Introduction
Calcification, a common pathological characteristic
of thyroid diseases, occurs in the foci of 30% of benign thyroid
nodules and 65% of malignant thyroid nodules (1). Calcifications in thyroid nodules can be
classified as microcalcifications, coarse calcifications, annular
calcifications and mixed calcifications, according to their size,
but some studies have manifested that microcalcification presents
the greatest differences between benign and malignant thyroid
nodules (2,3). Microcalcification can only be examined
through high-resolution ultrasound due to its very small volume
(maximum diameter of 2.0 mm). Therefore, there are few reports on
the diagnosis of benign and malignant thyroid nodules with
microcalcification as the indicator. By virtue of infusing an
ultrasound contrast agent into the blood circulation,
contrast-enhanced ultrasound can enhance the differences in the
ultrasonographic images between tissues and blood vessels, improve
the resolution of ultrasonographic image results and increase the
application range and depth of ultrasound (4). The development of contrast-enhanced
ultrasound technique and its wide application in clinic make the
accurate imaging examination of microcalcification in thyroid
nodules possible. Therefore, in this study, contrast-enhanced
ultrasound was applied to examine the microcalcification in benign
and malignant thyroid nodules, and the differences in the
quantitative parameters of contrast-enhanced ultrasound were
compared, so as to explore the differential diagnosis value of
contrast-enhanced ultrasound in benign and malignant thyroid
nodules with microcalcification.
Patients and methods
Research subjects
A total of 184 patients with thyroid nodules
accompanied by microcalcification, treated in People's Hospital of
Shanxi Province (Taiyuan, China) from April 2015 to March 2017,
were selected, including 100 males and 84 females. The age of
patients ranged from 20 to 70 years, with an average age of
47.5±8.9 years. The foci in thyroid nodules of all patients were
examined via pathological sections, including 104 cases of benign
thyroid nodules and 80 cases of malignant thyroid nodules. This
research was reviewed and approved by the Ethics Committee of
People's Hospital of Shanxi Province. All patients enrolled were
aware of this research and each one and/or their guardians signed
an informed consent.
Methods
Main reagents and instruments
Ultrasonic apparatus (Philips GmbH Healthcare,
Hamburg, Germany), with built-in specialized analysis software for
time-intensity curve (TIC), contrast-enhanced ultrasound-matching
imaging techniques and variable frequency linear-array probe
(frequency, 2–5 MHz) was utilized. Also, SonoVue contrast agent
(Bracco SpA, Milan, Italy) composed of sulphur hexafluoride was
used. During application, 2.4 ml of freeze-dried powder of SonoVue
contrast agent were dissolved in 5.0 ml normal saline to prepare
microbubble suspension.
Contrast-enhanced ultrasound
examination for thyroid nodules
The research subjects were put in supine position,
with their neck stretched sufficiently to expose the anterior
region of the neck. Firstly, the conventional ultrasound was
adopted to examine the thyroid nodules, so as to determine their
characteristics, such as morphology, boundary and internal echo.
Then, color Doppler ultrasound was applied to inspect the thyroid
nodules and surrounding tissues, so as to determine the blood flow
inside the foci and the surrounding tissues. The optimal sections
for observation of the thyroid nodules were confirmed by
integrating the examination findings of conventional ultrasound and
color Doppler ultrasound. After that, the probe was fixed, and the
contrast pulse sequence of the apparatus was switched on. Then, 2.4
ml of prepared microbubble suspension of SonoVue contrast agent
were rapidly injected into the blood vessels through the cubital
vein, after which 5.0 ml normal saline was injected immediately.
Timer was started, and the data were collected while the contrast
agent was injected. Moreover, constant and dynamic data (120 sec)
of every research subject were acquired. If the obtained
contrast-enhanced images of the thyroid nodules were not
satisfactory, the second injection of contrast agent through the
cubital vein was performed for contrast-enhanced ultrasonography
for the second time. The built-in data processing software of the
ultrasonic apparatus was utilized to analyze the data collected.
Three regions of interest were drawn at the positions with the
strongest ultrasound imaging in the nodule center and edge, as well
as the surrounding normal tissues of thyroid gland, for which the
TIC, time to peak (Tp), peak intensity (Peak), area under curve
(AUC) and mean transit time (MTT) were obtained separately.
Statistical analysis
SPSS v.20.0 software (IBM Corp., Armonk, NY, USA)
was used for the statistical analysis of the collected data.
Measurement data were expressed as mean ± standard deviation.
t-test was performed for the statistical analysis of the
differences of the measurement data between two groups, and ANOVA
was performed for the comparison of the measurement data between
multiple groups. Least Significant Difference test was the post hoc
test used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of contrast-enhanced
ultrasound images of benign and malignant thyroid nodules with
microcalcification
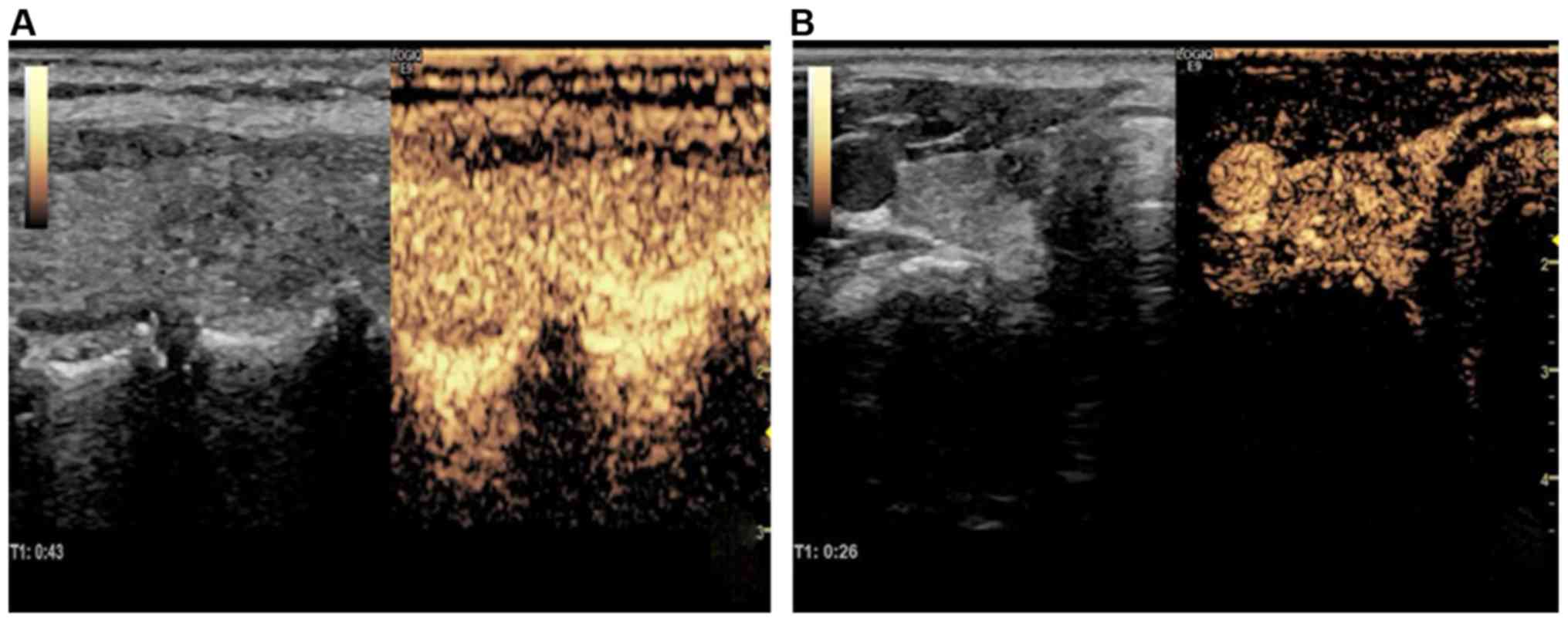
The features of contrast-enhanced ultrasound images
of benign and malignant thyroid nodules with microcalcification
were compared. The results indicated that the foci of benign
thyroid nodules had regular edges and clear boundaries and
displayed equal or slightly high enhancement in general, without
blood perfusion defect inside the foci. The malignant thyroid
nodules were manifested as irregular focus edges, unclear
boundaries and low fiber reinforcement of the whole foci, uneven
distribution of images and blood perfusion defect inside the foci,
especially severe blood perfusion defect in the nodule center
(Fig. 1).
Comparison of TICs for benign and
malignant thyroid nodules with microcalcification
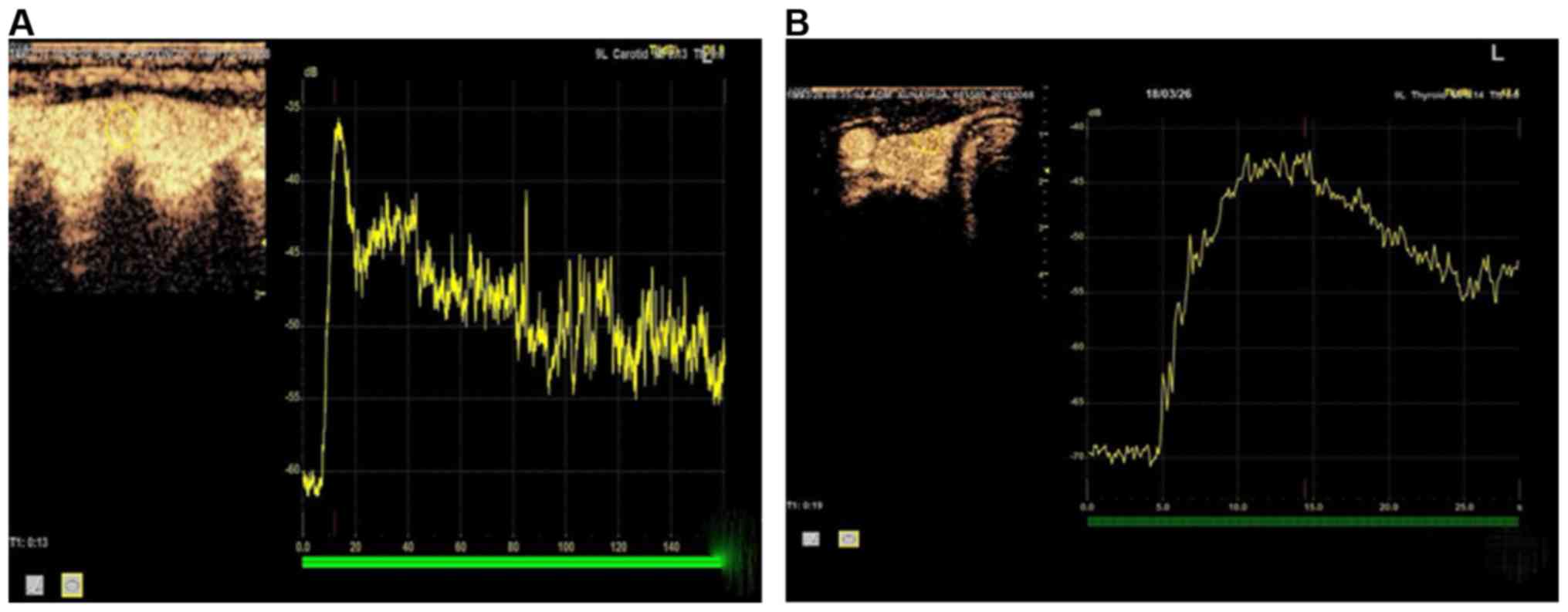
The features of TICs for benign and malignant
thyroid nodules with microcalcification were compared. The TIC for
malignant thyroid nodules showed a slow ascending and slow
descending trend in general: sluggish ascending section, smooth
descending section, prolonged Tp and low and flat curve. The
overall TIC for benign thyroid nodules was similar to that for
surrounding normal tissues of thyroid gland, which was manifested
as a rapid ascending and rapid descending trend: it rose and
declined fast, with short Tp and narrow and high curve (Fig. 2).
Comparisons of quantitative parameters
of TICs for benign and malignant thyroid nodules with
microcalcification
The quantitative parameters of TICs for benign and
malignant thyroid nodules with microcalcification were compared.
According to the results, there were no significant differences in
the Peak, Tp, AUC and MTT in the center and edge of benign thyroid
nodules, as well as the surrounding normal tissues of thyroid gland
(P>0.05). No significant differences in the Tp and MTT were
presented in the center and edge of malignant thyroid nodules or in
the surrounding normal tissues of thyroid gland (P>0.05).
Compared with those in the surrounding normal tissues of thyroid
gland, the Peak was remarkably shorter, and the AUC was notably
smaller in the center and edge of malignant thyroid nodules
(P<0.05); the nodule center had obviously shorter Peak and
smaller AUC than the nodule edge (P<0.05). In addition, the Tp
and MTT in the center and edge of benign thyroid nodules were not
significantly different from those of malignant thyroid nodules
(P>0.05). In comparison with those of malignant thyroid nodules,
the Peak was extended and AUC was enlarged markedly in the center
and edge of benign thyroid nodules (P<0.05; Table I).
 | Table I.Comparisons of quantitative parameters
of TICs for benign and malignant thyroid nodules with
microcalcification. |
Table I.
Comparisons of quantitative parameters
of TICs for benign and malignant thyroid nodules with
microcalcification.
|
| Benign thyroid
nodules with microcalcification | Malignant thyroid
nodules with microcalcification |
|---|
|
|
|
|
|---|
| Location | Peak (%) | Tp (sec) | AUC (%, sec) | MTT (sec) | Peak (%) | Tp (sec) | AUC (%, sec) | MTT (sec) |
|---|
| Nodule center | 28.63±5.24 | 32.67±6.87 | 1,863.61±472.72 | 48.72±10.62 |
16.31±3.56a,b | 32.04±6.88 |
916.37±257.62a,b | 48.25±10.32 |
| Nodule edge | 28.77±5.62 | 33.26±6.89 | 1,876.62±426.21 | 49.01±11.63 |
23.56±4.78a | 32.48±7.01 |
1,353.63±421.42a | 49.62±11.52 |
| Surrounding normal
tissues of thyroid gland | 29.67±5.81 | 33.89±6.18 | 1,883.63±482.50 | 49.63±11.28 | 29.54±5.30 | 33.56±6.32 | 1,873.29±463.71 | 49.98±10.89 |
Discussion
Gravel-like microcalcification has attracted close
attention in clinical practice. Studies have indicated that
microcalcification possesses a fairly high incidence rate in
papillary thyroid carcinoma, becoming a major morphological
characteristic of the disease, while papillary thyroid carcinoma is
a primary form of malignant lesion of the thyroid nodules (5,6). In
examinations, if the instruments are not sensitive enough, the
pathologically puny foreign bodies of collagen and fibrosis, as
well as a small amount of colloid, produce strong echoes in
ultrasound, which may be confused with the echoes of true
microcalcification, thus generating false-positive results. It has
been reported that the thresholds of microcalcification diagnosed
via ultrasound include maximum diameters ≤2.0, ≤1.0 and ≤0.5 mm, of
which the threshold of the maximum diameter ≤2.0 mm is used most
extensively. The smaller the maximum diameter of microcalcification
is, the higher the correlation with malignant thyroid nodules will
be (7,8). In consideration of the sensitivity of
contrast-enhanced ultrasound examination, the maximum diameter of
2.0 mm was also selected as the threshold of microcalcification in
this investigation. Major limitations exist in conventional
ultrasound because the volume of early microcalcification is too
small, and the sensitivity of conventional two-dimensional
ultrasound is low. It is difficult to take the microcalcification
as the basis for differential diagnosis of benign and malignant
thyroid nodules. In contrast-enhanced ultrasound, the contrast
between the blood vessels of foci and surrounding tissues is
enhanced by perfusion of ultrasound contrast agent in the blood
vessels of foci, thereby clearly reflecting the blood perfusion and
vascular distribution inside the thyroid nodules (9,10). The
sensitivity of ultrasound in detecting the microcalcification can
be improved significantly by virtue of contrast-enhanced
ultrasound. Therefore, contrast-enhanced ultrasound was adopted to
study the lesions of thyroid nodules with microcalcification.
In this study, the features of contrast-enhanced
ultrasound for malignant thyroid nodules were manifested as
irregular focus edge, unclear boundary, low fiber reinforcement of
the whole focus, uneven distribution of images and blood perfusion
defect inside the focus, especially severe blood perfusion defect
in the nodule center. TIC showed a slow ascending and slow
descending trend in general: sluggish ascending section, smooth
descending section, prolonged Tp and low and flat curve. The TIC
features and the features of contrast-enhanced ultrasound for
malignant thyroid nodules were prominently different from those for
benign thyroid nodules. Moreover, there were significant
differences in the quantitative parameters of TICs for the center
and edge of benign and malignant thyroid nodules. These results are
related to the mechanism of microcalcification formation in the
thyroid nodules and the pathological characteristics of benign and
malignant lesions. The main component of microcalcification is
calcium oxalate crystal (11). The
major mechanism of microcalcification formation is as follows
(12,13): i) thrombus is formed in the
fibrovascular core of thyroid nodules, which develops into
infarction, or necrosis of metastatic tumor cell nests in the lymph
vessels occurs and triggers calcium deposit in dead tumor cells,
thus forming gravel-like microcalcification. ii) The normal tumor
cells in the thyroid nodules can release metabolic products and
lead to dystrophic calcification of tumor cells, but such a
calcification has no association with the apoptosis and necrosis of
the tumor cells. iii) A large amount of bone morphogenetic
protein-1 is expressed at the position of thyroid nodules, and the
macrophages can produce osteopontin. In the malignant thyroid
nodules with microcalcification, the existence of gravel-like
calcium oxalate crystals can inhibit neovascularization inside the
thyroid nodules and cause a lack of blood supply in the tumor
(14). Furthermore, due to the very
rapid cell proliferation of malignant thyroid tumors, the massive
blood vessels inside the tumor have relatively small diameters and
tortuous courses, which generate a great resistance to reduce the
flow of blood and contrast agent toward the center of the nodules
with microcalcification (15).
In conclusion, contrast-enhanced ultrasound can
preferably compare the lesions of benign and malignant thyroid
nodules with microcalcification, which possesses certain value in
the differential diagnosis of benign and malignant thyroid
nodules.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG and JY contributed to the contrast-enhanced
ultrasound examination of thyroid nodules. QL and JY acquired,
analyzed and interpreted the general data of patients. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the People's Hospital of Shanxi Province (Taiyuan, China). Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J
and Lu J: Clinical value of using ultrasound to assess
calcification patterns in thyroid nodules. World J Surg.
35:122–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Zhang H, Zhang P, He L and Dong W:
Diagnostic value of ultrasound-detected calcification in thyroid
nodules. Ann Acad Med Singapore. 43:102–106. 2014.PubMed/NCBI
|
|
3
|
Jiang J, Shang X, Zhang H, Ma W, Xu Y,
Zhou Q, Gao Y, Yu S and Qi Y: Correlation between maximum intensity
and microvessel density for differentiation of malignant from
benign thyroid nodules on contrast-enhanced sonography. J
Ultrasound Med. 33:1257–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemec U, Nemec SF, Novotny C, Weber M,
Czerny C and Krestan CR: Quantitative evaluation of
contrast-enhanced ultrasound after intravenous administration of a
microbubble contrast agent for differentiation of benign and
malignant thyroid nodules: Assessment of diagnostic accuracy. Eur
Radiol. 22:1357–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim D, Kim DW, Heo YJ, Baek JW, Lee YJ,
Park YM, Baek HJ and Jung SJ: Computed tomography features of
benign and malignant calcified thyroid nodules: a single-center
study. J Comput Assist Tomogr. 41:937–940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tugendsam C, Petz V, Buchinger W,
Schmoll-Hauer B, Schenk IP, Rudolph K, Krebs M and Zettinig G:
Ultrasound criteria for risk stratification of thyroid nodules in
the previously iodine deficient area of Austria - a single centre,
retrospective analysis. Thyroid Res. 11:32018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ning CP, Ji QL, Fang SB, Wang HQ, Zhong YM
and Niu HT: Distribution patterns of microcalcifications in
suspected thyroid carcinoma: A classification method helpful for
diagnosis. Eur Radiol. 28:2612–2619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koltin D, O'Gorman CS, Murphy A, Ngan B,
Daneman A, Navarro OM, García C, Atenafu EG, Wasserman JD, Hamilton
J, et al: Pediatric thyroid nodules: ultrasonographic
characteristics and inter-observer variability in prediction of
malignancy. J Pediatr Endocrinol Metab. 29:789–794. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Shang X, Wang H, Xu YB, Gao Y and
Zhou Q: Diagnostic value of contrast-enhanced ultrasound in thyroid
nodules with calcification. Kaohsiung J Med Sci. 31:138–144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Sloun RJG, Demi L, Postema AW, Jmch De
La Rosette J, Wijkstra H and Mischi M: Entropy of
ultrasound-contrast-agent velocity fields for angiogenesis imaging
in prostate cancer. IEEE Trans Med Imaging. 36:826–837. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Na DG, Kim DS, Kim SJ, Ryoo JW and Jung
SL: Thyroid nodules with isolated macrocalcification: Malignancy
risk and diagnostic efficacy of fine-needle aspiration and core
needle biopsy. Ultrasonography. 35:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Özemir IA, Bayraktar B, Anılır E, Orhun K,
Eren T, Sağıroğlu J, Ceyran AB, Yiğitbaşı R and Alimoğlu O: The
association of papillary thyroid cancer with microcalcification in
thyroidnodules with indeterminate cytology based on fine-needle
aspiration biopsy. Turk J Med Sci. 46:1719–1723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majstorov V: Ultrasonographic findings in
patients with benign and malignant thyroid nodules who underwent
ultrasound guided fine needle aspiration cytology. Open Access
Maced J Med Sci. 3:689–693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khoo ML, Asa SL, Witterick IJ and Freeman
JL: Thyroid calcification and its association with thyroid
carcinoma. Head Neck. 24:651–655. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MJ, Men YM, Zhang YL, Zhang YX and Liu
H: Improvement of diagnostic efficiency in distinguishing the
benign and malignant thyroid nodules via conventional ultrasound
combined with ultrasound contrast and elastography. Oncol Lett.
14:867–871. 2017. View Article : Google Scholar : PubMed/NCBI
|