticancer effects
of BF211 are mediated by proteasome inhibition and it is possible
that the contribution of proteasome inhibition to the cytotoxicity
of BF211 is minimal.
Clinical trials regarding the use of BF in
patients
Chansu, the galenical preparation of the dried white
venom of Chinese Bufo gargarizans, has been widely used for
the treatment of cancer at oncology clinics in China (55). Huachansu, one of the main
biologically active components of BF, is a Chinese medicine derived
from dried toad venom from the skin glands of Bufo bufo
gargarizans Cantor or Bufo melanotictus Schneider
(55). Huachansu is manufactured by
Anhui Jinchan Biochemistry Company Ltd., in Huaibei, China (Chinese
FDA no. ISO9002) and includes 2 primary biologically active
chemical components: Indole alkaloids (bufotenidine, bufotenine,
serotonin and cinobufotenine) and steroidal cardiac glycosides
(>28 have been identified, including BF, cinobufagin,
resibufogenin, marinobufagin, cinobufotalin and bufotalin)
(55). A previous study confirmed
that BF, cinobufagin and resibufogenin are the 3 major cardiac
glycosides, which the anticancer activity of Huachansu can be
attributed to (56). A pilot study
investigating the use of Huachansu in patients with advanced cancer
was performed by Meng et al (57), using a phase I trial design.
Huachansu was intravenously administered for 2 weeks followed by 1
week off, for each cycle. Without significant adverse events or
progressive disease, treatment continued beyond two cycles. A total
of 15 patients (11 with hepatocellular cancer, 2 with pancreatic
cancer and 2 with non-small cell lung cancer) were included in the
trial. Overall 6 patients (40%) had stable disease (median
duration, 6.0 months; range, 3.5–11.1 months). In addition, 1
patient with hepatocellular cancer had a 20% reduction in tumor
mass (duration of disease stability in response to Huachansu alone,
11 months). The plasma BF concentration reached maximal levels at
the end of the 2-h infusion and was proportional to the amount of
drug administered. Notably, a dose-dependent increase in BF levels
was observed in all the patients, with no evidence of drug
accumulation in the plasma, which may have been due to the short
half-life of the drug. Although there was no correlation between
the plasma BF level and the anticancer effect of Huachansu, the
small number of patients studied precluded drawing an association
between the drug dose and response. One limitation of the study by
Meng et al (57) was the
absence of a control group. Significantly, Huachansu has been found
to be well tolerated, even at doses eight times those normally
administered (normally administered doses: Level l, 10
ml/m2; level 2, 20 ml/m2; level 3, 40
ml/m2; level 4, 60 ml/m2; and level 5, 90
ml/m2) in China, and can lead to disease stabilization
in certain patients according to the results of a phase I clinical
trial (57).
Conclusion
In summary, the potential roles of BF in various
cancer types have been increasingly recognized, but the specific
mechanisms have not been fully clarified (Fig. 1). The present review demonstrates
that BF has marked antitumor activity. BF could exert an antitumor
effect by inducing apoptosis and triggering autophagic cell death
in various human cancer cells. The anti-inflammatory activities of
BF are also important for its antitumor function. Notably, an
increasing number of BF derivatives have shown potent anticancer
activity, including PEGS-BF and BF211. Clinical trials regarding
the use of BF in cancer patients have supported the positive effect
of BF on cancer treatment.
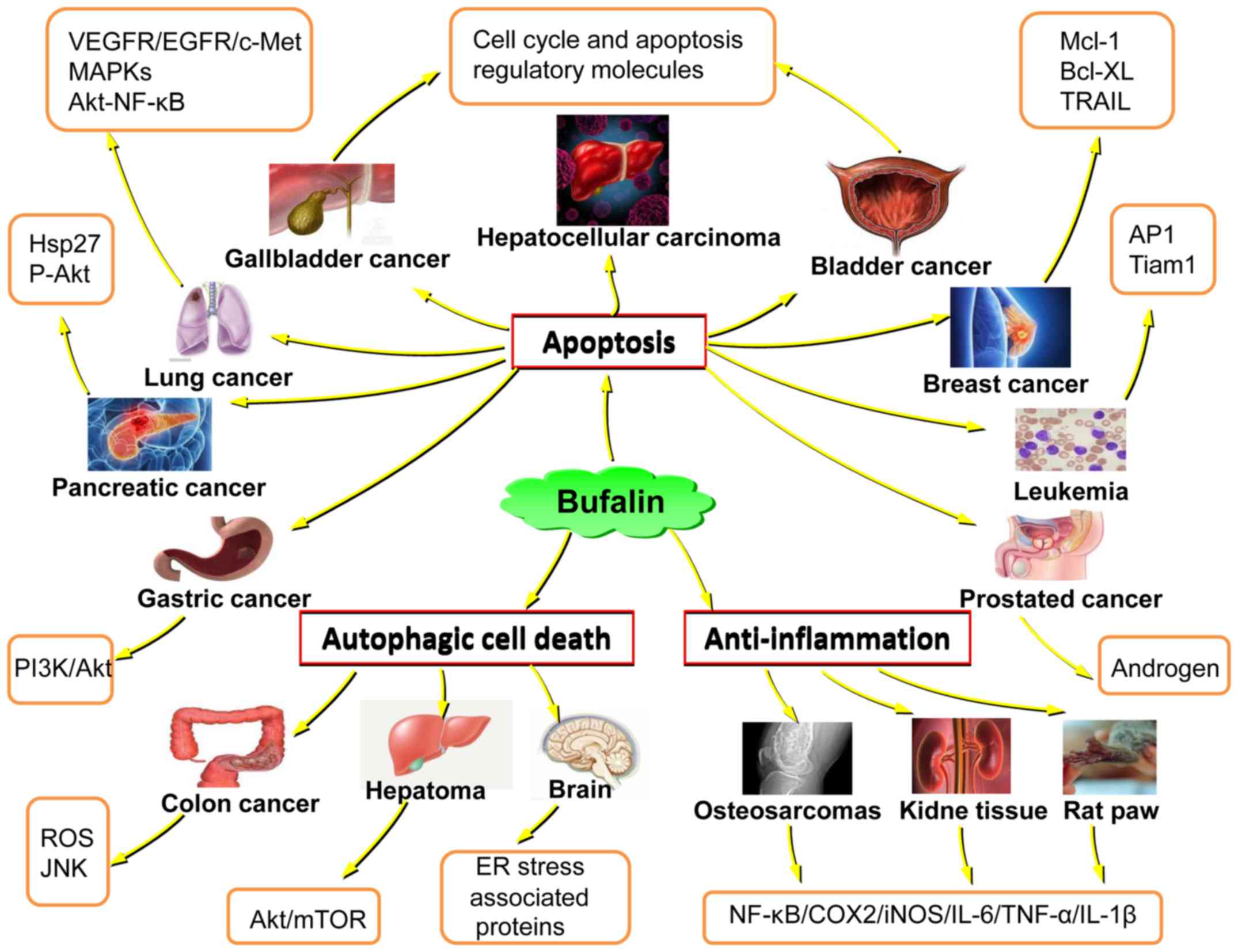 | Figure 1.Schematic diagram displaying the
anticancer properties of bufalin, from the perspective of the
emerging treatment targets for various cancer types. Hsp27, heat
shock protein 27; P-Akt, phosphorylated protein kinase B; VEGFR,
vascular endothelial growth factor receptor; EGFR, epidermal growth
factor receptor, cMet, mesenchymal-epithelial transition factor;
MAPKs, mitogen-activated protein kinases; NF-κB, necrosis factor-κ
B; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen
species; JNK, c-Jun N-terminal kinases; mTOR, mechanistic target or
rapamycin; ER, endoplasmic reticulum; COX2, cyclooxygenase 2; iNOS,
inducible nitric oxide synthase; IL, interleukin; TNF-α, tumor
necrosis factor-α; AP1, activating protein 1; Tiam1, T-lymphoma
invasion and metastasis-inducing protein; Mcl-1, induced myeloid
leukemia cell differentiation protein; Bcl-XL, B-cell
lymphoma-extra large; TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand. |
An increasing number of clinical studies will focus
on investigating the use of BF in cancer treatment in the near
future. Research has revealed that BF has potential as a potent,
novel and effective anticancer therapy for cancer patients.
However, more BF derivatives with stronger anticancer effects and
lower toxicity levels should be identified to promote the
development of additional novel anticancer agents. Furthermore,
large-scale randomized studies are required to investigate the
efficacy of BF in various cancer types. This review provides
critical information for the design of larger and more focused
clinical studies that are necessary to systematically and
definitively evaluate the role of BF in cancer treatments.
Acknowledgements
Not applicable.
Funding
This study is supported by grants from the National
Natural Science Foundation of China (nos. 81372714 and 81672480),
the Liaoning Provincial Natural Science Foundation of China (nos.
201602244 and 2016010217-30), the Distinguished Professor Project
of Liaoning Province, the Special Grant for Translational Medicine,
the Dalian Medical University (no. 2015002) and the Basic research
projects in colleges and universities of Liaoning Province (no.
LQ2017033).
Availability data and materials
Not applicable.
Author contributions
YL, JL, XJ, XW, JX, SL and BZ contributed in the
design and writing of the review. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Xia Y, Zuo Q and Chen T: Molecular
mechanisms underlying the antimetastatic activity of bufalin. Mol
Clin Oncol. 8:631–636. 2018.PubMed/NCBI
|
|
2
|
Dai XY, Zhou BF, Xie YY, Lou J and Li KQ:
Bufalin and 5-fluorouracil synergistically induce apoptosis in
colorectal cancer cells. Oncol Lett. 15:8019–8026. 2018.PubMed/NCBI
|
|
3
|
Cui X, Inagaki Y, Xu H, Wang D, Qi F,
Kokudo N, Fang D and Tang W: Anti-hepatitis B virus activities of
cinobufacini and its active components bufalin and cinobufagin in
HepG2.2.15 cells. Biol Pharm Bull. 33:1728–1732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu CX, Nan KJ and Lei Y: Agents from
amphibians with anticancer properties. Anticancer Drugs.
19:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F,
Yagasaki K and Zhang G: Effects of bufalin on the proliferation of
human lung cancer cells and its molecular mechanisms of action.
Cytotechnology. 62:573–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q,
Wang X and Li J: Bufalin exerts antitumor effects by inducing cell
cycle arrest and triggering apoptosis in pancreatic cancer cells.
Tumor Biol. 35:2461–2471. 2014. View Article : Google Scholar
|
|
8
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13:185–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu YY, Hu Q, Tang QF, Feng W, Hu SJ,
Liang B, Peng W and Yin PH: MicroRNA-497 and bufalin act
synergistically to inhibit colorectal cancer metastasis. Tumor
Biol. 35:2599–2606. 2014. View Article : Google Scholar
|
|
10
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie XB, Yin JQ, Wen LL, Gao ZH, Zou CY,
Wang J, Huang G, Tang QL, Colombo C, He WL, Jia Q and Shen JN:
Critical role of heat shock protein 27 in bufalin-induced apoptosis
in human osteosarcomas: A proteomic-based research. PLoS One.
7:e473752012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takai N, Kira N, Ishii T, Yoshida T,
Nishida M, Nishida Y, Nasu K and Narahara H. Bufalin: a traditional
oriental medicine, induces apoptosis in human cancer cells. Asian
Pac J Cancer Prev. 13:399–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang DM, Feng LX, Liu M, Jin WH, Luo J,
Nie AY, Zhou Y, Li Y, Wu WY, Jiang BH, et al: Possible
target-related proteins and signal network of bufalin in A549 cells
suggested by both iTRAQ-based and label-free proteomic analysis.
Proteomics. 16:935–945. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SH, Hsiao YT, Chen JC, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin alters gene
expressions associated DNA damage, cell cycle and apoptosis in
human lung cancer NCI-H460 cells in vitro. Molecules. 19:6047–6057.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
18
|
Dong Y, Yin S, Li J, Jiang C, Ye M and Hu
H: Bufadienolide compounds sensitize human breast cancer cells to
TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway.
Apoptosis. 16:394–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Z, Li E and Liu Y, Gao Y, Sun H, Wang
Y, Wang Z, Liu X, Wang Q and Liu Y: Bufalin induces the apoptosis
of acute promyelocytic leukemia cells via the downregulation of
survivin expression. Acta Haematol. 128:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai X, Lu J, Wang Y, Fang F, Li B and Gu
W: Reversal effect of bufalin on multidrug resistance in K562/VCR
vincristine-resistant leukemia cell line. J Tradit Chin Med.
34:678–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LP, Zhao YN, Sun X and Gao RL:
Effects of bufalin on up-regulating methylation of Wilm's tumor 1
gene in human erythroid leukemic cells. Chin J Integr Med.
23:288–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14:1193–1198. 1994.PubMed/NCBI
|
|
23
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of androgen
dependen and independent prostate cancer cells. Prostate.
54:112–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and-independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan YL, Wang X, Lou JC, Xing JS, Yu ZL,
Wang H, Zou S, Ma X and Zhang B: Bufalin inhibits glioblastoma
growth by promoting proteasomal degradation of the
Na+/K+ -ATPase α1 subunit. Biomed
Pharmacother. 103:204–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naumann P, Fortunato F, Zentgraf H,
Büchler MW, Herr I and Werner J: Autophagy and cell death signaling
following dietary sulforaphane act independently of each other and
require oxidative stress in pancreatic cancer. Int J Oncol.
39:101–109. 2011.PubMed/NCBI
|
|
30
|
Rabinowitz JD and White E: Autophagy and
metabolism. Science. 330:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: Cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinez-Borra J and Lopez-Larrea C:
Autophagy and self-defense. Adv Exp Med Biol. 738:169–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anders HJ and Schlondorff DO: Innate
immune receptors and autophagy: Implications for autoimmune kidney
injury. Kidney Int. 78:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ropolo A, Bagnes CI, Molejon MI, Lo Re A,
Boggio V, Gonzalez CD and Vaccaro MI: Chemotherapy and
autophagy-mediated cell death in pancreatic cancer cells.
Pancreatology. 12:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yousefi S and Simon HU: Autophagy in
cancer and chemotherapy. Results Probl Cell Differ. 49:183–190.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsai SC, Yang JS, Peng SF, Lu CC, Chiang
JH, Chung JG, Lin MW, Lin JK, Amagaya S, Wai-Shan, Chung C, et al:
Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell
death in SK-HEP-1 human hepatocellular carcinoma cells. Int J
Oncol. 41:1431–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen S, Zhang Y, Wang Z, Liu R and Gong X:
Bufalin induces the interplay between apoptosis and autophagy in
glioma cells through endoplasmic reticulum stress. Int J Biol Sci.
10:212–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsujimoto Y and Shimizu S: Another way to
die: Autophagic programmed cell death. Cell Death Differ. 12 Suppl
2:S1528–S1534. 2005. View Article : Google Scholar
|
|
40
|
Chen KK and Kovaríková A: Pharmacology and
toxicology of toad venom. J Pharm Sci. 56:1535–1541. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahman A and Fazal F: Blocking NF-κB: An
inflammatory issue. Proc Am Thorac Soc. 8:497–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye J, Chen S and Maniatis T: Cardiac
glycosides are potent inhibitors of interferon-β gene expression.
Nat Chem Biol. 7:25–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wen L, Huang Y, Xie X, Huang W, Yin J, Lin
W, Jia Q and Zeng W: Anti-inflammatory and antinociceptive
activities of bufalin in rodents. Mediators Inflamm.
2014:1718392014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Colombo MP and Mantovani A: Targeting
myelomonocytic cells to revert inflammation-dependent cancer
promotion. Cancer Res. 65:9113–9116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang Y, Snuderl M and Jain RK:
Polarization of tumor-associated macrophages: A novel strategy for
vascular normalization and antitumor immunity. Cancer Cell. 19:1–2.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang Y, Yuan J, Righi E, Kamoun WS,
Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR,
Vianello F, et al: Vascular normalizing doses of antiangiogenic
treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci USA. 109:17561–17566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu Q, Liang B, Sun Y, Guo XL, Bao YJ, Xie
DH, Zhou M, Duan YR, Yin PH and Peng ZH: Preparation of
bufalin-loaded pluronic polyetherimide nanoparticles, cellular
uptake, distribution, and effect on colorectal cancer. Int J
Nanomedicine. 9:4035–4041. 2014.PubMed/NCBI
|
|
48
|
Yang Z, Teng Y, Wang H and Hou H:
Enhancement of skin permeation of bufalin by limonene via reservoir
type transdermal patch: Formulation design and biopharmaceutical
evaluation. Int J Pharm. 447:231–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pettit GR, Houghton LE, Knight JC and
Bruschwe F: Synthesis of bufalin. J Chem Soc D Chem Commun.
2:93–94. 1970. View Article : Google Scholar
|
|
50
|
Yuan XF, Tian HY, Li J, Jin L, Jiang ST,
Liu KW, Luo C, Middleton DA, Esmann M, Ye WC and Jiang RW:
Synthesis of bufalin derivatives with inhibitory activity against
prostate cancer cells. Nat Prod Res. 28:843–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu T, Yuan X, Jia T, Liu C, Ni Z, Qin Z
and Yuan Y: Polymeric prodrug of bufalin for increasing solubility
and stability: Synthesis and anticancer study in vitro and in vivo.
Int J Pharm. 506:382–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lei M, Xiao Z, Ma B, Chen Y, Liu M, Liu J,
Guo D, Liu X and Hu L: Synthesis and biological evaluation of
bufalin-3-yl nitrogen-containing-carbamate derivatives as
anticancer agents. Steroids. 108:56–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu M, Feng LX, Sun P, Liu W, Wu WY, Jiang
BH, Yang M, Hu LH, Guo DA and Liu X: A novel bufalin derivative
exhibited stronger apoptosis-inducing effect than bufalin in A549
lung cancer cells and lower acute toxicity in mice. PLoS One.
11:e01597892016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun P, Feng LX, Zhang DM, Liu M, Liu W, Mi
T, Wu WY, Jiang BH, Yang M, Hu LH, et al: Bufalin derivative BF211
inhibits proteasome activity in human lung cancer cells in vitro by
inhibiting β1 subunit expression and disrupting proteasome
assembly. Acta Pharmacol Sin. 37:908–918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Su YH and Nu X: Evaluation of
pharmacodynamic effect of pharmaceutical agents of Chan Su. J
Beijing Univ TCM. 24:51–54. 2001.
|
|
56
|
Su YH, Huang XQ, Zhang DZ, Zhang YN, Xie
JM and Linh CQ: HPLC separation and determination of bufadienolide
in cinobufacini injection. Chin Trad Patent Med. 25:24–27.
2003.
|
|
57
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|















