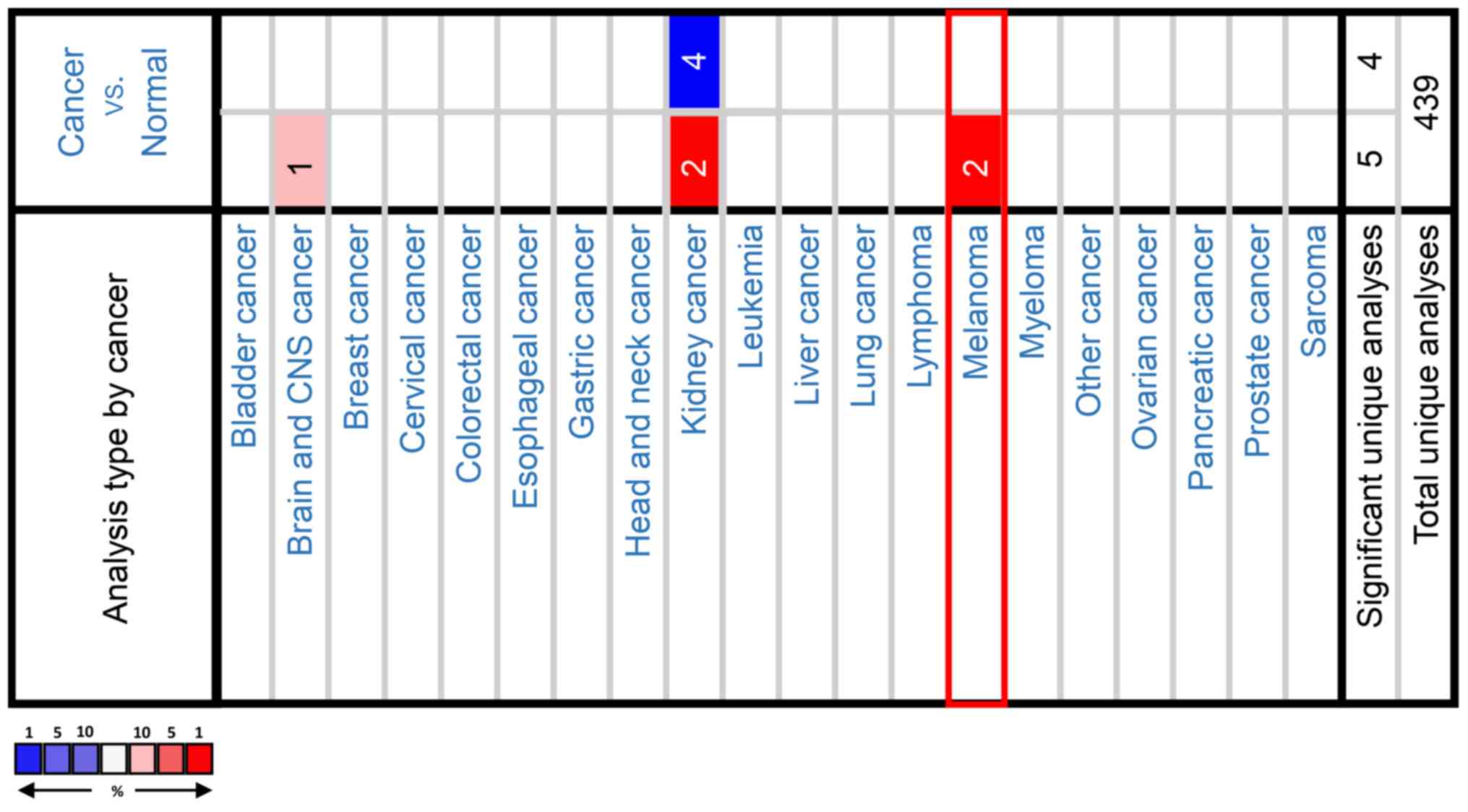
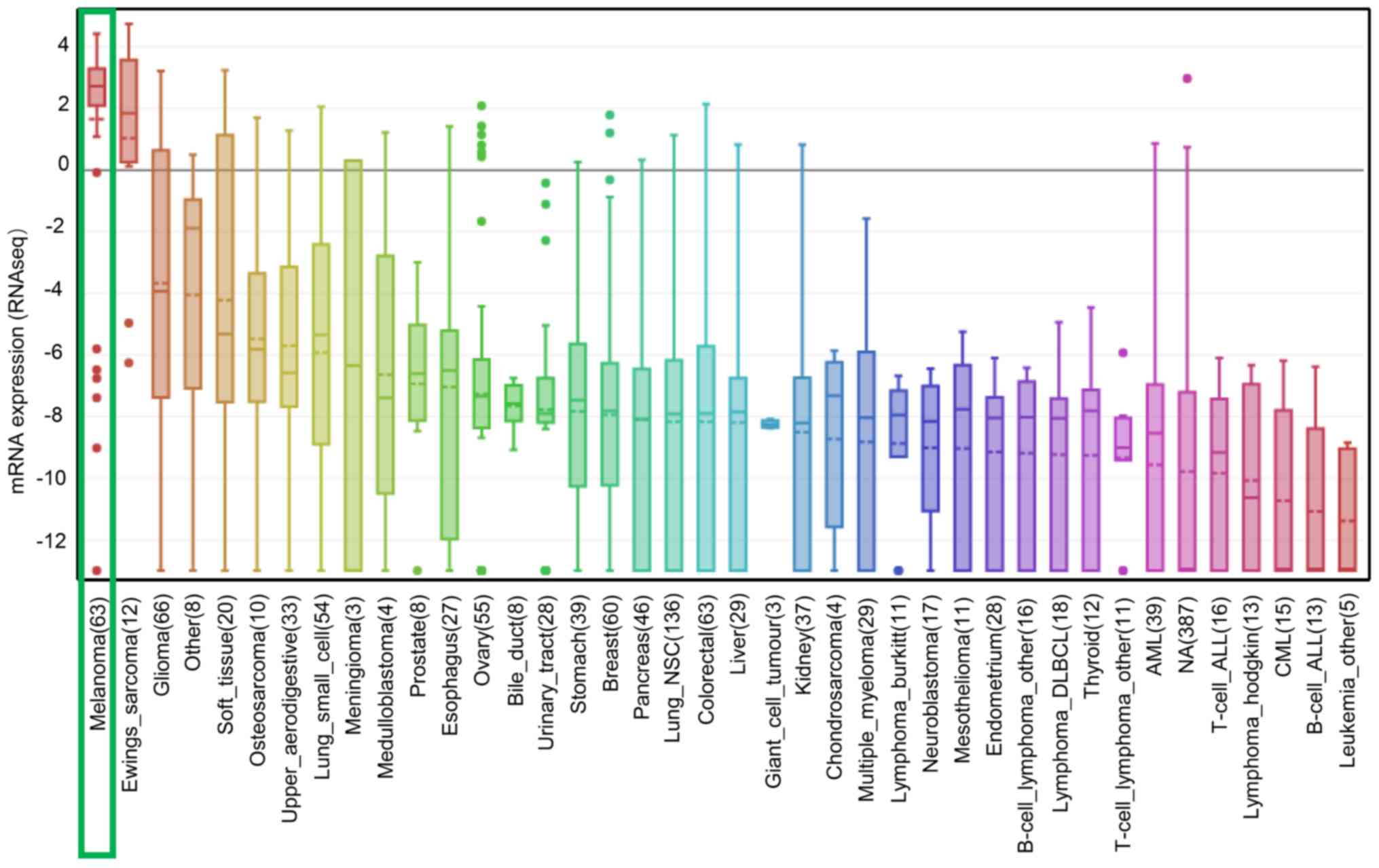
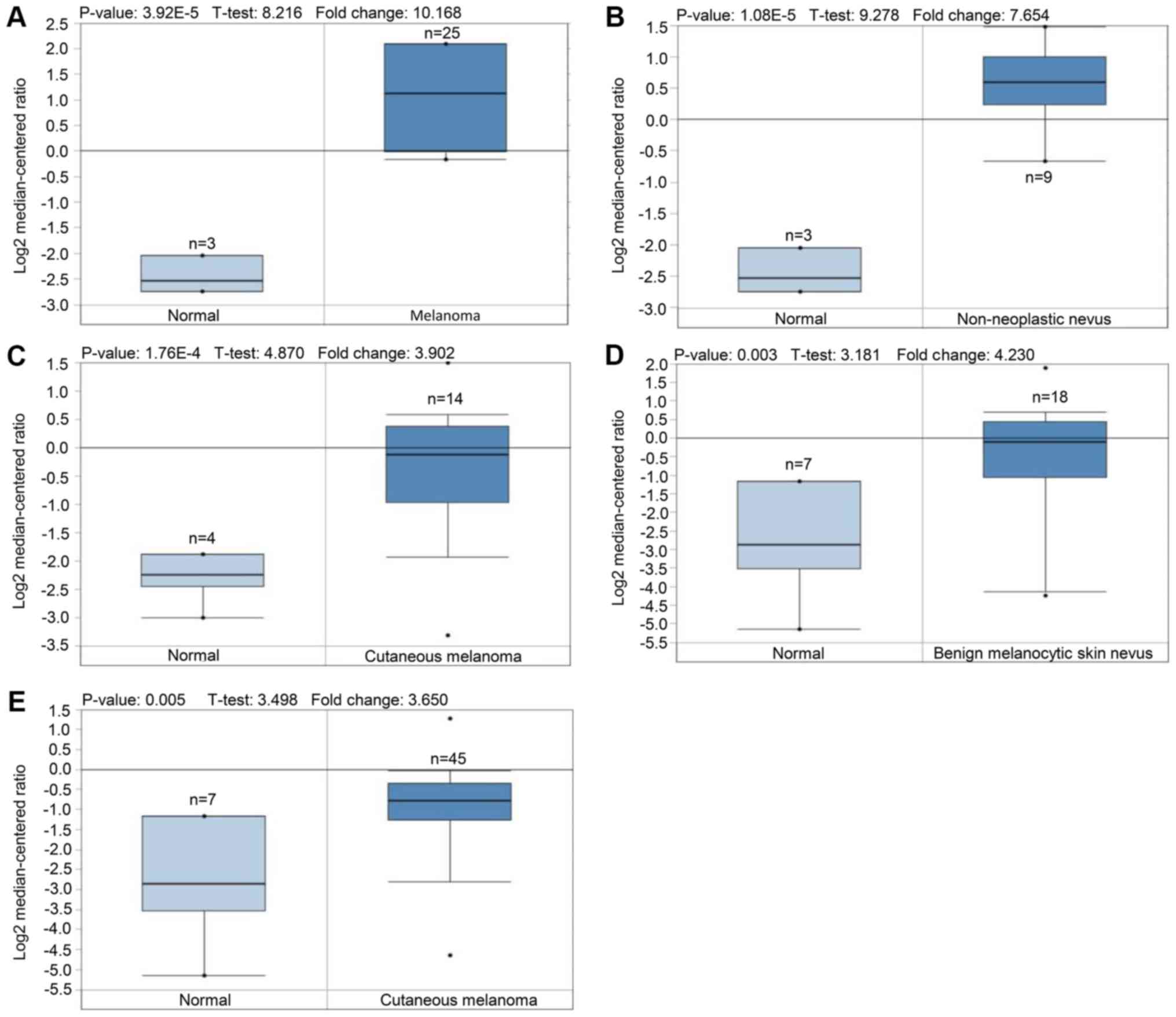
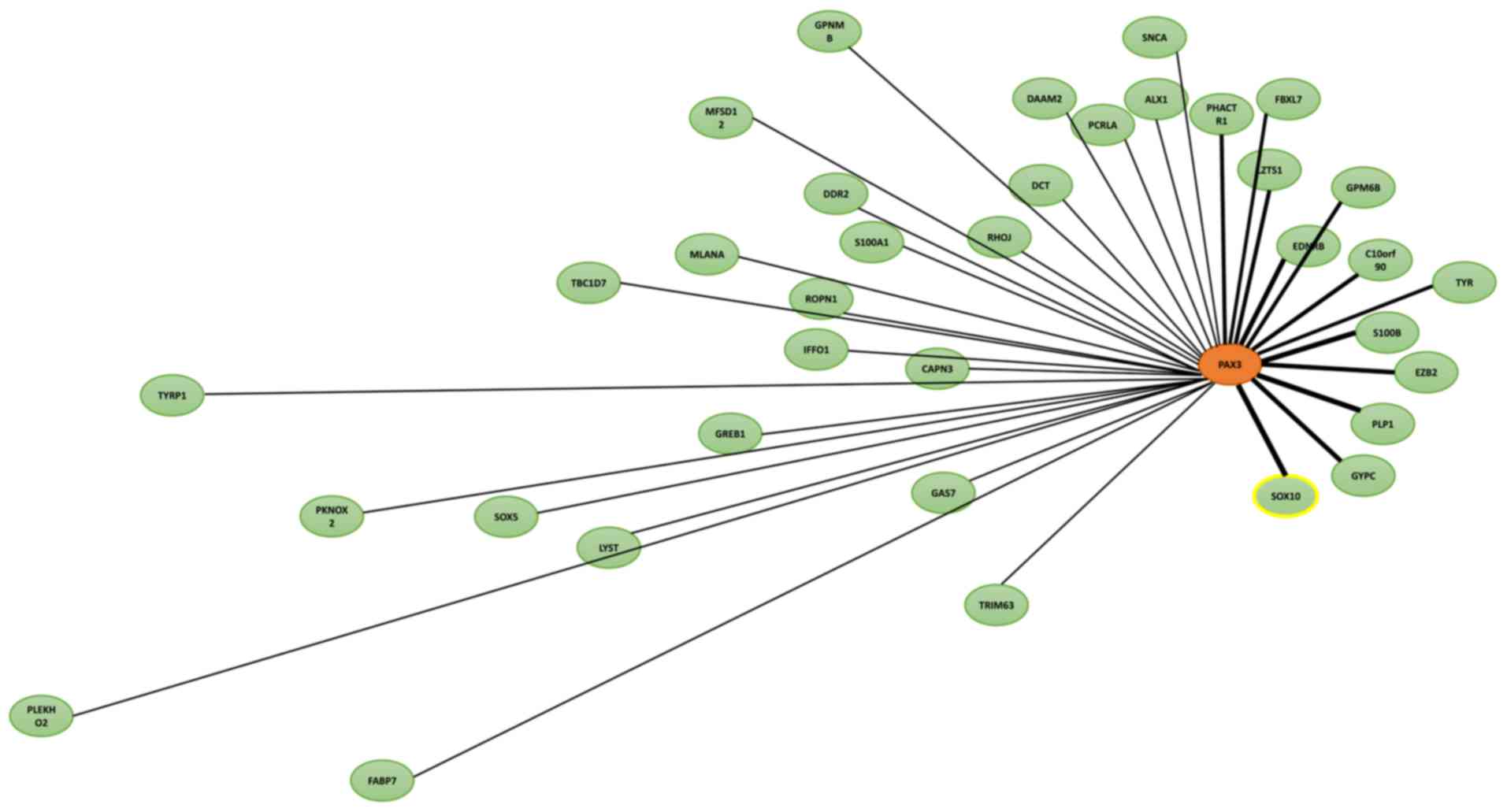
Since the 1980s, the incidence of melanoma has been
increasing at an annual rate of ~2.8% (1). Out of all patients diagnosed with
cutaneous malignant melanoma, ~20% will succumb to metastatic
disease, and the prognosis is significantly worse for those
patients who are diagnosed with regional and distant metastases,
with a 10-year survival rate of 64 and 16%, respectively (2,3).
Paired box 3 (PAX3) protein is known to be involved
in the development of cancer (4).
PAX3 protein contains two DNA binding domains; a paired domain and
a homeodomain, which may function alone or in combination to bind
downstream target genes (5–8).
Previous studies have demonstrated that PAX3 can
drive and activate C-X-C motif chemokine receptor 4 (CXCR4)/MET
proto-oncogene receptor tyrosine kinase expression, and may promote
melanoma metastasis and rapid tumor growth (15,16). E3
ligase APC/C (Cadherin 1) promotes ubiquitination-mediated PAX3
proteolysis and inhibits the proliferation of melanoma cells and
melanoma growth (17). In addition,
phosphorylation of PAX3 affects the melanoma phenotype (18). These findings may contribute to the
further diagnosis, prognosis and potential treatment of
melanoma.
In the present study, large databases of melanoma
genetic information were analyzed to investigate the expression
pattern of PAX3 in melanoma compared with normal tissues, and its
association with characteristic molecular markers and their
corresponding prognostic value in melanoma.
The mRNA expression levels of PAX3 and SRY-box 10
(SOX10) in various types of cancer were analyzed using CCLE
(https://portals.broadinstitute.org/ccle/home), which
is an online database of gene expression, chromosomal copy number
and massively parallel sequencing data from 1,000 human cancer cell
lines, aimed to facilitate the identification of genetic lineages
and predictors of drug sensitivity.
Differences in PAX3 expression between normal tissue
and melanoma tissue were examined by unpaired t-test, and survival
analysis for different groups was performed using the Kaplan-Meier
method with log-rank test. The median of all sample expression
values was calculated using descriptive statistical analyses. The
data were expressed as the mean ± standard deviation. All data were
analyzed using GraphPad Prism v7 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Since PAX3 was identified to be specific to
melanoma, the potential role of PAX3 in melanoma was further
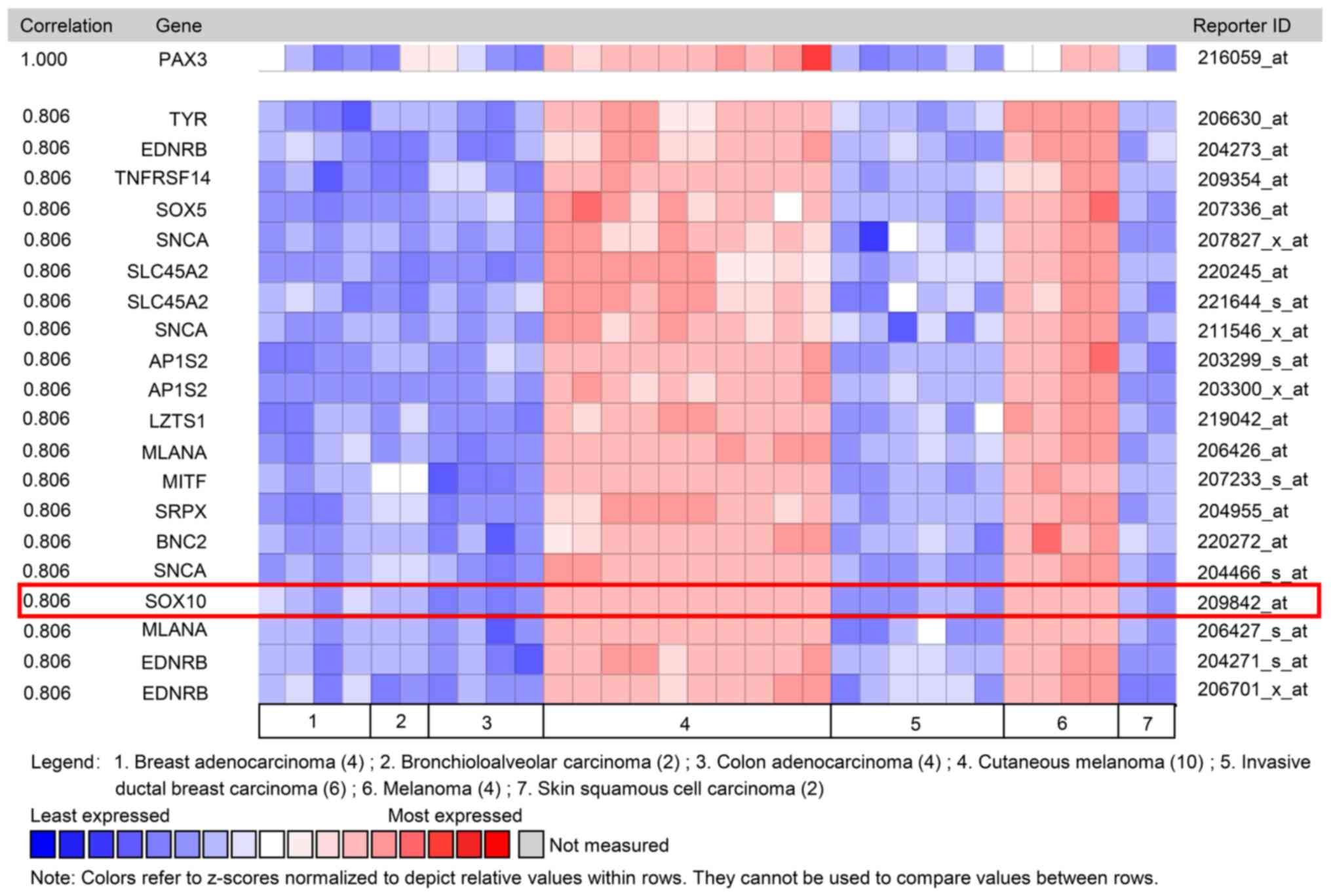
investigated. In a dataset from Wagner et al (22), Coexpedia co-expression analysis
suggested that SOX10 ranked first with a score of 2.778 (Table I and Fig.
4). In Oncomine co-expression analysis, and the dataset from
Pratilas et al (23), PAX3
expression was identified to be significantly associated with SOX10
(r=0.806; Table II and Fig. 5). As shown in Tables I and II, co-expression analysis data indicated
that PAX3 expression may be clearly associated with SOX10
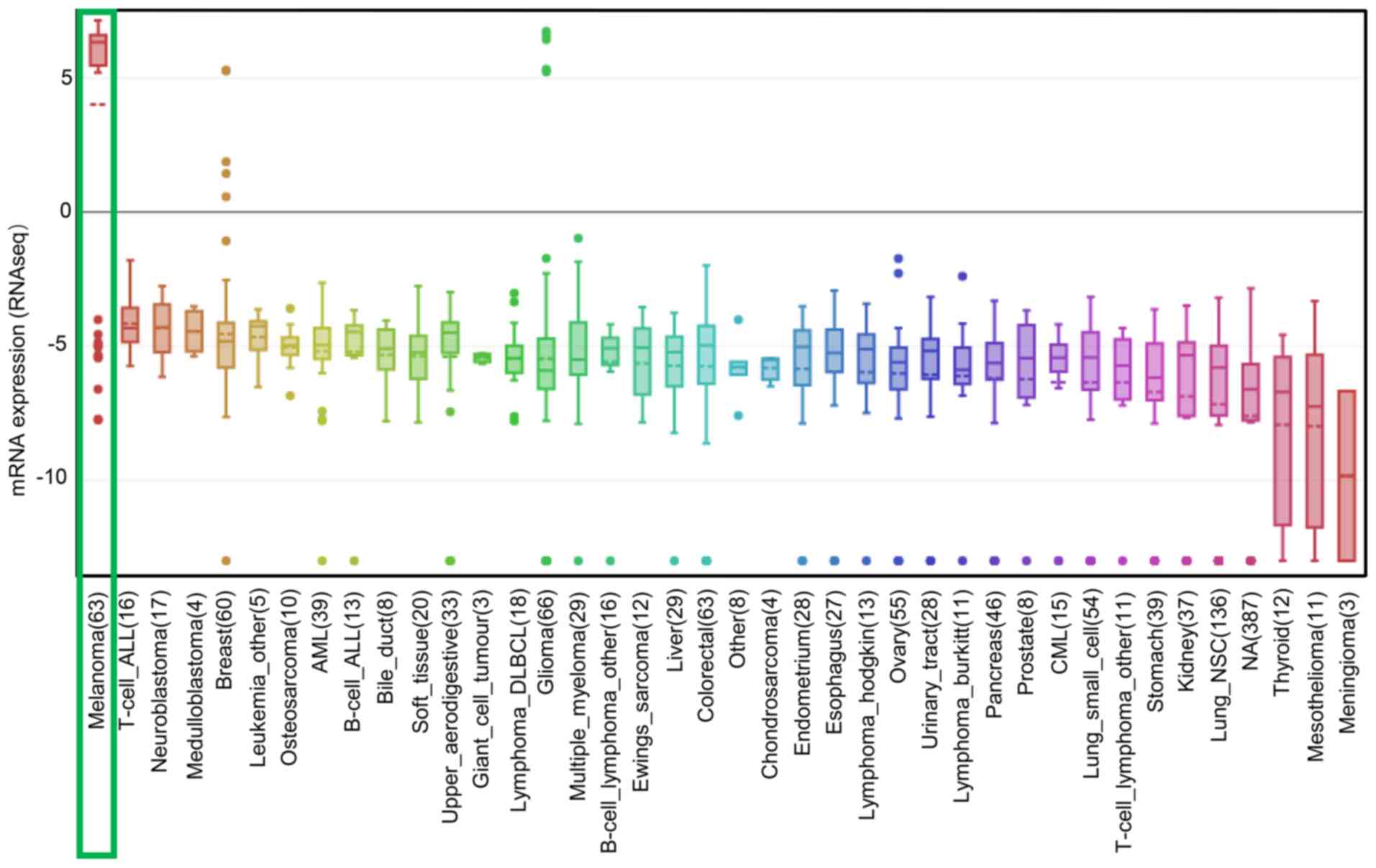
expression. Additionally, similar results were obtained in the CCLE
analysis, in which the mRNA expression level of SOX10 was ranked
highest in melanoma cell lines (Fig.
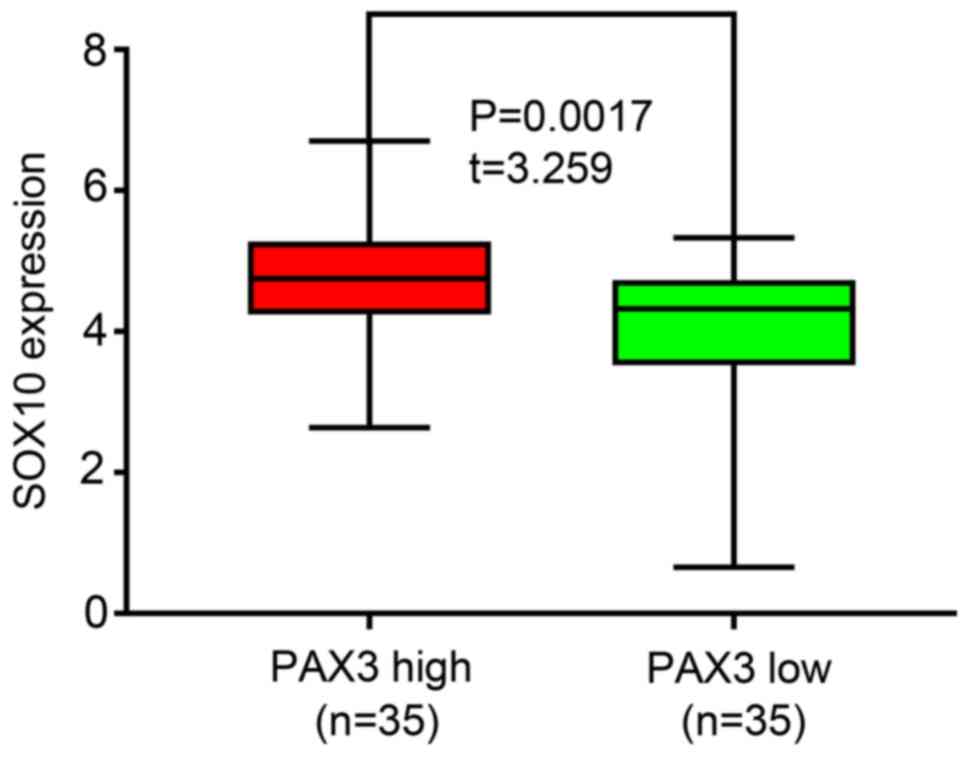
6). In addition, SOX10 overexpression was observed in melanoma
cell lines with high PAX3 expression, while low expression was
observed in melanoma cell lines with low PAX3 expression (P=0.0017;
Fig. 7). The aforementioned results
suggested that SOX10 may be a co-expressed gene of PAX3.
During previous years, the understanding of melanoma
development and biology has improved. It has become clear that the
progression from premalignant lesions to fully developed melanoma
does not represent a single evolutionary pattern. Each melanoma
subtype can develop from different precursor lesions and may
exhibit different stages of gene mutation and transformation
(25). However, some patients
relapse with disseminated disease, and ~10% of melanoma cases are
diagnosed during late stages and are either unresectable or have
metastasized (26). Therefore, a
number of studies have investigated the development of melanoma to
improve targeted therapies (26–28).
PAX3 serves a vital regulatory role in pigment cell
development during embryonic development (29). Medic et al (9) suggested that there is no statistically
significant difference in the expression of PAX3 in melanocytes and
melanoma cells; however, Bailey et al (30) demonstrated that PAX3 expression is
significantly inhibited in adult melanocytes and the expression of
PAX3 mRNA in melanoma cells is 200-times that of normal skin
(31). Notably, PAX3, particularly
PAX3E, significantly inhibits the proliferation and increases
chemosensitivity of melanoma cells (15,32–35),
meanwhile, treatment with PAX3 inhibitors resulted in a significant
decrease in PAX3 expression in melanoma cells, whereas PAX3
expression had no change in melanocytes (36). PAX3 may not only drive the expression
of genes that promote cellular metastasis and invasion, but may
also regulate the mRNA expression levels of genes involved in
melanoma differentiation, proliferation and survival (9,34,37).
Reid et al (14) revealed
that PAX3 expression is evident at all stages of melanoma
progression, including primary lesions, circulating melanoma cells
and metastatic lesions. Medic et al (9) suggested that PAX3 directly targets the
transforming growth factor β1 promoter in metastatic melanoma cell
lines, as well as other genes associated with cell migration,
including melanoma cell adhesion molecule, chondroitin sulfate
proteoglycan 4 and CXCR4. Additionally, PAX3 may drive CXCR4
expression to promote melanoma metastasis (18). In addition, silencing PAX3 with RNA
interference can inhibit proliferation and induce terminal
differentiation and apoptosis, according to the activation of
caspase-3 and p53 in melanoma cells (38–40).
Notably, >2.76 copies/µl of PAX3d mRNA in the bloodstream
predicts recurrence of cutaneous malignant melanoma (41). Furthermore, the human PAX3 gene may
serve a role in other human malignancies, including
rhabdomyosarcoma and Ewing's sarcoma (42). Co-expression analysis using Oncomine
revealed a positive association between PAX3 expression and SOX10
expression. The findings of Bondurand et al (43) are consistent with the complex
functional roles of PAX3 and SOX10 in neural crest stem
cell-derived melanocyte development, and SOX10 has been
demonstrated to markedly activate melanocyte inducing transcription
factor (MITF) expression in cultured cell lines, while PAX3
synergistically transactivates the promoter of MITF with SOX10 to
influence the maintenance of melanocyte stem cells (43–45).
Additionally, a small number of PAX3 transcriptional cofactors have
been identified, but only SOX10 and ETS proto-oncogene 1
transcription factor have been verified within melanoma cells
(46). Furthermore, there is
increasing evidence that signaling proteins tend to form
interaction networks rather than simple linear pathways, meaning
PAX3 and SOX10 may use specific interaction networks to regulate
the proliferation, differentiation and migration of melanocyte
precursors (47–51).
In the present study, the incidence rate of melanoma
was identified to be higher in men than in women. Using Oncomine
analysis, the expression of PAX3 in male patients with melanoma was
significantly higher than in female patients (P=0.0286, t=2.232).
Some studies have suggested that this increased male susceptibility
may be associated with androgens (52–54) and
hyperandrogenism may result in variants in melanoma-associated
pigmentary genes (55–58). According to Kocarnik et al
(59), the solute carrier family 45
member 2 single nucleotide polymorphism rs16891982, which has a
non-synonymous mutation (F374L) located in exon 5, may be
responsible for imparting a higher melanoma risk in men, possibly
through alterations in pigmentation and melanogenesis (60). Therefore, the present study
demonstrated that the differential expression of PAX3 and its
downstream targets may be a potential predictor of sex-specific
genetic risks in melanoma.
In conclusion, PAX3 was highly expressed in melanoma
and predicted a worse survival rate for patients with melanoma.
PAX3 expression was positively associated with SOX10 expression,
and the expression of PAX3 in male patients with melanoma was
significantly higher than that in female patients. The
aforementioned evidence indicated that PAX3 may act as a potential
modulator in melanoma.
The authors would like to thank Dr H. Nikki March,
for editing the English text of a draft of this manuscript.
This study was partly supported by the National
Natural Science Foundation of China (grant no. 81704087), the
National Disease Research Program of TCM (grant no. 20160524), the
Innovative Talents Promotion Project-Key Scientific and
Technological Innovation Team Project (grant no. 2017KCT-27) and
the Shaanxi Province Clinical Medical Research Center Project
(grant no. 2016LCZX-12).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
YL and SC designed the study and drafted the
manuscript. WL and YZ were primarily dedicated to collecting and
statistically analyzing data. XY and JX supervised the scientific
work, interpreted the data, revised the manuscript, provided
financial support and agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Little EG and Eide MJ: Update on the
current state of melanoma incidence. Dermatol Clin. 30:355–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buzaid AC and Atkins M: Practical
guidelines for the management of biochemotherapy-related toxicity
in melanoma. Clin Cancer Res. 7:2611–2619. 2001.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robson EJ, He SJ and Eccles MR: A PANorama
of PAX genes in cancer and development. Nat Rev Cancer. 6:52–62.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corry GN and Underhill DA: Pax3 target
gene recognition occurs through distinct modes that are
differentially affected by disease-associated mutations. Pigment
Cell Res. 18:427–38. 2005.PubMed/NCBI
|
|
6
|
Chalepakis G and Gruss P: Identification
of DNA recognition sequences for the Pax3 paired domain. Gene.
162:267–270. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chalepakis G, Jones FS, Edelman GM and
Gruss P: Pax-3 contains domains for transcription activation and
transcription inhibition. Proc Natl Acad Sci USA. 91:12745–12749.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epstein DJ, Vogan KJ, Trasler DG and Gros
P: A mutation within intron 3 of the Pax-3 gene produces aberrantly
spliced mRNA transcripts in the splotch (Sp) mouse mutant. Proc
Natl Acad Sci USA. 90:532–536. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medic S, Rizos H and Ziman M: Differential
PAX3 functions in normal skin melanocytes and melanoma cells.
Biochem Biophys Res Commun. 411:832–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barber TD, Barber MC, Cloutier TE and
Friedman TB: PAX3 gene structure, alternative splicing and
evolution. Gene. 237:311–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barr FG, Fitzgerald JC, Ginsberg JP,
Vanella ML, Davis RJ and Bennicelli JL: Predominant expression of
alternative PAX3 and PAX7 forms in myogenic and neural tumor cell
lines. Cancer Res. 59:5443–5448. 1999.PubMed/NCBI
|
|
12
|
Takeuchi H, Morton DL, Kuo C, Turner RR,
Elashoff D, Elashoff R, Taback B, Fujimoto A and Hoon DS:
Prognostic significance of molecular upstaging of paraffin embedded
sentinel lymph nodes in melanoma patients. J Clin Oncol.
22:2671–2680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galibert MD, Yavuzer U, Dexter TJ and
Goding CR: Pax3 and regulation of the melanocyte-specific
tyrosinase-related protein-1 promoter. J Biol Chem.
274:26894–26900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reid AL, Millward M, Pearce R, Lee M,
Frank MH, Ireland A, Monshizadeh L, Rai T, Heenan P, Medic S, et
al: Markers of circulating tumour cells in the peripheral blood of
patients with melanoma correlate with disease recurrence and
progression. Br J Dermatol. 168:85–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubic JD, Little EC, Lui JW, Iizuka T and
Lang D: PAX3 and ETS1 synergistically activate MET expression in
melanoma cells. Oncogene. 34:4964–4974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubic JD, Lui JW, Little EC, Ludvik AE,
Konda S, Salgia R, Aplin AE and Lang D: PAX3 and FOXD3 promote
CXCR4 expression in melanoma. J Biol Chem. 290:21901–21914. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao J, Dai X, Wan L, Wang H, Zhang J, Goff
PS, Sviderskaya EV, Xuan Z, Xu Z, Xu X, et al: The E3 ligase
APC/C(Cdh1) promotes ubiquitylation-mediated proteolysis of PAX3 to
suppress melanocyte proliferation and melanoma growth. Sci Signal.
8:ra872015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iyengar AS, Miller PJ, Loupe JM and
Hollenbach AD: Phosphorylation of PAX3 contributes to melanoma
phenotypes by affecting proliferation, invasion, and
transformation. Pigment Cell Melanoma Res. 27:846–848. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haqq C, Nosrati M, Sudilovsky D, Crothers
J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR III, Allen RE,
Singer MI, et al: The gene expression signatures of melanoma
progression. Proc Natl Acad Sci USA. 102:6092–6097. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riker AI, Enkemann SA, Fodstad O, Liu S,
Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, et al: The
gene expression profiles of primary and metastatic melanoma yields
a transition point of tumor progression and metastasis. BMC Med
Genomics. 1:132008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Talantov D, Mazumder A, Yu JX, Briggs T,
Jiang Y, Backus J, Atkins D and Wang Y: Novel genes associated with
malignant melanoma but not benign melanocytic lesions. Clin Cancer
Res. 11:7234–7242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wagner KW, Punnoose EA, Januario T,
Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF,
Totpal K, et al: Death-receptor O-glycosylation controls tumor-cell
sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med.
13:1070–1077. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pratilas CA, Taylor BS, Ye Q, Viale A,
Sander C, Solit DB and Rosen N: (V600E)BRAF is associated with
disabled feedback inhibition of RAF-MEK signaling and elevated
transcriptional output of the pathway. Proc Natl Acad Sci USA.
106:4519–4524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu L, Shen SS, Hoshida Y, Subramanian A,
Ross K, Brunet JP, Wagner SN, Ramaswamy S, Mesirov JP and Hynes RO:
Gene expression changes in an animal melanoma model correlate with
aggressiveness of human melanoma metastases. Mol Cancer Res.
6:760–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shain AH and Bastian BC: From melanocytes
to melanomas. Nat Rev Cancer. 16:345–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luke JJ, Flaherty KT, Ribas A and Long GV:
Targeted agents and immunotherapies: Optimizing outcomes in
melanoma. Nat Rev Clin Oncol. 14:463–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amann VC, Ramelyte E, Thurneysen S,
Pitocco R, Bentele-Jaberg N, Goldinger SM, Dummer R and Mangana J:
Developments in targeted therapy in melanoma. Eur J Surg Oncol.
43:581–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christiansen SA, Khan S and Gibney GT:
Targeted therapies in combination with immune therapies for the
treatment of metastatic melanoma. Cancer J. 23:59–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lang D, Lu MM, Huang L, Engleka KA, Zhang
M, Chu EY, Lipner S, Skoultchi A, Millar SE and Epstein JA: Pax3
functions at a nodal point in melanocyte stem cell differentiation.
Nature. 433:884–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bailey CM, Morrison JA and Kulesa PM:
Melanoma revives an embryonic migration program to promote
plasticity and invasion. Pigment Cell Melanoma Res. 25:573–583.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medic S and Ziman M: PAX3 expression in
normal skin melanocytes and melanocytic lesions (naevi and
melanomas). PLoS One. 5:e99772010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hathaway-Schrader JD, Doonan BP, Hossain
A, Radwan FFY, Zhang L and Haque A: Autophagy-dependent crosstalk
between GILT and PAX-3 influences radiation sensitivity of human
melanoma cells. J Cell Biochem. 119:2212–2221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Kumar S, Slevin M and Kumar P:
Functional analysis of alternative isoforms of the transcription
factor PAX3 in melanocytes in vitro. Cancer Res. 66:8574–8580.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu F, Cao J, Lv J, Dong L, Pier E, Xu GX,
Wang RA, Xu Z, Goding C and Cui R: TBX2 expression is regulated by
PAX3 in the melanocyte lineage. Pigment Cell Melanoma Res.
26:67–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu F, Cao J, Wu J, Sullivan K, Shen J,
Ryu B, Xu Z, Wei W and Cui R: Stat3-targeted therapies overcome the
acquired resistance to vemurafenib in melanomas. J Invest Dermatol.
133:2041–2049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith MP, Ferguson J, Arozarena I, Hayward
R, Marais R, Chapman A, Hurlstone A and Wellbrock C: Effect of
SMURF2 targeting on susceptibility to MEK inhibitors in melanoma. J
Natl Cancer Inst. 105:33–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartlett D, Boyle GM, Ziman M and Medic S:
Mechanisms contributing to differential regulation of PAX3
downstream target genes in normal human epidermal melanocytes
versus melanoma cells. PLoS One. 10:e01241542015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He S, Li CG, Slobbe L, Glover A, Marshall
E, Baguley BC and Eccles MR: PAX3 knockdown in metastatic melanoma
cell lines does not reduce MITF expression. Melanoma Res. 21:24–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He SJ, Stevens G, Braithwaite AW and
Eccles MR: Transfection of melanoma cells with antisense PAX3
oligonucleotides additively complements cisplatin-induced
cytotoxicity. Mol Cancer Ther. 4:996–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scholl FA, Kamarashev J, Murmann OV,
Geertsen R, Dummer R and Schäfer BW: PAX3 is expressed in human
melanomas and contributes to tumor cell survival. Cancer Res.
61:823–826. 2001.PubMed/NCBI
|
|
41
|
Autilio C, Paolillo C, Lavieri MM, Pocino
K, De Paolis E, Di Stasio E, Marchetti P, Gian Carlo CA and
Capoluongo E: PAX3d mRNA over 2.76 copies/µl in the bloodstream
predicts cutaneous malignant melanoma relapse. Oncotarget.
8:85479–85491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Fang WH, Krupinski J, Kumar S,
Slevin M and Kumar P: Pax genes in embryogenesis and oncogenesis. J
Cell Mol Med. 12:2281–2294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bondurand N, Pingault V, Goerich DE,
Lemort N, Sock E, Le Caignec C, Wegner M and Goossens M:
Interaction among SOX10, PAX3 and MITF, three genes altered in
Waardenburg syndrome. Hum Mol Genet. 9:1907–1917. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Potterf SB, Furumura M, Dunn KJ, Arnheiter
H and Pavan WJ: Transcription factor hierarchy in Waardenburg
syndrome: Regulation of MITF expression by SOX10 and PAX3. Hum
Genet. 107:1–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe A, Takeda K, Ploplis B and
Tachibana M: Epistatic relationship between Waardenburg syndrome
genes MITF and PAX3. Nat Genet. 18:283–286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mascarenhas JB, Littlejohn EL, Wolsky RJ,
Young KP, Nelson M, Salgia R and Lang D: PAX3 and SOX10 activate
MET receptor expression in melanoma. Pigment Cell Melanoma Res.
23:225–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hou L and Pavan WJ: Transcriptional and
signaling regulation in neural crest stem cell-derived melanocyte
development: Do all roads lead to Mitf? Cell Res. 18:1163–1176.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Otręba M, Miliński M, Buszman E, Wrześniok
D and Beberok A: Hereditary hypomelanocytoses: The role of PAX3,
SOX10, MITF, SNAI2, KIT, EDN3 and EDNRB genes. Postepy Hig Med Dosw
(Online). 67:1109–1118. 2013.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pingault V, Ente D, Dastot-Le Moal F,
Goossens M, Marlin S and Bondurand N: Review and update of
mutations causing Waardenburg syndrome. Hum Mutat. 31:391–406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Otręba M, Rok J, Buszman E and Wrześniok
D: Regulation of melanogenesis: The role of cAMP and MITF. Postepy
Hig Med Dosw (Online). 66:33–40. 2012.(In Polish). PubMed/NCBI
|
|
51
|
Lin JY and Fisher DE: Melanocyte biology
and skin pigmentation. Nature. 445:843–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li WQ, Cho E, Weinstock MA, Mashfiq H and
Qureshi AA: Epidemiological assessments of skin outcomes in the
nurses' health studies. Am J Public Health. 106:1677–1683. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang M, Qureshi AA, Geller AC, Frazier L,
Hunter DJ and Han J: Use of tanning beds and incidence of skin
cancer. J Clin Oncol. 30:1588–1593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li WQ, Qureshi AA, Ma J, Goldstein AM,
Giovannucci EL, Stampfer MJ and Han J: Personal history of prostate
cancer and increased risk of incident melanoma in the United
States. J Clin Oncol. 31:4394–4399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nair-Shalliker V, Egger S, Chrzanowska A,
Mason R, Waite L, Le Couteur D, Seibel MJ, Handelsman DJ, Cumming
R, Smith DP and Armstrong BK: Associations between sun sensitive
pigmentary genes and serum prostate specific antigen levels. PLoS
One. 13:e01938932018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chia SE, Wong KY, Cheng C, Lau W and Tan
PH: Sun exposure and the risk of prostate cancer in the singapore
prostate cancer study: A case-control study. Asian Pac J Cancer
Prev. 13:3179–3185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nair-Shalliker V, Smith DP, Egger S,
Hughes AM, Kaldor JM, Clements M, Kricker A and Armstrong BK: Sun
exposure may increase risk of prostate cancer in the high UV
environment of New South Wales, Australia: A case-control study.
Int J Cancer. 131:E726–E732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bonilla C, Gilbert R, Kemp JP, Timpson NJ,
Evans DM, Donovan JL, Hamdy FC, Neal DE, Fraser WD, Davey SG, et
al: Using genetic proxies for lifecourse sun exposure to assess the
causal relationship of sun exposure with circulating vitamin d and
prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 22:597–606.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kocarnik JM, Park SL, Han J, Dumitrescu L,
Cheng I, Wilkens LR, Schumacher FR, Kolonel L, Carlson CS, Crawford
DC, et al: Replication of associations between GWAS SNPs and
melanoma risk in the Population Architecture Using Genomics and
Epidemiology (PAGE) Study. J Invest Dermatol. 134:2049–2052. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hernando B, Ibarrola-Villava M, Fernandez
LP, Peña-Chilet M, Llorca-Cardeñosa M, Oltra SS, Alonso S, Boyano
MD, Martinez-Cadenas C and Ribas G: Sex-specific genetic effects
associated with pigmentation, sensitivity to sunlight, and melanoma
in a population of Spanish origin. Biol Sex Differ. 7:172016.
View Article : Google Scholar : PubMed/NCBI
|