Introduction
Bone tumor (BT) (1)
is a tumor that occurs in the bone or its subsidiary tissues. BT
are classified as benign BT and malignant BT. Benign BT is easy to
cure and has a good prognosis, while malignant BT develops rapidly
with poor prognosis and high mortality. The incidence of BT in the
world (2) is low; but the absence of
obvious symptoms or the neglect of minor symptoms in the early
stage leads to misdiagnosis and missed diagnosis. Sometimes, it
even develops into malignant BT at the time of a visit. PET/CT
examination (3) is a common imaging
detection method for the diagnosis of tumor. It is widely used in
the differential diagnosis of various diseases. The diagnosis of BT
has become a difficult problem in clinic because of its diverse
causes and complex components. The clinical value of PET/CT in
differential diagnosis of bone tumors and tumor-like lesions has
also become a hot research topic (4).
PET/CT, a scanner combined by positron emission
tomography and X-ray computed tomography, combines the two imaging
techniques perfectly to form a complementary advantage (5). PET (positron emission tomography)
provides functional and metabolic information (6) and CT (computed tomography) (7) provides detailed anatomical and
pathological information. The pathophysiological and morphological
changes of the disease can be obtained by the fusion of these two
techniques. PET/CT is an advanced examination method. Its
application in the diagnosis of tumors, especially BT, and the
clinical value of differential diagnosis cannot be ignored. In
addition, it is also non-invasive (8). Because of the existence of false
positive and false negative, the result should be judged
synthetically. In this study, CT was used as a control to evaluate
the diagnostic efficacy of PET/CT in different stages of bone
tumors.
Patients and methods
Clinical information
Fifty-four patients, including 34 males and 20
females with an age range of 15–75 years, with bone tumors (BT)
treated in Henan Province Luoyang Orthopedic Traumatological
Hospital (Henan Provincal Orthopedic Hospital) (Luoyang, China)
from August, 2016 to February, 2018 were selected into this study.
There were 14 cases of benign BT patients, 15 cases of stage I, 10
cases of stage II and 15 cases of stage III in malignant BT
patients with clinical diagnosis (Table
I).
 | Table I.Clinical information of BT
patients. |
Table I.
Clinical information of BT
patients.
| Factors | n=54 [n (%)] |
|---|
| Age (years) |
|
| ≤36 | 21 (38.89) |
|
>36 | 33 (61.11) |
| Sex |
|
| Male | 34 (62.96) |
|
Female | 20 (37.04) |
| Weight index
(kg) |
|
| Male
(65.21±6.48) | 34 (62.96) |
| Female
(45.18±5.36) | 20 (37.04) |
| Glycemic indices
(mmol/l) |
|
|
<7.8 | 54 (100.00) |
| Lesion location |
|
|
Thigh-bone | 8 (14.81) |
|
Humerus | 5 (9.26) |
| Shin
bone | 9 (16.67) |
|
Radius | 10 (18.52) |
| Ulna | 5 (9.26) |
|
Spine | 8 (14.81) |
|
Pelvis | 9 (16.67) |
| Pathogenic
condition |
|
|
Benign | 14 (25.93) |
|
Malignant | 40 (74.07) |
| Lymphatic
metastasis |
|
| Yes | 35 (64.81) |
| No | 19 (35.19) |
Inclusion and exclusion criteria for the study were:
i) Only BT patients admitted to Henan Province Luoyang Orthopedic
Traumatological Hospital (Henan Provincal Orthopedic Hospital),
lesions examined by pathology department and patients diagnosed as
malignant BT and benign BT were included. ii) Pregnant women and
patients with allergic reactions to contrast agents, claustrophobia
and other contraindications were excluded. Informed consent was
signed in advance by patients and their families. The present study
was approved by the Ethics Committee of Henan Province Luoyang
Orthopedic Traumatological Hospital (Henan Provincal Orthopedic
Hospital).
Main reagents and instruments
PET/CT imaging agent: 18F-deoxyglucose
(18FDG) was purchased from ACCDON Inc. (Waltham, MA,
USA). PET/CT scanner was purchased from Royal Philips Electronics
Co., Ltd. (Eindhoven, The Netherlands) and 64-slice spiral CT was
purchased from Siemens AG (Munich, Germany).
Methods
PET/CT examination
Patients were weighed. The injection measurement of
image agent should be controlled according to the patients weight.
Patients with BT should fast for at least 6 h before the
examination. After 6 h, the venous blood glucose concentration of
BT patients was measured to ensure that the blood glucose
concentration was <7.8 mmol/l. The hospital needs to handle it
in time when blood glucose concentration is too high or too low.
18F-FDG imaging agent was injected into the patients
elbow vein after the patients blood glucose concentration was
within the normal range (the radiochemical purity should be
>95%). Patients needed to empty their urine first and then drink
600 ml purified water before PET/CT examination. CT
scan-fluoroscopic guidance was performed on the lesions of BT
patients first; the PET was used to scan the largest range of BT
lesions next, then the decay data of CT was corrected. The fusion
images of CT, PET and PET/CT in all directions were then
formed.
CT examination
All subjects were examined with 64-slice spiral CT
with a slice thickness of 5–10 mm, a matrix of 512 × 512 mm. The
soft tissue window and bone window parameters were set to
1,500–3,000 HU in window width and 300–700 HU in window level.
Judgement criterion
The diagnostic criteria for BT are as follows: i)
The history of BT patients is completely clear. ii) PCT/CT showed
that the concentration of bone nuclide was abnormal, the
distribution was irregular and the distribution range was enlarged
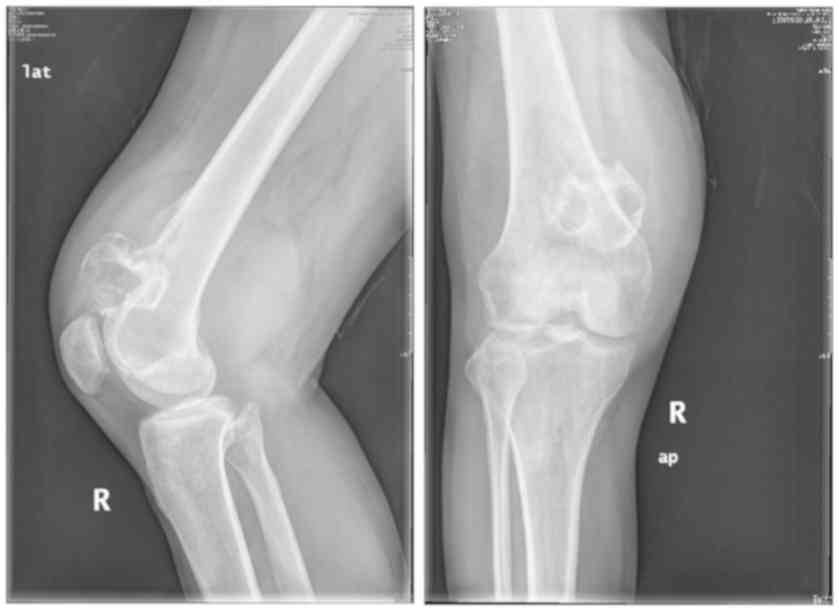
with time. Manifestations of BT in PET/CT are shown in Table II and Fig. 1. iii) CT or X-ray showed osteogenic
destruction or osteolytic lesions in some bone tissues.
Manifestations of BT in CT are shown in Table III. A patient who meets the first
or last two criteria can be diagnosed as a BT patient. All images
were evaluated by two or more relevant chief physicians.
 | Table II.Manifestations of BT in PET/CT. |
Table II.
Manifestations of BT in PET/CT.
| Variables | Manifestations in
PET/CT |
|---|
| Benign BT | Bone tissue grew
slowly, with no apparent or slight symptoms, clear periosteal
edges, no periosteal reaction and no bone scan radioactive
concentration. |
| Malignant BT | Periosteal edges were
unclear. The soft tissue mass was obviously enhanced and the edge
of the mass was clear. There were even cortical destruction,
pathological fractures, bone lesions or bone scan radioactive
concentration. |
 | Table III.Manifestations of BT in CT. |
Table III.
Manifestations of BT in CT.
| Variables | Manifestations in
CT |
|---|
| Benign BT | The edges between BT
lesion and periosteum was clear. Tumor invasion could be seen in
bone marrow, but there were still normal bone marrow tissues. |
| Malignant BT | The edges between BT
lesion and periosteum was unclear. There were changes in the
adjacent tissue and swells or lumps in soft tissue; bone marrow was
damaged and periosteal reaction occurred. Proliferation of tumor
cells in bone marrow made it difficult to see normal bone marrow
tissues. Tumor bone was produced. |
Statistical methods
SPSS 17.0 (Beijing Bizinsight Information Technology
Co., Ltd., Beijing, China) software system was used for statistical
analysis; The enumeration data were represented by [n (%)]. An
χ2 test was used for a comparison of diagnostic accuracy
of BT in different phases. Students' t-test was used for diagnostic
accordance rate of CT and PET/CT. The difference was statistically
significant at P<0.05.
Results
Analysis of diagnostic results
i) Comparison between PET/CT, CT scan results and
pathological diagnosis results: 30 cases of malignant BT were
detected by CT, and the positive predictive value was 85.71%, while
38 cases of malignant BT were detected by PET/CT, and the positive
predictive value was 95.00%. Because CT scan was insensitive to the
diagnosis of BT, the tissue imaging of adjacent disc was not clear;
it was easy to cause misdiagnosis and missed diagnosis. Eight
patients were screened as benign BT by CT, then screened as
malignant BT by PET/CT, and confirmed as malignant BT by pathology
at the same time (Tables IV and
V); ii) Comparison of the diagnostic
efficacy between PET/CT and CT in BT: The sensitivity, specificity,
diagnostic accordance rate, negative predictive value and positive
predictive value of CT screening were 75.00, 64.29, 72.22, 47.37
and 85.71%, respectively. While the sensitivity, specificity,
diagnostic accordance rate, negative predictive value and positive
predictive value of PET/CT screening were 95.00, 85.71, 92.59,
85.71 and 95.00%, respectively. There were significant differences
in sensitivity, negative predictive value, positive predictive
value and diagnostic accordance rate between PET/CT and CT
screening (P<0.05). There was no significant difference in
specificity between the two groups (P>0.05) (Table VI).
 | Table IV.Comparison between CT scan results and
pathological diagnosis results. |
Table IV.
Comparison between CT scan results and
pathological diagnosis results.
| Variables | Malignant BT
diagnosed by pathology | Benign BT diagnosed
by pathology | Total |
|---|
| Malignant BT
diagnosed by CT | 30 | 5 | 35 |
| Benign BT diagnosed
by CT | 10 | 9 | 19 |
| Total | 40 | 14 | 54 |
 | Table V.Comparison between PET/CT scan results
and pathological diagnosis results. |
Table V.
Comparison between PET/CT scan results
and pathological diagnosis results.
| Variables | Malignant BT
diagnosed by pathology | Benign BT diagnosed
by pathology | Total |
|---|
| Malignant BT
diagnosed by PET/CT | 38 | 2 | 40 |
| Benign BT diagnosed
by PET/CT | 2 | 12 | 14 |
| Total | 40 | 14 | 54 |
 | Table VI.Comparison of the diagnostic efficacy
between CT and PET/CT in BT. |
Table VI.
Comparison of the diagnostic efficacy
between CT and PET/CT in BT.
| Variables | CT [n (%)] | PET/CT [n (%)] | χ2 | P-value |
|---|
| Diagnostic accordance
rate | 39 (72.22) | 50
(92.59)a | 7.728 | 0.005 |
| Sensitivity | 30 (75.00) | 38
(95.00)a | 6.275 | 0.012 |
| Specificity | 9
(64.29) | 12
(85.71)b | 1.714 | 0.190 |
| Negative predictive
value | 9
(47.37) | 12
(85.71)a | 5.122 | 0.024 |
| Positive predictive
value | 30 (85.71) | 38
(95.00)a | 6.275 | 0.012 |
Comparison of the diagnostic efficacy
between CT and PET/CT in different stages of BT
i) The diagnostic accordance rates of CT in benign
BT and malignant BT were 64.29 and 75.00%, respectively. The
diagnostic accordance rates of PET/CT in benign BT and malignant BT
were 85.71 and 95.00%, respectively. The result showed that the
diagnostic accordance rates of PET/CT in benign BT and malignant BT
were higher than those of CT. The diagnostic rate of PET/CT in
malignant BT was significantly higher than that of CT (P<0.05),
and the difference was statistically significant (Table VII); ii) In comparison of the
positive diagnostic rate between CT and PET/CT in different stages
of malignant BT, the positive diagnostic rates of CT in stages
I–III of malignant BT were 46.67, 90.00 and 93.33%, respectively.
The positive diagnostic rates of PET/CT in the same stages were
86.67, 100.00 and 10.00%, respectively. Comparing the data of the
two groups, it was found that the positive diagnostic rate of
PET/CT in stages I–III of malignant BT was higher than that of CT,
and the difference in stage I of malignant BT was statistically
significant (P<0.05) (Table
VIII).
 | Table VII.Diagnostic accordance rate of CT and
PET/CT in benign BT and malignant BT (%). |
Table VII.
Diagnostic accordance rate of CT and
PET/CT in benign BT and malignant BT (%).
| Groups | CT | PET/CT | t | P-value |
|---|
| Benign group | 64.29 | 85.71b | 1.714 | 0.190 |
| Malignant
group | 75.00 | 95.00a | 6.275 | 0.012 |
 | Table VIII.Comparison of the positive diagnostic
rate between CT and PET/CT in different stages of malignant BT. |
Table VIII.
Comparison of the positive diagnostic
rate between CT and PET/CT in different stages of malignant BT.
| Stage | CT | PET/CT | χ2 | P-value |
|---|
| Stage I (n=15) | 7
(46.67) | 13
(86.67)a | 5.400 | 0.020 |
| Stage II
(n=10) | 9
(90.00) | 10
(100.00)b | 1.053 | 0.305 |
| Stage III
(n=15) | 14 (93.33) | 15
(100.00)b | 1.034 | 0.309 |
Discussion
The location of bone tumor (BT) is often in bone
tissue or bone subsidiary tissue. Since BT in different stages has
similar clinical and imaging manifestations, it is more difficult
to diagnose BT in clinic. Relevant BT pathology (9) result shows that the clinical
manifestations of partial benign BT are in a malignant state, and
the effect of some benign BT-like lesions under X-ray (10) is particularly like that of malignant
BT, and thus makes it more difficult to diagnose BT and BT-like
lesions (11). At present, X-ray
examination and CT scan are often used for early diagnosis or tumor
staging of BT. Clinical application data (12) show that although X-ray examination
can clearly reflect the location and size of BT in the BT staging
diagnosis, it cannot accurately diagnose whether the BT is benign
or malignant. Thus, the limitation of X-ray in the specific staging
diagnosis of malignant BT is more obvious. CT can better display
the fine anatomical structure of the location of BT when compared
with X-ray examination. However, for improving the early diagnosis
rate and the specific stage of BT patients, CT still lacks more
precise function, metabolism and other molecular information
(13) to assist BT staging
diagnosis. PET/CT technology, which can integrate body function,
metabolism and other molecular information, and accurate anatomical
and pathological information, has been put into clinical
application in recent years. A study (14) has confirmed that PET/CT is
particularly sensitive for benign tumor, malignant tumor and early
diagnosis of tumors.
The diagnostic efficacy of PET/CT and CT in BT was
measured, and the results were compared and analyzed in the present
study. It was found that the positive predictive value of CT in BT
patients was 85.71%, while the detection rate of PET/CT in BT
patients was 95.00%. In comparison, the detection rate of PET/CT in
BT was significantly higher than that of CT. CT examination results
of Janssen et al (15) found
that CT imaging of adjacent disc tissue (16,17) was
not very clear, and it was easy to cause misdiagnosis and missed
diagnosis. While the results of different diagnostic efficacy of
PET/CT and CT in BT showed that the sensitivity, specificity,
diagnostic accordance rate, negative predictive value and positive
predictive value of CT screening for BT were 75.00, 64.29, 72.22,
47.37 and 85.71%, respectively. While the sensitivity, specificity,
diagnostic accordance rate, negative predictive value and positive
predictive value of PET/CT screening were 95.00, 85.71, 92.59,
85.71 and 95.00% respectively. There were significant differences
in sensitivity, negative predictive value, positive predictive
value and diagnostic accordance rate between PET/CT and CT
screening (P<0.05). There was no significant difference in
specificity between the two groups (P>0.05). The diagnostic
efficacy of PET/CT in BT is better than that of CT. The results of
Guimaraes et al (18) were
consistent with ours. They also applied the PET/CT technique to the
comparative study of the diagnostic efficacy in BT, and compared it
with other scanning techniques. By analyzing the scanning results
of BT patients, they found that the sensitivity, specificity,
diagnostic accordance rate, negative predictive value and positive
predictive value of PET/CT were significantly higher than those of
other scanning techniques, which was a good complement to our
findings. Finally, the diagnostic efficacy of PET/CT in different
stages of BT was analyzed concretely. The result showed that the
diagnostic accordance rates of PET/CT in benign BT and malignant BT
were higher than those of CT. The diagnostic rate of PET/CT in
malignant BT was significantly higher than that of CT (P<0.05),
and the difference was statistically significant. Particularly in
the stages I–III of the malignant BT, it was found that the
positive diagnostic rate of PET/CT in stages I–III of malignant BT
was higher than that of CT, and the difference in stage I was
statistically significant (P<0.05). The accurate diagnostic
efficacy of PET/CT in BT staging (19) has been confirmed in the clinical
study of BT. The advantages of PET/CT in the diagnosis of BT or
other tumors were summarized by El-Galaly et al (20), through extensive clinical data
induction and comparison with other detection methods (21,22).
They considered that the advantages of PET/CT in the diagnosis of
different stages of tumor were that it could locate the lesion more
accurately, detect the smaller lesion, and distinguish the benign,
malignant and different stages of BT, abdominal neuroendocrine
tumor and ovarian cancer, hepatocellular carcinoma by PET/CT
imaging.
In this study, the selection of research objects was
strictly in accordance with the inclusion and exclusion criteria to
ensure the reliability of the results. However, due to the small
number of subjects included, there were still some missed diagnosis
and misdiagnosis for PET/CT in the detection of the diagnostic
efficacy of PET/CT.
In conclusion, the diagnostic efficacy of PET/CT
scan screening in different stages of BT is significantly better
than that of CT. When CT scan screening is not accurate enough to
judge BT staging, PET/CT can provide more precise tissue
physiological metabolism and imaging evidence of anatomical
structure of BT lesion. That is, PET/CT can accurately diagnose the
pathological nature of BT, effectively diagnose the clinical stage
of malignant BT, and provide more accurate clinical diagnosis basis
for BT treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
SH collected and interpreted the data, and wrote the
manuscript. SH and YaL were mainly involved in PET/CT examination.
YuL and MZ helped with the statistical analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Henan Province Luoyang Orthopedic Traumatological
Hospital (Henan Provincal Orthopedic Hospital) (Luoyang, China).
Signed informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ringe KI, Panzica M and von Falck C:
Thermoablation of bone tumors. Rofo. 188:539–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldsby R, Burke C, Nagarajan R, Zhou T,
Chen Z, Marina N, Friedman D, Neglia J, Chuba P and Bhatia S:
Second solid malignancies among children, adolescents, and young
adults diagnosed with malignant bone tumors after 1976: Follow-up
of a Children's Oncology Group cohort. Cancer. 113:2597–2604. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calais J, Fendler WP, Herrmann K, Eiber M
and Ceci F: Reply: Comparison of 68Ga-PSMA-11 and
18F-fluciclovine PET/CT in a case series of 10 patients
with prostate cancer recurrence: prospective trial is on its way. J
Nucl Med. 59:8612018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paskeviciute B, Bölling T, Brinkmann M,
Rudykina G, Ernst I, Stegger L, Schober O, Willich N, Weckesser M
and Könemann S: Impact of 18F-FDG-PET/CT on staging and
irradiation of patients with locally advanced rectal cancer.
Strahlenther Onkol. 185:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bundschuh RA, Dinges J, Neumann L,
Seyfried M, Zsótér N, Papp L, Rosenberg R, Becker K, Astner ST,
Henninger M, et al: Textural parameters of tumor heterogeneity in
¹8F-FDG PET/CT for therapy response assessment and
prognosis in patients with locally advanced rectal cancer. J Nucl
Med. 55:891–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fendler WP, Eiber M, Beheshti M, Bomanji
J, Ceci F, Cho S, Giesel F, Haberkorn U, Hope TA, Kopka K, et al:
68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure
guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med
Mol Imaging. 44:1014–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jo HJ, Kim SJ, Kim IJ and Kim S:
Predictive value of volumetric parameters measured by F-18 FDG
PET/CT for lymph node status in patients with surgically resected
rectal cancer. Ann Nucl Med. 28:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thorek DL, Ulmert D, Diop NF, Lupu ME,
Doran MG, Huang R, Abou DS, Larson SM and Grimm J: Non-invasive
mapping of deep-tissue lymph nodes in live animals using a
multimodal PET/MRI nanoparticle. Nat Commun. 5:30972014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hameed M and Dorfman H: Primary malignant
bone tumors - recent developments. Semin Diagn Pathol. 28:86–101.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azcona C, Burghard E, Ruza E, Gimeno J and
Sierrasesúmaga L: Reduced bone mineralization in adolescent
survivors of malignant bone tumors: Comparison of quantitative
ultrasound and dual-energy X-ray absorptiometry. J Pediatr Hematol
Oncol. 25:297–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knoeller SM, Uhl M, Gahr N, Adler CP and
Herget GW: Differential diagnosis of primary malignant bone tumors
in the spine and sacrum. The radiological and clinical spectrum:
Minireview. Neoplasma. 55:16–22. 2008.PubMed/NCBI
|
|
12
|
Westhovens R and Dequeker J:
Musculoskeletal manifestations of benign and malignant tumors of
bone. Curr Opin Rheumatol. 15:70–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maury CPJ: Amyloid and the origin of life:
Self-replicating catalytic amyloids as prebiotic informational and
protometabolic entities. Cell Mol Life Sci. 75:1499–1507. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ulaner GA, Castillo R, Wills J, Gönen M
and Goldman DA: 18F-FDG-PET/CT for systemic staging of
patients with newly diagnosed ER-positive and HER2-positive breast
cancer. Eur J Nucl Med Mol Imaging. 44:1420–1427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janssen JC, Meißner S, Woythal N, Prasad
V, Brenner W, Diederichs G, Hamm B and Makowski MR: Comparison of
hybrid 68Ga-PSMA-PET/CT and
99mTc-DPD-SPECT/CT for the detection of bone metastases
in prostate cancer patients: Additional value of morphologic
information from low dose CT. Eur Radiol. 28:610–619. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
London K, Stege C, Cross S, Onikul E, Graf
N, Kaspers G, Dalla-Pozza L and Howman-Giles R: 18F-FDG
PET/CT compared to conventional imaging modalities in pediatric
primary bone tumors. Pediatr Radiol. 42:418–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costelloe CM, Chuang HH, Chasen BA, Pan T,
Fox PS, Bassett RL and Madewell JE: Bone windows for distinguishing
malignant from benign primary bone tumors on FDG PET/CT. J Cancer.
4:524–530. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guimaraes JB, Facchetti L, Rigo L, Garcia
DL, Gama P, Franc BL and Nardo L: The role of PET/CT in the
assessment of primary bone tumors. Curr Radiol Rep. 4:532016.
View Article : Google Scholar
|
|
19
|
Kitajima K, Fukushima K, Yamamoto S, Kato
T, Odawara S, Takaki H, Fujiwara M, Yamakado K, Nakanishi Y,
Kanematsu A, et al: Diagnostic performance of
11C-choline PET/CT and bone scintigraphy in the
detection of bone metastases in patients with prostate cancer.
Nagoya J Med Sci. 79:387–399. 2017.PubMed/NCBI
|
|
20
|
El-Galaly TC, Gormsen LC and Hutchings M:
PET/CT for staging; Past, present, and future. Semin Nucl Med.
48:4–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Partovi S, Kohan A, Rubbert C,
Vercher-Conejero JL, Gaeta C, Yuh R, Zipp L, Herrmann KA, Robbin
MR, Lee Z, et al: Clinical oncologic applications of PET/MRI: A new
horizon. Am J Nucl Med Mol Imaging. 4:202–212. 2014.PubMed/NCBI
|
|
22
|
Al-Bulushi NK and Abouzied ME: Comparison
of 18F-FDG PET/CT scan and 99mTc-MDP bone
scintigraphy in detecting bone metastasis in head and neck tumors.
Nucl Med Commun. 37:583–588. 2016. View Article : Google Scholar : PubMed/NCBI
|