Introduction
Gastric cancer is the fourth most common cancer type
globally and was recorded to be the third highest cause of
cancer-associated mortality in 2015 (1). According to recent data, the incidence
rate of and mortality rate of gastric cancer in China in 2015 was
679.1 and 498.0, respectively, per 100,000 of the population
(1). Although mortality rates have
decreased since 2010, the outcome for patients with advanced stage
gastric cancer remains poor, with an estimated relative 5-year
survival rate of 30% (2).
Understanding the molecular mechanisms of carcinogenesis and
identifying molecular targets for the development of novel
treatments is required in order to improve the survival time of
patients with gastric cancer.
Although molecular targets have been identified in
gastric cancer, only a limited number have been used as diagnostic
biomarkers or therapeutic targets within the clinical setting. The
human epidermal growth factor receptor-2 protein (HER2) is a member
of the epidermal growth factor receptor family of receptor tyrosine
kinases (2,3). HER2 overexpression and/or amplification
have been determined in invasive breast cancer (3), and in other cancer types, including
colon, ovarian, lung and prostate cancer (4). Amplification of the HER2 gene and
overexpression of the HER2 protein is observed in ~20% of gastric
cancer cases (3,5). A number of clinical trials of varying
design are currently investigating the potential of anti-HER2
therapies in gastric cancer (2,3). A wide
range of response rates have been reported in patients with
HER2-positive gastric cancer treated with trastuzumab (6,7), and
these data prompted the identification of novel genes associated
with the diagnosis and treatment of this disease.
The ephrin type-A receptor 5 (EphA5), previously
referred to as brain specific kinase or Cek7, was first isolated
from adult mouse brain (8). The Eph
family of receptors, named due to the first family member being
identified in an erythropoietin-producing human hepatocellular
carcinoma cell line, is the largest subfamily of receptor
protein-tyrosine kinases (4). Eph
receptors and the Eph ligands have been implicated in various
aspects of development, particularly nervous system patterning
(9–11). The Eph receptors and Eph ligands are
divided into two subclasses, A and B, according to their sequence
homology, structure and binding affinity (12). Previously, the Ephreceptors and Eph
ligands have been determined to be differentially expressed in
various human cancer types, including colorectal, lymphoma,
prostate, ovarian and lung cancer (13–18).
Changes in the expression patterns of these receptors and their
ligands may be associated with alterations in tumor behavior,
including increased invasiveness, metastatic potential and
angiogenesis, and consequently these changes may also be associated
with poor patient outcome. EphA5 is differentially expressed in
breast, prostate, lung, ovarian and colorectal cancer types
(18–22). In a previous study, it was determined
that EphA5 was expressed in the normal fallopian tube (100%),
benign epithelial ovarian tumor cases (100%) and the majority of
ovarian serous borderline tumor cases (76%) (5). Reduction of EphA5 expression was
observed in ovarian serous carcinoma cases (31%) and was associated
with tumor grade and International Federation of Gynecology and
Obstetrics stage (21). In the
present study, immunohistochemistry (IHC) was performed to evaluate
EphA5 protein expression in a set of specimens from patients with
gastric cancer. The association of EphA5 expression with
clinicopathological parameters was analyzed. To the best of our
knowledge, the present study is the first to investigate the role
of EphA5 in gastric cancer.
Materials and methods
Patients and tissue samples
The study group was comprised of 110 consecutive
patients with primary gastric adenocarcinomas. All patients
underwent surgical resection without prior chemo- or radiotherapy
between January 2015 and December 2016 at the Department of
Surgery, Taixing People Hospital (Taixing, Jiangsu, China) and
Jinling Hospital (Nanjing, Jiangsu, China). Each tumor was
classified according to the Tumor-Node-Metastasis (TNM) system of
the World Health Organization Classification of Tumors, Pathology
and Genetics of Tumors of the Digestive System (23) and Lauren classification (24). Data were acquired with approval from
the Ethics Committees of the Taixing People's Hospital and Jinling
Hospital. The formalin-fixed, paraffin-embedded samples were
retrospectively collected from the Department of Pathology of
Taixing People's Hospital and the Sir Run Run Hospital (Nanjing,
Jiangsu, China). Of the 110 patients, 80 were male and 30 were
female. Mean patient age was 65.7 years (range, 43–82 years).
Patient details are summarized in Table
I.
 | Table I.Association between the expression of
EphA5 and clinicopathological parameters. |
Table I.
Association between the expression of
EphA5 and clinicopathological parameters.
|
| EphA5 protein
expression |
|
|---|
|
|
|
|
|---|
| Parameters | − (n=30) | + (n=80) | P-valuea |
|---|
| Patients, % | 27.3 | 72.7 |
|
| Age, n |
| <70
years | 22 | 48 | 0.266 |
| ≥70
years | 8 | 32 |
|
| Sex, n |
|
Female | 6 | 24 | 0.345 |
| Male | 24 | 56 |
|
| Primary location,
n |
|
Lower | 22 | 44 | 0.125 |
| Middle or
upper | 8 | 36 |
|
| Lauren, n |
|
Intestinal | 8 | 40 | 0.032 |
|
Diffuse | 22 | 40 |
|
| Depth of invasion,
n |
|
T1/2 | 8 | 26 | 0.647 |
|
T3/4 | 22 | 54 |
|
| Lymph node, n |
|
N0/1 | 9 | 55 | <0.001 |
|
N2/3 | 21 | 25 |
|
| TNM stage, n |
|
I/II | 9 | 33 | 0.379 |
|
III/IV | 21 | 47 |
|
| HER2, n |
|
Negative | 22 | 74 | 0.020 |
|
Positive | 8 | 6 |
|
| Ki-67, n |
|
<14% | 6 | 40 | 0.005 |
|
≥14% | 24 | 40 |
|
Hematoxylinand eosin staining
Hematoxylin and eosin staining is the most common
staining technique use in routine histology. The technique uses a
combination of two dyes, hematoxylin and eosin, for indication of
nuclei and cytoplasmic inclusions in clinical specimens. Briefly,
the tissue sections were deparaffinized in xylene for 5 min at room
temperature, then the sections were hydrate by passing through
decreasing concentrations of alcohol baths and water (100, 90, 80
and 70%). The sections were stained in hematoxylin for 3–5 min,
washed with water for 5 min and differentiated in 1% acid alcohol
for 5 min. One percent eosin Y staining was applied for 10 min
prior to washing and dehydrating.
IHC analysis of EphA5, HER2 and Ki-67
protein expression
IHC for EphA5, HER2, and Ki-67 was performed using a
previously described protocol (6).
In brief, tissue samples were cut into 4-µm thick sections. Gastric
cancer tissues were prepared on slides and fixed with 95% ethanol
for 1 min. The sections were deparaffinized using xylene,
dehydrated by an ethanol gradient (95, 80 and 70%) and then
rehydrated with deionized water. Antigen retrieval was performed by
autoclave treatment (120°C for 2 min in 1 mmol/l EDTA, pH 8.0).
Endogenous peroxidase was quenched with 3% hydrogen peroxide in
methanol. Samples were then blocked with 10% goat serum (Abcam,
Cambridge, UK) for 10 min at room temperature to prevent
nonspecific binding. Incubation with polyclonal antibodies for
EphA5 (dilution, 1:400; cat. no. AM7610a; Abgent, Inc., San Diego,
CA, USA), HER2 (dilution, 1:100; cat. no. A0485; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) and a monoclonal antibody
for Ki-67 (dilution, 1:100; cat. no. ab15580; Abcam) was performed
overnight at 4°C. Following washing with PBS (pH 7.4), the sections
were then incubated with a secondary antibody against rabbit and
mouse immunoglobulin (peroxidase-conjugated; Dako; Agilent
Technologies, Inc.) for 30 min at room temperature.
Immunoreactivity was visualized using the colorimetric detection
reagent 3,3′-diaminobenzidine. Nuclei were counterstained with
hematoxylin at room temperature for 40 sec. For EphA5 protein
detection, PBS was used in place of a primary antibody as the
negative control. Results were visualized using an Olympus light
microscope (Olympus Corporation, Tokyo, Japan) at ×200
magnification.
Evaluation of IHC data
EphA5 protein expression was semi-quantitatively
assessed. Tissue specimens were assigned one of four scores
according to the intensity of antibody staining (0, none; 1, weakly
positive/weak light yellow; 2, moderately positive/medium
brown-yellow; and 3, strongly positive/dark brown). Staining extent
was graded according to the percentage of stained tumor cells and
was defined as follows: 0, 0%; 1, 0<n<25%; 2, 25–50%; and 3,
>50% positively-stained cells. Staining intensity and staining
extent values were added together and these final scores were then
used to define expression status as follows: 0–2, negative (−); and
3–6, positive (+) (23). HER2
immunostaining was scored according to the system previously
reported for gastric cancer (25)
and was as follows: 0 (negative), no reactivity or <10%; 1+
(negative), faint with partial membrane staining ≥10%; 2+
(positive), weak-to-moderate with complete or basolateral staining
>10%; and 3+ (positive), moderate-to-strong with complete or
basolateral staining ≥10%. Ki-67 positive tumor cells demonstrated
punctate yellow-brown nuclear staining. High Ki-67 expression was
defined as ≥14% positive tumor cell staining. Immunostained
specimens were evaluated independently by two pathologists in a
blinded manner. Any discrepancies in the scores were resolved by
consensus following further evaluation.
Fluorescence in situ hybridization
(FISH)
FISH was performed to determine the HER2 status of
gastric cancer tissue specimens. The test was performed according
to the manufacturer's protocol for the HER2 probe (Beijing GP
Medical Technologies, Ltd., Beijing, China). The HER2 gene was
labeled with Spectrum Orange and the chromosome-17 centromere
(CEP17) with Spectrum Green, which is part of the kit from Beijing
GP Medical Technology. Two independent observers blinded to the
study scored HER2 and CEP17 labeling from an analysis of 100 nuclei
per tissue specimen, and the HER2:CEP17 signal ratio was
subsequently calculated. Tissues were scored as HER2-positive or
HER2-negative depending on whether their signal ratio was ≥2 or
<2, respectively.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The
χ2 test was used to analyze the association of EphA5
protein expression with clinicopathological parameters. The data
are presented as the mean ± standard error of the mean. A total of
10 high-power fields (×400) were used for microscopy. P<0.05 was
considered to indicate a statistically significant difference.
Results
EphA5 expression in gastric carcinoma
samples
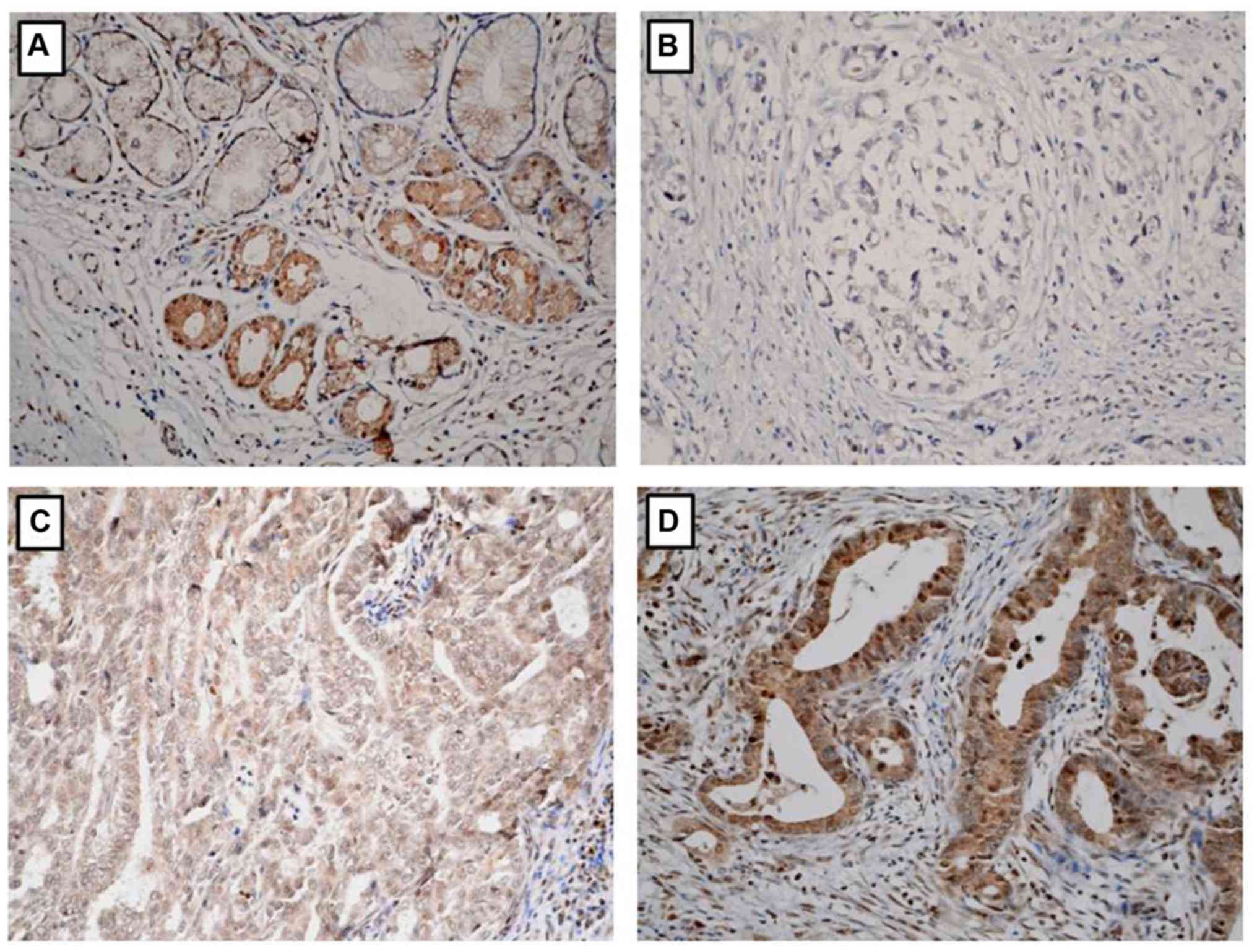
IHC was performed to evaluate EphA5 protein
expression in tumor tissue specimens obtained from 110 consecutive
patients with primary gastric adenocarcinoma. EphA5 protein
staining was predominantly located in the cytoplasm and weakly
stained in the nucleus. EphA5 was positively expressed in all
non-tumor gastric epithelia and differentially expressed among
gastric cancer tissue samples (Fig.
1). EphA5 was negatively expressed in 30/110 (27.3%) and
positively expressed in 80/110 (72.7%) patient specimens.
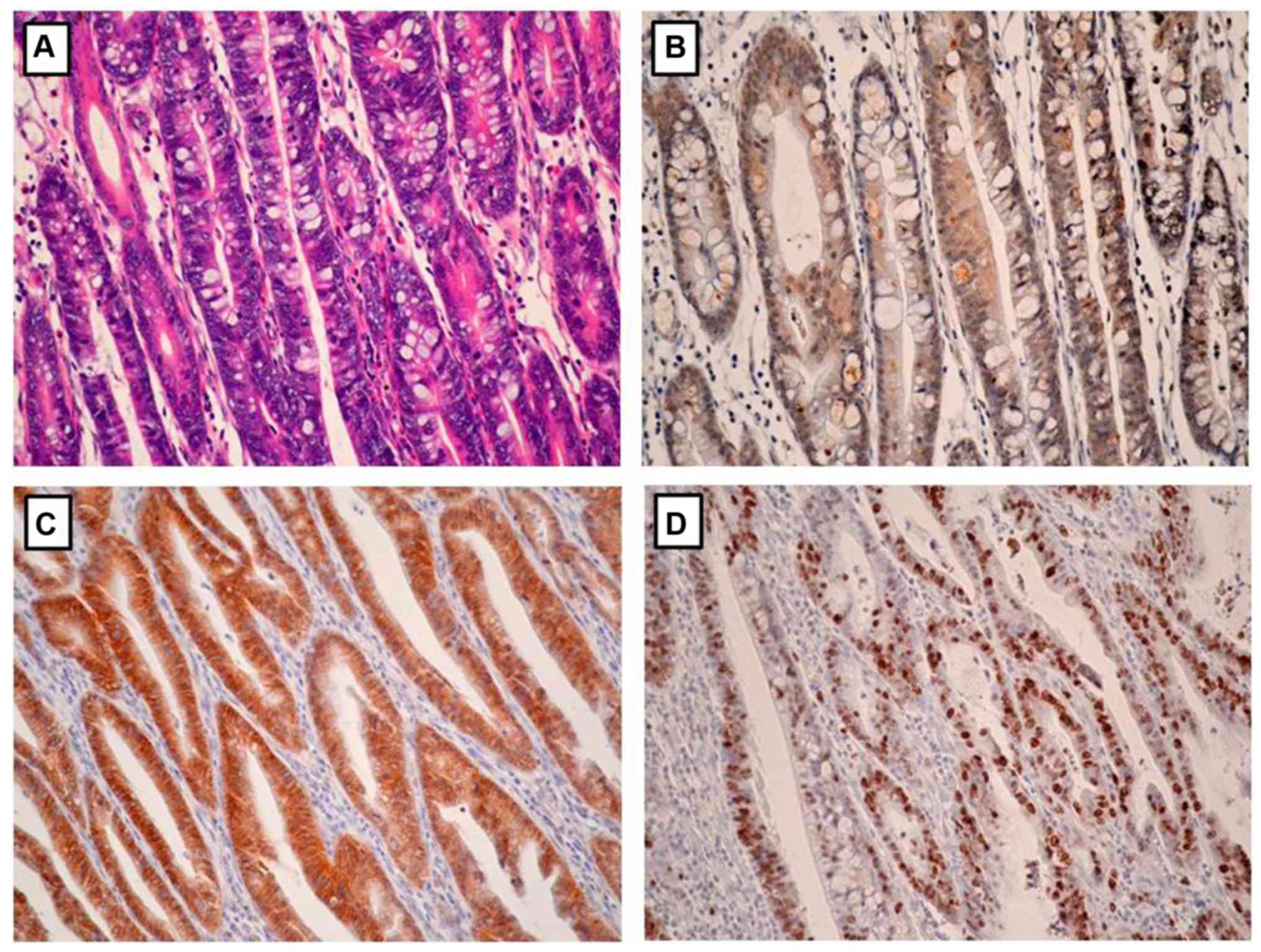
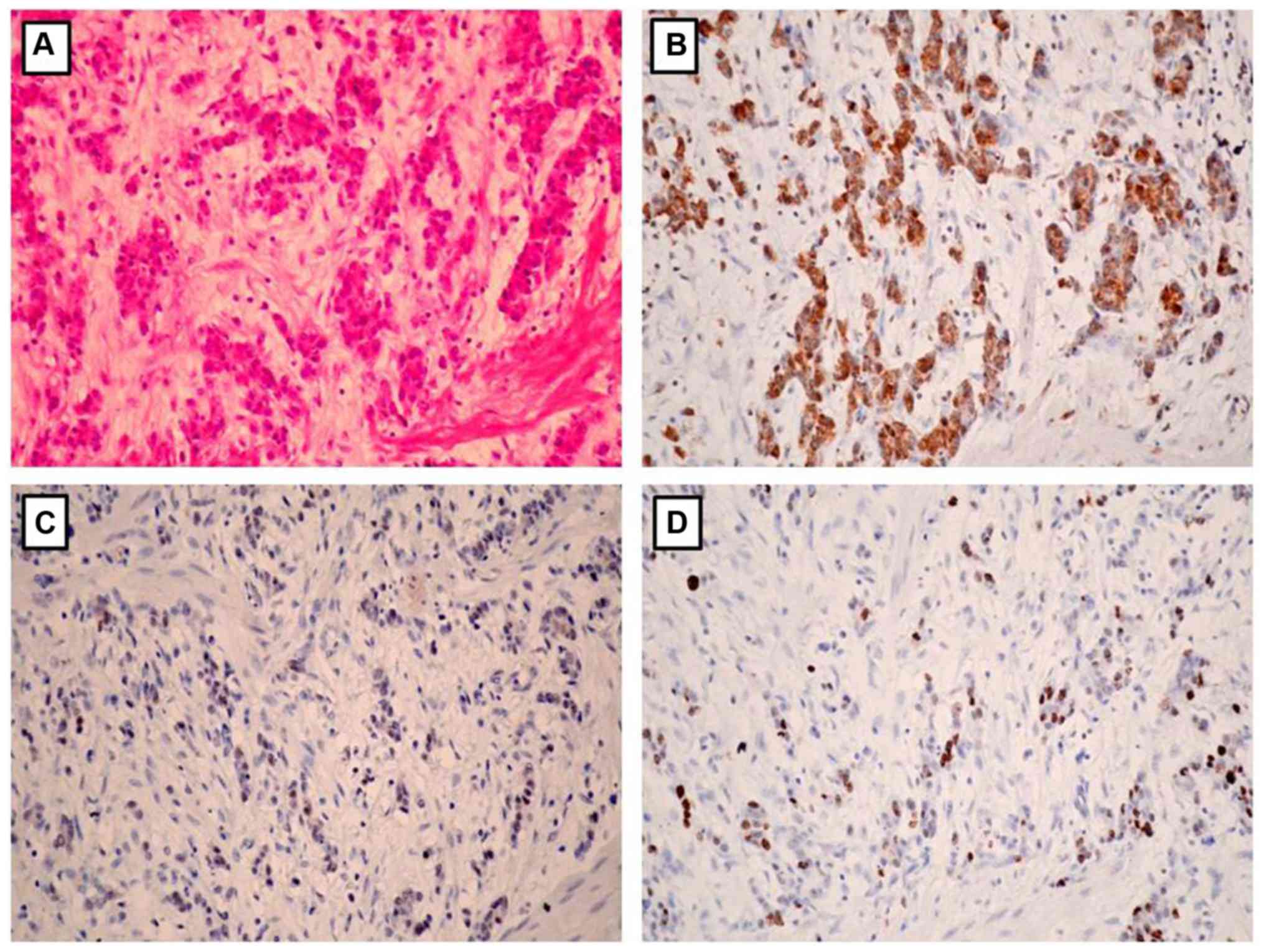
Association of EphA5 expression with
clinicopathological parameters
The association between EphA5 expression and
clinicopathological parameters was statistically analyzed (Table I). EphA5 expression was significantly
associated with Lauren classification (P=0.032), lymph node
metastasis (P<0.001), HER2 expression (P=0.020) and Ki-67
expression (P=0.005). Notably, EphA5 expression was negatively
associated with HER2 expression and Ki-67 index in the gastric
cancer tissues (Figs. 2 and 3). No significant association was
determined between EphA5 expression and age, sex, primary location,
depth of invasion and TNM stage.
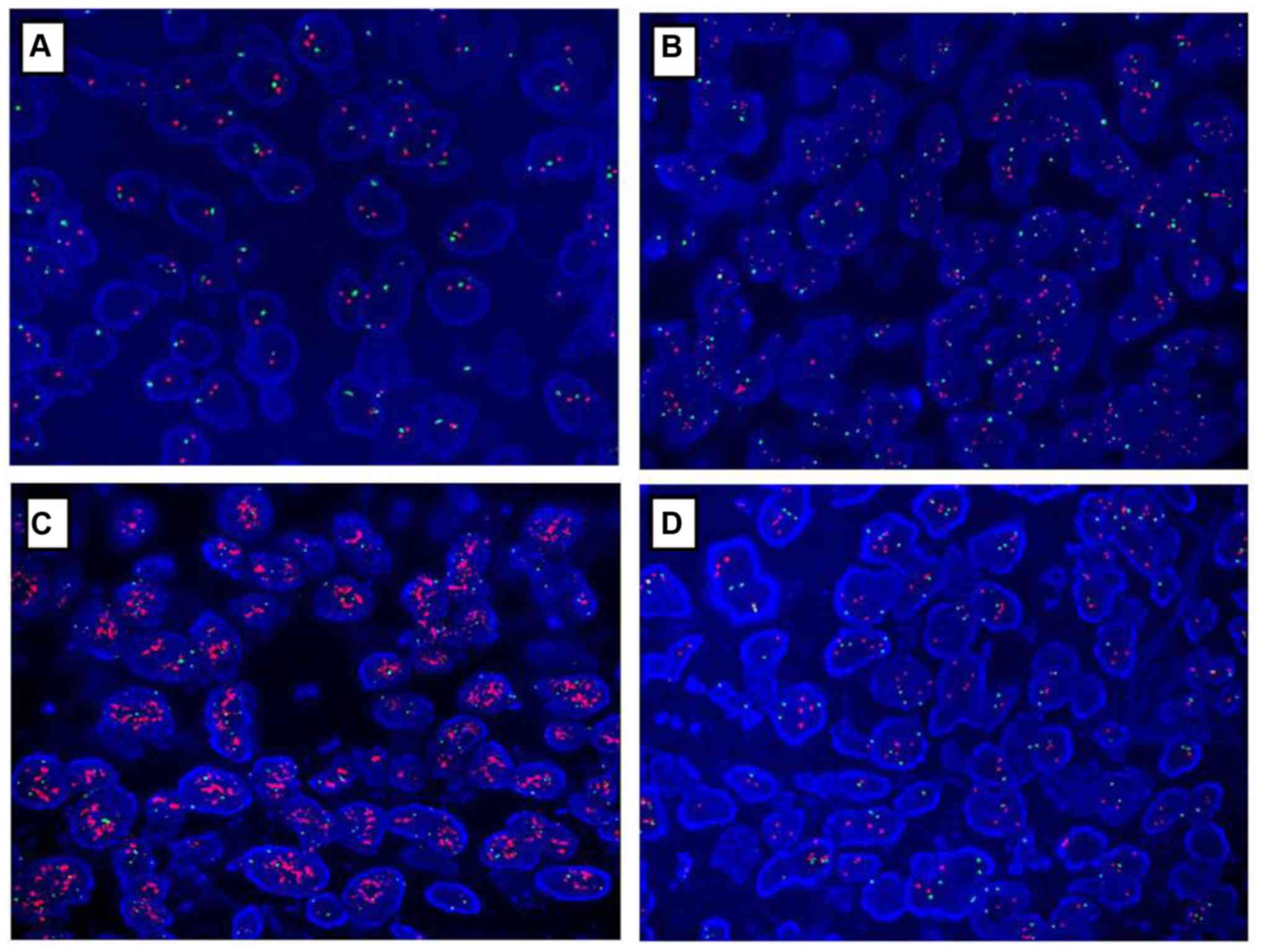
Confirmation of HER2 amplification by
FISH
HER2 status in gastric cancer specimens was
confirmed by FISH analysis as depicted in Table II and Fig. 4. A total of 96 gastric cancer samples
that were HER2-negative (score of 0 or 1+), as determined by IHC,
were also negative for HER2 amplification as confirmed by FISH. Out
of five samples that had an IHC score of 2+, four of them
demonstrated HER2 amplification by FISH, and one of these samples
exhibited polyploidy. A total of nine samples with an IHC score of
3+ demonstrated HER2 amplification by FISH.
 | Table II.Human epidermal growth factor
receptor 2 status in gastric cancer detected by IHC and FISH. |
Table II.
Human epidermal growth factor
receptor 2 status in gastric cancer detected by IHC and FISH.
|
| IHC score (24) |
|---|
|
|
|
|---|
| Parameter | 0 | 1+ | 2+ | 3+ |
|---|
| FISH-negative | 9 | 6 | 1 | 0 |
| FISH-positive | 0 | 0 | 4 | 9 |
| Total no. | 9 | 6 | 5 | 9 |
Discussion
In the present study, the expression of EphA5 in
gastric cancer tissues and its association with clinicopathological
parameters were examined. EphA5 protein was expressed in all
non-tumor gastric epithelia and was differentially expressed among
gastric cancer tissue samples. EphA5 was negatively expressed in
30/110 (27.3%) and positively expressed in 80/110 (72.3%) samples
from patients with gastric cancer. Although the specificity of the
anti-EphA5 antibody was confirmed in an ovarian cancer study
(21), the absence of a positive
control is a limitation of the present study. EphA5 protein
expression was reduced in the majority of gastric cancer tissue
samples, compared with non-tumor gastric epithelia. Gene
downregulation in cancer can be attributed to a number of molecular
mechanisms, including mutation, deletion, hypermethylation and
microRNA regulation. Aberrant methylation of CpG islands in the
promoter region of genes is a frequent mechanism for their
downregulation in cancer (26–29).
Hypermethylation of the CpG island in the EphA5
promoter has been reported in human cancer types (19). EphA5 mRNA expression has been
detected in breast cancer cell lines and human breast cancer
(19). EphA5 transcripts have been
detected in the HBL-100 human breast epithelial galactophore cell
line, and the ZR-75–30 and SKBR-3 breast cancer cell lines, while
reduction of EphA5 expression has been observed in the MCF-7, T47D,
MDA-MB-231, MDA-MB-435S and Bacp37 breast cancer cell lines
(19). In primary breast cancer
tissues, EphA5 mRNA levels were determined to be reduced in 67% of
tumor cases, compared with corresponding normal breast tissues.
Hypermethylation of the CpG island in the EphA5 promoter was
detected in breast cancer cell lines by direct sequencing following
bisulfite modification of genomic DNA. Epigenetic silencing of the
EphA5 gene was also confirmed in primary breast cancer by
methylation-specific polymerase chain reaction (19). Downregulation and promoter
hypermethylation of EphA5 have also been observed in human prostate
cancer (20). EphA5 expression was
negatively detected in 27.3% and weakly detected in 45.4% gastric
cancer cases. It was determined that hypermethylation of CpG island
in the promoter region of EphA5 is one of the molecular mechanisms.
The expression profile in gastric cancer cell lines was not
checked, based on reports that human cell lines used in China may
be contaminated (30), which is a
limitation of the present study. In the present study, the
methylation status of the CpG island within the EphA5 promoter
region in gastric cancer cell lines and tissues was not examined,
which is another limitation of the present study. In future
studies, the methylation status of this promoter in gastric cancer
tissue samples will be examined and the association between
methylation status and clinicopathological parameters will be
investigated.
The present data demonstrated that EphA5 expression
is associated with Lauren classification and lymph node metastasis.
EphA5 expression may therefore have clinical utility as an
indicator for lymph node metastasis. HER2 overexpression is
increasingly recognized as a frequent molecular event in gastric
cancer. The study by Bang et al (2) indicated that the addition of the
HER2-targeted agent trastuzumab with chemotherapy significantly
improved the survival of patients with HER2-positive metastatic
gastric cancer. HER2 expression status is also associated with
patient outcome (31). The present
data indicated that EphA5 expression is negatively associated with
HER2 expression. Notably, a recent study (32) demonstrated that the EphA1/EphA2
signaling axis regulates glutamine metabolism in HER2-positive
breast cancer. Future studies are now warranted to elucidate the
crosstalk between EphA5 and HER2.
Ki-67 is a nuclear protein associated with
proliferation that is an established prognostic biomarker in
various human tumor types, including breast cancer, lymphoma and
neuroendocrine neoplasia (33–35).
Previously, a high Ki-67 index was reported to predict reduced
disease-free and overall survival in intestinal-type gastric cancer
(35) and advanced gastric cancer
(33). In the present study, it was
determined that EphA5 expression was inversely associated with the
Ki-67 index. The present data indicated that EphA5 may be a
molecular marker for gastric cancer metastasis. In the present
study, the significance of EphA5 expression in survival was not
analyzed due to the tissue specimens analyzed being from patients
who underwent surgery recently. In the future, the association
between EphA5 expression and survival in gastric cancer will be
investigated and its utility in the prognosis of this disease will
be evaluated.
In conclusion, EphA5 is differentially expressed in
gastric cancer. Downregulation of EphA5 was determined in
approximately one third of gastric cancer cases, compared with
normal gastric epithelia. Hypermethylation of the CpGisland within
the EphA5 promoter provides a possible mechanism for this
downregulation. The present data indicated that EphA5 may function
as a tumor suppressor in gastric cancer. EphA5 may therefore be a
potential therapeutic target and a metastatic marker in gastric
cancer.
Acknowledgements
The authors would like to thank Dr James Monypenny
(Biochemistry-Cancer Research UK London Research Institute) for
editing the original English grammar within this manuscript.
Funding
This study was supported in part by the National
Natural Science Foundation of China (grant no. 81371611) and the
National Basic Research Priorities Program 973 Project (grant no.
2014CB744501) from the Ministry of Science and Technology of
China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ collected tissue samples. XW conducted FISH. SG
conducted IHC. JW analyzed the data. JL designed the project. HW
interpreted the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Taixing People's Hospital (Taixing, China) and Jinling Hospital
(Nanjing, China).
Patient consent for publication
The manuscript was approved by all patients for
publication in Oncology Letters.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szöllösi J, Balázs M, Feuerstein BG, Benz
CC and Waldman FM: ERBB-2 (HER2/neu) gene copy number, p185HER-2
overexpression, and intratumor heterogeneity in human breast
cancer. Cancer Res. 55:5400–5407. 1995.PubMed/NCBI
|
|
4
|
Ramieri MT, Murari R, Botti C, Pica E,
Zotti G and Alo PL: Detection of HER2 amplification using the SISH
technique in breast, colon, prostate, lung and ovarian carcinoma.
Anticancer Res. 30:1287–1292. 2010.PubMed/NCBI
|
|
5
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shitara K, Yatabe Y, Matsuo K, Sugano M,
Kondo C, Takahari D, Ura T, Tajika M, Ito S and Muro K: Prognosis
of patients with advanced gastric cancer by HER2 status and
trastuzumab treatment. Gastric Cancer. 16:261–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An E, Ock CY, Kim TY, Lee KH, Han SW, Im
SA, Kim TY, Liao WL, Cecchi F, Blackler A, et al: Quantitative
proteomic analysis of HER2 expression in the selection of gastric
cancer patients for trastuzumab treatment. Ann Oncol. 28:110–115.
2017.PubMed/NCBI
|
|
8
|
Zhou R, Copeland TD, Kromer LF and Schulz
NT: Isolation and characterization of Bsk, a growth factor
receptor-like tyrosine kinase associated with the limbic system. J
Neurosci Res. 37:129–143. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Symonds AC, King CE, Bartlett CA, Sauvé Y,
Lund RD, Beazley LD, Dunlop SA and Rodger J: EphA5 and ephrin-A2
expression during optic nerve regeneration: A ‘two-edged sword’.
Eur J Neurosci. 25:744–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Washburn CP, Cooper MA and Zhou R:
Expression of the tyrosine kinase receptor EphA5 and its ligand
ephrin-A5 during mouse spinal cord development. Neurosci Bull.
23:249–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cooper MA, Crockett DP, Nowakowski RS,
Gale NW and Zhou R: Distribution of EphA5 receptor protein in the
developing and adult mouse nervous system. J Comp Neurol.
514:310–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unified nomenclature for Eph family
receptors and their ligands, the ephrins. Eph nomenclature
committee. Cell. 90:403–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Kataoka H, Suzuki M, Sato N,
Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M, et
al: Downregulation of EphA7 by hypermethylation in colorectal
cancer. Oncogene. 24:5637–5647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dawson DW, Hong JS, Shen RR, French SW,
Troke JJ, Wu YZ, Chen SS, Gui D, Regelson M, Marahrens Y, et al:
Global DNA methylation profiling reveals silencing of a secreted
form of Epha7 in mouse and human germinal center B-cell lymphomas.
Oncogene. 26:4243–4252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen P, Huang Y, Zhang B, Wang Q and Bai
P: EphA2 enhances the proliferation and invasion ability of LNCaP
prostate cancer cells. Oncol Lett. 8:41–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Wen J, Wang H, Guo Q, Shi S, Shi
Q, Zhou X, Liu Q, Lu G and Wang J: Loss of expression of EphB1
protein in serous carcinoma of ovary associated with metastasis and
poor survival. Int J Clin Exp Pathol. 7:313–321. 2013.PubMed/NCBI
|
|
17
|
Wu R, Wang H, Wang J, Wang P, Huang F, Xie
B, Zhao Y, Li S and Zhou J: EphA3, induced by PC-1/PrLZ,
contributes to the malignant progression of prostate cancer. Oncol
Rep. 32:2657–2665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Staquicini FI, Qian MD, Salameh A, Dobroff
AS, Edwards JK, Cimino DF, Moeller BJ, Kelly P, Nunez MI, Tang X,
et al: Receptor tyrosine kinase EphA5 is a functional molecular
target in human lung cancer. J Biol Chem. 290:7345–7359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu DY, Wang ZM, Wang BL, Chen L, Yang WT,
Shen ZZ, Huang W and Shao ZM: Frequent epigenetic inactivation of
the receptor tyrosine kinase EphA5 by promoter methylation in human
breast cancer. Hum Pathol. 41:48–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Zhu Y, Ma C, Qiu Z, Zhang X, Kang Z,
Wu Z, Wang H, Xu X, Zhang H, et al: Downregulation of EphA5 by
promoter methylation in human prostate cancer. BMC Cancer.
15:182015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Wang X, Wei X and Wang J: EphA5
protein, a potential marker for distinguishing histological grade
and prognosis in ovarian serous carcinoma. J Ovarian Res. 9:832016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu S, Feng J, Jin Q, Wang W and Zhang S:
Reduced expression of EphA5 is associated with lymph node
metastasis, advanced TNM stage, and poor prognosis in colorectal
carcinoma. Histol Histopathol. 32:491–497. 2017.PubMed/NCBI
|
|
23
|
Aaltonen LA and Hamilton SR: Pathology and
Genetics of Tumors of the Digestive System. The
Tumor-Node-Metastasis (TNM) System of the World Health Organization
Classification of Tumors. IARC Press; Lyon: 2000
|
|
24
|
Laurén P: Pekka Laurén and histological
classification of gastric carcinoma. Interview and comment by Timo
J Nevalainen. Gut. 62:1230–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woo CG, Ho WJ, Park YS, Park SR, Ryu MH,
Jung HY and Kang YK: A potential pitfall in evaluating HER2
immunohistochemistry for gastric signet ring cell carcinomas.
Pathology. 49:38–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cabrero M, Yu Y, Verma A, Yang H, Colla S,
Jia Y, Zheng H, Bohannan Z, Ganan-Gomez I, Futreal A, et al:
Downregulation of protection of telomeres 1 expression in
myelodysplastic syndromes with 7q deletion. Br J Haematol.
173:161–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coccaro N, Zagaria A, Orsini P, Anelli L,
Tota G, Casieri P, Impera L, Minervini A, Minervini CF, Cumbo C, et
al: RARA and RARG gene downregulation associated with EZH2 mutation
in acute promyelocytic-like morphology leukemia. Hum Pathol.
80:82–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shuai F, Wang B and Dong S: MicroRNA-204
inhibits the growth and motility of colorectal cancer cells by
downregulation of CXCL8. Oncol Res. 26:1295–1305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ulker D, Ersoy YE, Gucin Z, Muslumanoglu M
and Buyru N: Downregulation of SCARA5 may contribute to breast
cancer via promoter hypermethylation. Gene. 673:102–106. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye F, Chen C, Qin J, Liu J and Zheng C:
Genetic profiling reveals an alarming rate of cross-contamination
among human cell lines used in China. FASEB J. 29:4268–4272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chua TC and Merrett ND: Clinicopathologic
factors associated with HER2-positive gastric cancer and its impact
on survival outcomes-a systematic review. Int J Cancer.
130:2845–2856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Youngblood VM, Kim LC, Edwards DN, Hwang
Y, Santapuram PR, Stirdivant SM, Lu P, Ye F, Brantley-Sieders DM
and Chen J: The Ephrin-A1/EPHA2 signaling axis regulates glutamine
metabolism in HER2-positive breast cancer. Cancer Res.
76:1825–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang G, Chen S, Wang D, Wang R, Lin L,
Chen S, Wang L and Huang Q: High Ki67 expression has prognostic
value in surgically-resected T3 gastric adenocarcinoma. Clin Lab.
62:141–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yagi T, Inoue N, Yanai A, Murase K,
Imamura M, Miyagawa Y, Enomoto Y, Nishimukai A, Takatsuka Y, Hirota
S, et al: Prognostic significance of geminin expression levels in
Ki67-high subset of estrogen receptor-positive and HER2-negative
breast cancers. Breast Cancer. 23:224–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Min KW, Kim DH, Son BK, Kim DH, Kim EK,
Seo J, Ahn SB, Jo YJ, Park YS and Ha J: A High Ki67/BCL2 index
could predict lower disease-free and overall survival in
intestinal-type gastric cancer. Eur Surg Res. 58:158–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|