Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumour types worldwide and has the highest
incidence rates of all types of cancer (1). In general, HCC development is silent
and is, therefore, usually diagnosed only when it reaches the late
stage of disease (2,3). Although HCC treatment has made marked
progress with the development of modern medical science and
technology, there are a limited number of effective treatment
options. Surgical resection and liver transplantation remain the
first choices for treating HCC (4).
The 5-year survival rate following hepatectomy can reach 50–70%
(5–7), but >70% of patients with HCC
following surgery will have tumour recurrence (8). Therefore, identifying effective
predictors of recurrence and metastasis following HCC surgery is
important for guiding postoperative treatment and improving patient
prognosis.
In previous years, tumour necrosis factor-α (TNF-α),
interleukin (IL)-6, IL-17, IL-10 and other cytokines associated
with inflammation or immunity have received extensive attention.
These cytokines can promote and/or inhibit tumour development
(9), and they are important for
evaluating the risk of recurrence and long-term survival following
HCC surgery. Among these, TNF-α is a pro-inflammatory cytokine that
has a variety of biological activities and is produced primarily by
macrophages and monocytes (10).
TNF-α serves an important role in tumour development and
progression. Moore et al (11) reported that TNF-α serves a role in
promoting skin cancer development; however, Joseph et al
(12) identified that TNF-α can
cause damage to tumour vascular endothelial cells, resulting in
blood vessel rupture, dysfunction or thrombosis. Effects such as
these can block local blood flow to the tumour tissue and cause
haemorrhage or hypoxic necrosis. These conflicting studies suggest
that the role of TNF-α in tumours needs to be investigated further.
Nevertheless, the conclusion that TNF-α is associated with
tumorigenesis, development, recurrence and metastasis is
consistent.
The mitogen-activated protein kinase (MAPK) family
is an important signal transduction system in cells. This family
includes extracellular signal regulated protein kinase, p38MAPK and
c-Jun N-terminal kinase (13). The
p38MAPK signalling pathway is an important part of the MAPK cascade
and leads to different biological functions by mediating signal
transduction (14). There is
evidence that TNF-α can activate MAPK kinase kinase (MAPKKK) and
ultimately activate p38MAPK and induce apoptosis (15). Furthermore, Meldrum et al
(16) reported that p38MAPK can
induce apoptosis by increasing TNF-α expression. In addition,
Valladares et al (17)
revealed that p38MAPK-mediated TNF-α induced apoptosis and cell
cycle arrest in rat embryonic brown adipose tissue. The
aforementioned studies indicate that TNF-α and p38MAPK are involved
in apoptosis regulation and possibly tumour inhibition. TNF-α
induces apoptosis by activating p38MAPK, and p38MAPK enhances TNF-α
expression to induce apoptosis, which constitutes a positive
feedback pathway.
The present study used an integrated bioinformatics
analysis based on two datasets available from the Oncomine™
database (www.oncomine.org) to determine the
association between TNF-α and/or p38MAPK expression and prognosis
in HCC samples. TNF-α and p38MAPK expression was investigated in
patients with HCC, alongside the associations between TNF-α and
p38MAPK expression and clinicopathological characteristics and
prognosis.
Materials and methods
Oncomine™ database
Oncomine™ is a bioinformatics database with an
abundance of collected and standardized DNA microarray data. As an
analysis platform, Oncomine™ facilitates functional discovery using
genome-wide expression analyses (18,19). The
present study used the Oncomine™ database to collect data on TNF-α
and p38 gene abundance values and HCC prognosis. By searching
‘Gene: TNF (Search: TNF-alpha) or MAPK14 (Search: p38)’; ‘Cancer
Type: Hepatocellular Carcinoma’; and ‘Clinical Outcome: Survival
Status or Recurrence Status’ and setting ‘P-value: <0.0001’;
‘Fold Change: 2’; and ‘Gene rank: = top 10%’, two datasets were
obtained: Guichard Liver and Guichard Liver 2. A total of 74
samples in the Guichard Liver dataset had complete follow-up data,
whereas 25 samples in the Guichard Liver 2 dataset had complete
follow-up data. More importantly, none of these data had detailed
Tumour-Node-Metastasis (TNM) staging (20). The data were divided into high and
low TNF-α- and p38MAPK-expression groups on the basis of the median
of the abundance values. SPSS software (version 19.0; IBM Corp.,
Armonk, NY, USA) was used to perform the survival analysis.
Patient information
Paraffin-embedded specimens that had been surgically
removed from 83 patients with HCC between January 2000 and December
2012 at the Department of Hepatobiliary and Pancreatic Surgery of
The Affiliated Hospital of Qingdao University (Qingdao, China) were
retrospectively collected. The patients included 67 males and 16
females with a mean age of 55.8 years (range, 31–83 years). The
inclusion criteria were as follows: i) Postoperative pathological
diagnosis of HCC, and ii) T1N0M0
stage. The exclusion criteria were as follows: i) Anticancer
treatment prior to surgery, ii) serious complications or death
within 30 days following surgery or iii) unavailable follow-up
information. The study was approved by the Ethics Committee of The
Affiliated Hospital of Qingdao University. Written informed consent
was obtained from all patients.
Immunohistochemistry
Immunuohistochemistry (IHC)was performed as
described previously (21). Briefly,
paraffin-embedded specimens obtained from patients with HCC were
cut into 4-µm-thick sections. Paraffin was removed with xylene
(Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) for 15
min and the sections were rehydrated through a graded alcohol
series (anhydrous ethanol I, 5 min; anhydrous ethanol II, 5 min;
95% ethanol, 3 min; 90% ethanol, 3 min; 80% ethanol, 2 min; and 70%
ethanol, 2 min) (Shanghai Macklin Biochemical Co., Ltd.). Following
antigen retrieval with citrate buffer (10 mM, pH 6.0), endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 10
min. Subsequently, the paraffin slides were stained with anti-TNF-α
(1:100 dilution; catalogue no. Ab6671; Abcam, Cambridge, MA, USA)
or anti-p38 (1:50 dilution; catalogue no. Ab197348; Abcam) primary
antibodies at 4°C overnight. The MaxVision kit (Fuzhou Maixin
Biotech Co., Ltd., Fujian, China) was used to detect primary
antibodies, and the colour was developed using
3,3′-diaminobenzidine chromogen substrate. The slides were
counterstained with haematoxylin for 1 min, cleared in water and
mounted with neutral balsam.
IHC evaluation
As described previously (21), two pathologists at the Affiliated
Hospital of Qingdao University (Qingdao, China) who had been
blinded to the clinical data interpreted the results
simultaneously. Under a light microscope, markedly stained cells
exhibited brown staining in the cytoplasm. Overall, 10 fields were
observed under high magnification (magnification, ×400), and the
degree of immunostaining was reviewed and scored according to the
intensity of staining and the percentage of immunoreactive cells.
Staining intensity was graded according to the following criteria:
Cells without staining were scored as 0, whereas that stained light
yellow, yellowish brown and brown were scored as 1, 2 and 3 points,
respectively. The extent of immunoreactivity was graded as follows:
<5%, ≥5%, ≥26%, ≥51% and ≥75% were scored as 0, 1, 2, 3 and 4
points, respectively. The two scores were summed, and a total score
of ≤4 represented low expression, whereas >4 represented high
expression. All patients were divided into high- and low-expression
groups according to the IHC score results. Patients with high
expression levels of both TNF-α and p38MAPK were included in the
high-expression group, whereas those with low expression of both
TNF-α and p38MAPK were included in the low-expression group.
Patients with high TNF-α and low p38MAPK expression and patients
with low TNF-α and high p38MAPK expression were included in the
TNF-α high-expression group and the p38MAPK high-expression group,
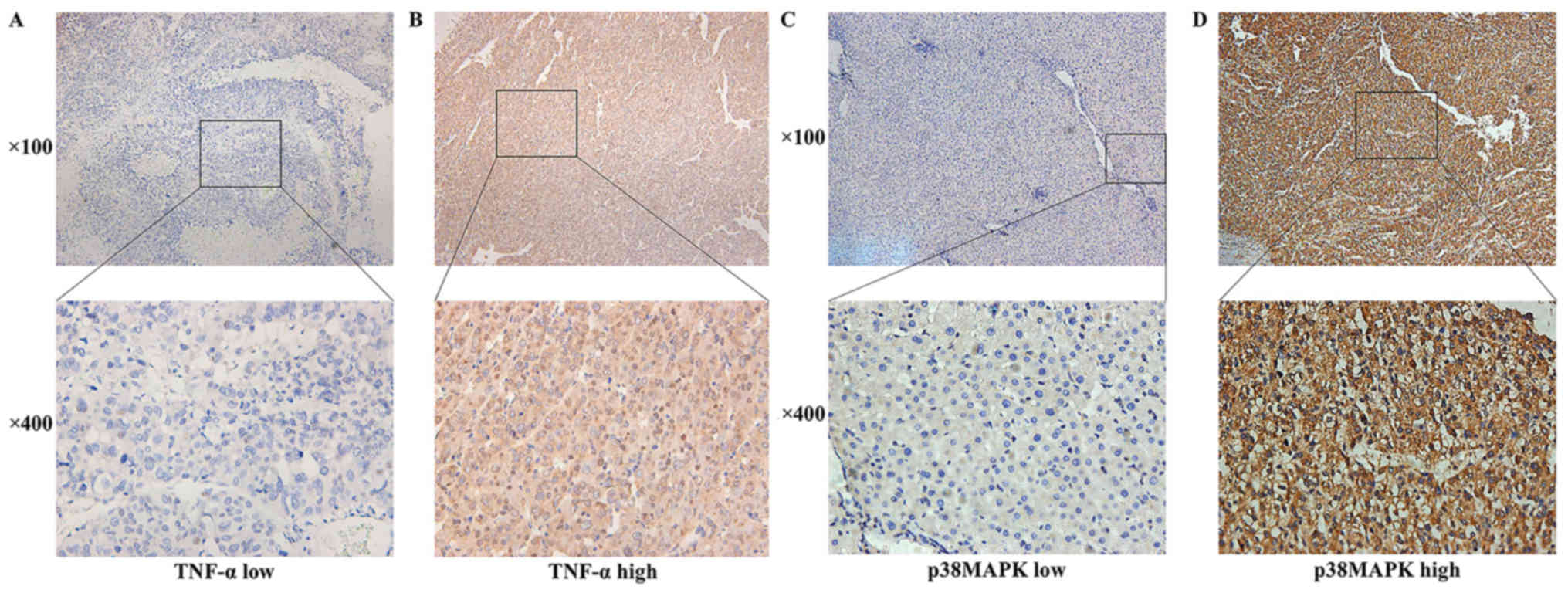
respectively. Representative images are presented in Fig. 1.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). The χ2 test, continuous
correction χ2 test or Fisher's exact test was used to analyse the
association between TNF-α or p38MAPK expression and the
clinicopathological characteristics. The association between TNF-α
and p38MAPK expression was determined by Pearson's contingency
analysis. The data were censored at the last follow-up (March 2017)
for patients without recurrence, metastases or mortality.
Disease-free survival (DFS) and overall survival (OS) rates were
assessed using Kaplan-Meier curves. The log-rank test was used to
compare TNF-α and/or p38MAPK expression with recurrence and
survival. The Cox proportional hazards model was used to screen
variables for unilateral and multivariate analyses of HCC
prognosis. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
TNF-α/p38MAPK expression in HCC and
clinicopathological characteristics
By reference to the Oncomine™ database, TNF-α and
p38MAPK exhibited high expression rates in the HCC samples. TNF-α
and p38MAPK were positively expressed in 50.51 (50/99) and 50.51%
(50/99) of the samples, respectively.
In the T1N0M0 HCC
tissues, TNF-α and p38MAPK were distributed in a diffuse manner and
were present primarily in the cytoplasm of the tumour cells
(Fig. 1). TNF-α and p38MAPK were
highly expressed in the HCC microenvironment. TNF-α and p38MAPK
were positively expressed in 62.65 (52/83) and 69.88% (58/83) of
the samples, respectively.
The association between TNF-α/p38MAPK expression in
the HCC microenvironment and various clinicopathological
characteristics of the patients with HCC were analysed using the
χ2 test as presented in Table
I. High p38MAPK expression in the HCC microenvironment was
significantly associated with low aspartate aminotransferase (AST)
levels (P=0.026; Table I). However,
no significant associations were observed between TNF-α or p38MAPK
expression and the other clinicopathological features. Pearson's
contingency analysis indicated a positive association between TNF-α
and p38MAPK expression (r=0.253; P=0.021; Table II).
 | Table I.Association between TNF-α/p38MAPK
expression and clinicopathological characteristics. |
Table I.
Association between TNF-α/p38MAPK
expression and clinicopathological characteristics.
|
|
| TNF-α | p38MAPK |
|---|
|
|
|
|
|
|---|
| Parameter | n (%) | High | Low | P-value | High | Low | P-value |
|---|
| Sex |
|
|
| 0.989a |
|
| 0.846b |
|
Male | 67 (80.7) | 42 | 25 |
| 46 | 21 |
|
|
Female | 16 (19.3) | 10 | 6 |
| 12 | 4 |
|
| Age, years |
|
|
| 0.803a |
|
| 0.567a |
|
<50 | 20 (24.1) | 13 | 7 |
| 15 | 5 |
|
|
≥50 | 63 (75.9) | 39 | 24 |
| 43 | 20 |
|
| Alcohol abuse |
|
|
| 0.835a |
|
| 0.268a |
|
Yes | 23 (27.7) | 14 | 9 |
| 14 | 9 |
|
| No | 60 (72.3) | 38 | 22 |
| 44 | 16 |
|
| HBV infection |
|
|
| 0.919b |
|
| 0.169b |
|
Yes | 74 (89.2) | 47 | 27 |
| 54 | 20 |
|
| No | 9 (10.8) | 5 | 4 |
| 4 | 5 |
|
| TBIL level,
µmol/l |
|
|
| 0.684b |
|
| 1.000b |
|
≤22 | 72 (86.7) | 44 | 28 |
| 50 | 22 |
|
|
>22 | 11 (13.3) | 8 | 3 |
| 8 | 3 |
|
| ALB level, g/l |
|
|
| 0.530b |
|
| 0.169b |
|
<35 | 9 (10.8) | 7 | 2 |
| 4 | 5 |
|
|
≥35 | 74 (89.2) | 45 | 29 |
| 54 | 20 |
|
| ALT level, U/l |
|
|
| 0.722a |
|
| 0.542b |
|
≤60 | 68 (81.9) | 42 | 26 |
| 49 | 19 |
|
|
>60 | 15 (18.1) | 10 | 5 |
| 9 | 6 |
|
| AST level, U/l |
|
|
| 0.803a |
|
| 0.026a |
|
≤42 | 63 (75.9) | 39 | 24 |
| 48 | 15 |
|
|
>42 | 20 (24.1) | 13 | 7 |
| 10 | 10 |
|
| PLT level,
×109 cells/l |
|
|
| 0.304a |
|
| 0.467b |
|
<100 | 19 (22.9) | 10 | 9 |
| 12 | 7 |
|
|
≥100 | 64 (77.1) | 42 | 22 |
| 46 | 18 |
|
| Liver
cirrhosis |
|
|
| 1.000b |
|
| 0.871b |
|
Yes | 9 (10.8) | 6 | 3 |
| 7 | 2 |
|
| No | 74 (89.2) | 46 | 28 |
| 51 | 23 |
|
| AFP level,
ng/l |
|
|
| 0.779a |
|
| 0.257a |
|
≤400 | 63 (75.9) | 40 | 23 |
| 42 | 21 |
|
|
>400 | 20 (24.1) | 12 | 8 |
| 16 | 4 |
|
| Child-Pugh
grade |
|
|
| 1.000c |
|
| 0.301c |
| A | 82 (98.8) | 51 | 31 |
| 58 | 24 |
|
| B | 1 (1.2) | 1 | 0 |
| 0 | 1 |
|
| Tumor size, cm |
|
|
| 0.363b |
|
| 0.231b |
| ≤5 | 76 (91.6) | 46 | 30 |
| 55 | 21 |
|
|
>5 | 7 (8.4) | 6 | 1 |
| 3 | 4 |
|
| Tumor margin,
cm |
|
|
| 0.530b |
|
| 0.089b |
| ≤2 | 74 (89.2) | 45 | 29 |
| 49 | 25 |
|
|
>2 | 9 (10.8) | 7 | 2 |
| 9 | 0 |
|
| Pathological
differentiation |
|
|
| 0.694b |
|
| 0.377b |
|
High | 8 (9.6) | 4 | 4 |
| 4 | 4 |
|
| Middle
and low | 75 (90.4) | 48 | 27 |
| 54 | 21 |
|
| Microvascular tumor
thrombus |
|
|
| 0.722a |
|
| 0.991b |
|
Yes | 15 (18.1) | 10 | 5 |
| 11 | 4 |
|
| No | 68 (81.9) | 42 | 26 |
| 47 | 21 |
|
| Capsule
invasion |
|
|
| 0.470b |
|
| 1.000c |
|
Yes | 76 (91.6) | 49 | 27 |
| 53 | 23 |
|
| No | 7 (8.4) | 3 | 4 |
| 5 | 2 |
|
 | Table II.Association between the expression of
TNF-α and p38MAPK. |
Table II.
Association between the expression of
TNF-α and p38MAPK.
|
| TNF-α | Pearson's
contingency |
|---|
|
|
|
|
|---|
| p38MAPK | High | Low | Coefficient | P-value |
|---|
| High | 41 | 17 | 0.253 | 0.021 |
| Low | 11 | 14 |
|
|
Survival analysis
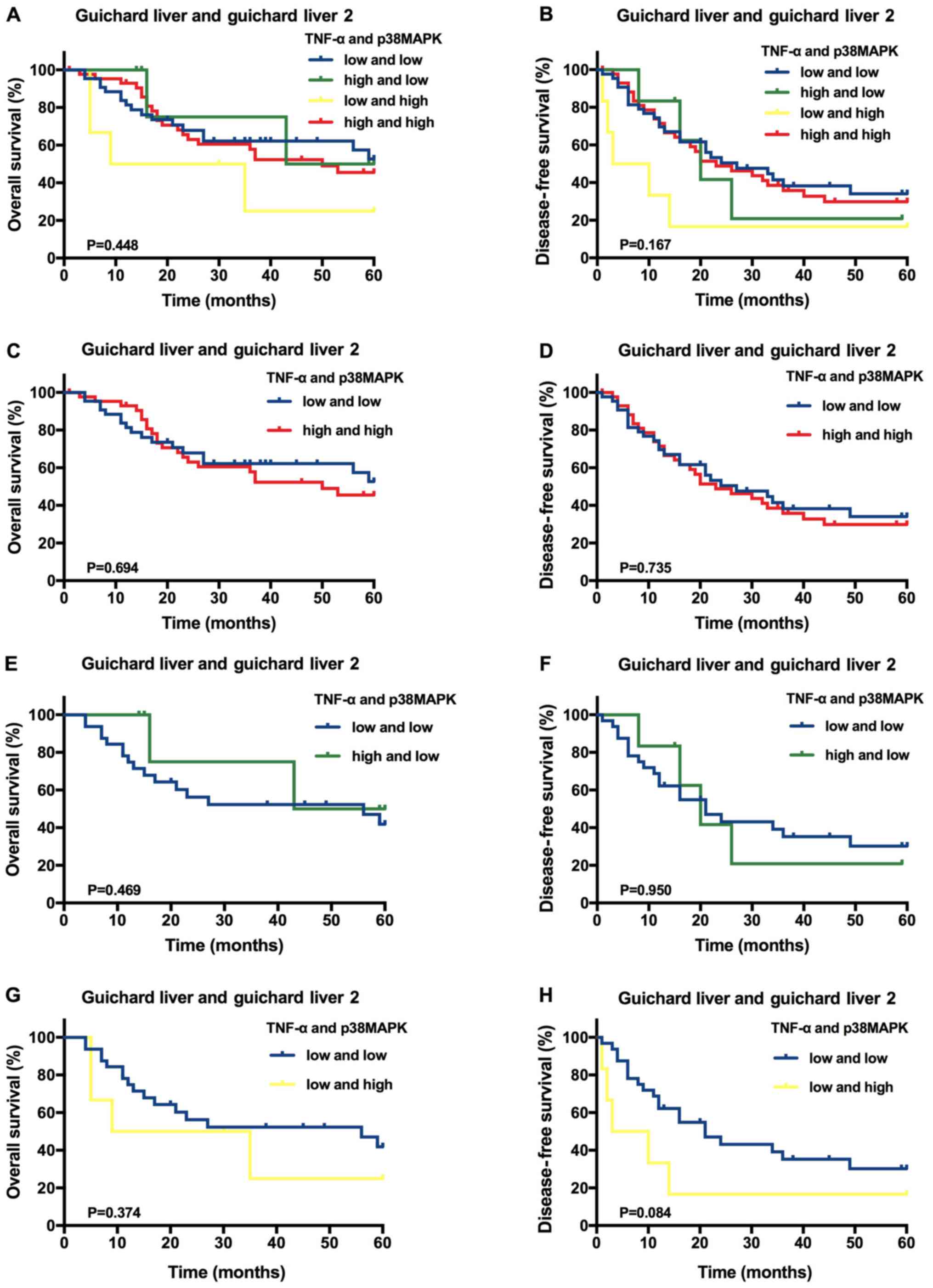
Using the Oncomine™ datasets, it was identified that
the average follow-up time was 33.19±20.64 months (range, 1.0–60.0
months), and the 1-, 3- and 5-year OS rates were 83.84, 45.45 and
24.24%, respectively. The DFS rates at 1, 3 and 5 years were 70.00,
29.30 and 16.16%, respectively. A Kaplan-Meier analysis of 99 HCC
samples indicated that, compared with the other expression
categories, high TNF-α and p38MAPK expression levels were not
significantly associated with higher OS or DFS rates (P>0.05;
Fig. 2A and B). Furthermore,
compared with HCC samples with low expression of both TNF-α and
p38MAPK, the samples with high expression of either TNF-α or
p38MAPK, as well as those with high expression of both TNF-α and
p38MAPK, were not significantly associated with improved OS and DFS
rates (P>0.05; Fig. 2C-H).
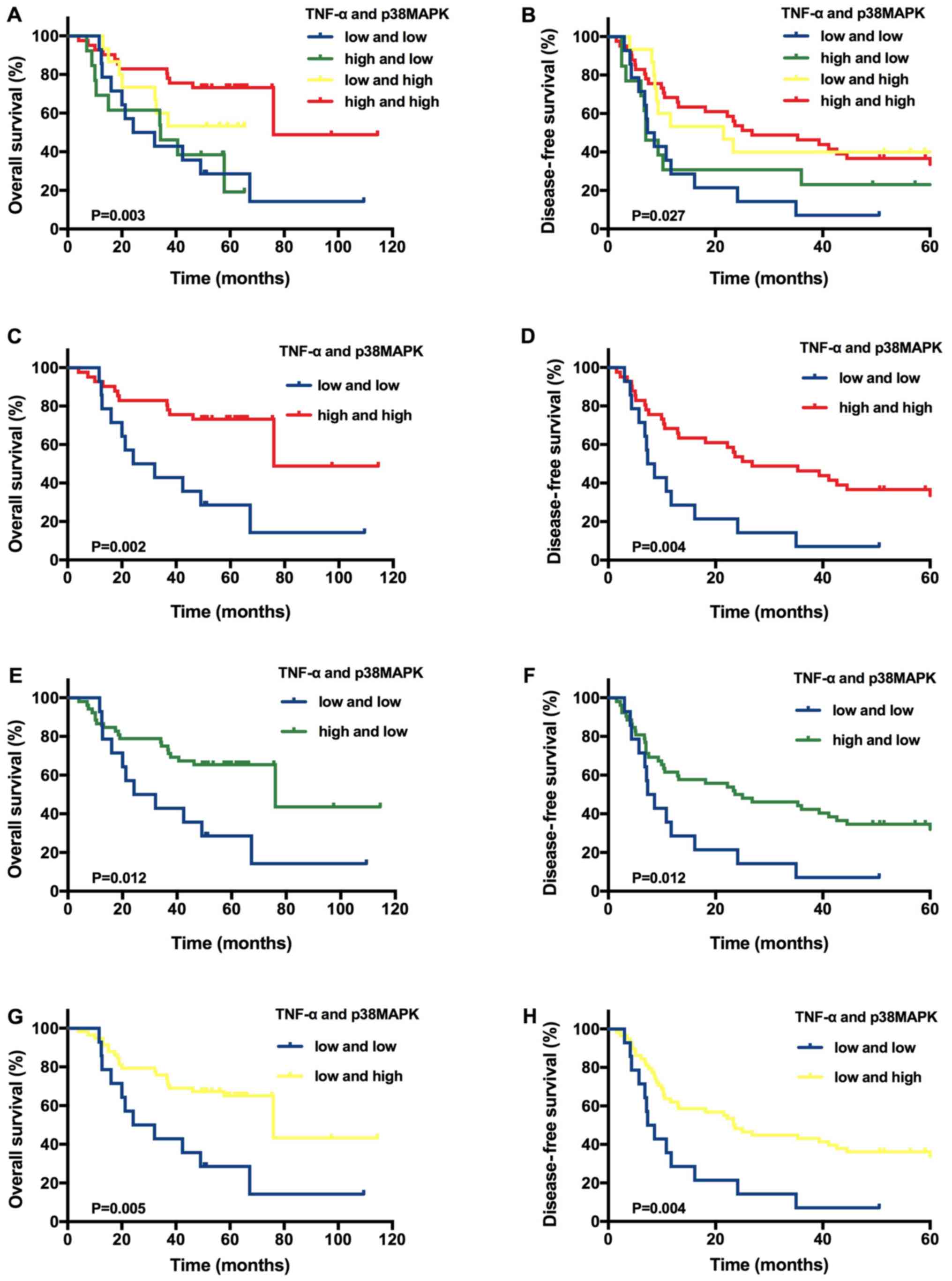
However, in the tissues from patients with
T1N0M0 HCC, the average follow-up
time was 44.85±24.11 months (range, 4.0–114.5 months), and the 1-,
3- and 5-year OS rates were 90.36, 66.27 and 31.33%, respectively.
The DFS rates at 1, 3 and 5 years were 53.01, 36.14 and 19.28%,
respectively. A Kaplan-Meier analysis of 83 patients with
T1N0M0 HCC indicated significantly
improved OS (P=0.003) and DFS (P=0.027) rates for the group with
high levels of both TNF-α and p38MAPK expression compared with the
other groups (Fig. 3A and 3B). On the basis of these results, another
Kaplan-Meier analysis was performed using 83 patients with
T1N0M0 HCC. Patients with high
expression levels of both TNF-α and p38MAPK had higher OS (P=0.002)
and DFS (P=0.004) rates compared with patients with low expression
levels of both TNF-α and p38MAPK (Fig.
3C and D). Furthermore, the patients with high TNF-α expression
alone had improved OS (P=0.012) and DFS (P=0.012) rates compared
with patients with low expression levels of both TNF-α and p38MAPK
(Fig. 3E and F). The patients with
high p38MAPK expression alone had higher OS (P=0.005) and DFS
(P=0.004) rates compared patients with low expression levels of
both TNF-α and p38MAPK (Fig. 3G and
H).
In addition, multiple univariate and multivariate
Cox proportional hazards analyses were performed, including
analyses of the high TNF-α only, high p38MAPK only, and high TNF-α
and p38MAPK expression groups. The multivariate analysis of the
TNF-α-expression group indicated that no hepatitis B virus
infection (P=0.019), platelets ≥100×109 cells/l
(P=0.031) and high TNF-α expression (P=0.0497) were independent
predictive factors for an improved OS rate (Table III). Tumour size ≤5 cm (P=0.044),
no microvascular tumour thrombus (P=0.010) and high TNF-α
expression (P=0.005) also independently indicated an improved DFS
rate (Table IV). Furthermore, the
multivariate analysis of the p38MAPK-expression group identified
that high p38MAPK expression (P=0.004) was an independent
predictive factor for a higher OS rates (Table III). No microvascular tumour
thrombus (P=0.031) and high p38MAPK expression (P=0.003) were
independent predictive factors for a higher DFS rates (Table IV). In addition, the multivariate
Cox regression model of the TNF-α and p38MAPK-expression group
(both low vs. both high) revealed that no microvascular tumour
thrombus (P=0.025 and P=0.011, respectively) and high TNF-α and
p38MAPK expression (P=0.001 and P=0.002, respectively) were
independent indicators for improved OS and DFS rates (Tables III and IV, respectively).
 | Table III.Multivariate analysis of variables
potentially associated with overall survival (Cox regression
model). |
Table III.
Multivariate analysis of variables
potentially associated with overall survival (Cox regression
model).
| Variable | HR (95% CI) | P-value |
|---|
| TNF-α |
|
|
| HBV
infection (no vs. yes) | 3.296
(1.212–8.967) | 0.019 |
| PLT
level (<100 vs. ≥100×109 cells/l) | 0.417
(0.188–0.921) | 0.031 |
| TNF-α
(both low vs. TNF-α high) | 0.461
(0.213–0.999) |
0.0497 |
| p38MAPK |
|
|
| p38MAPK
(both low vs. p38MAPK high) | 0.332
(0.157–0.702) | 0.004 |
| TNF-α and
p38MAPK |
|
|
|
Microvascular tumor thrombus
(no vs. yes) | 2.863
(1.141–7.185) | 0.025 |
| TNF-α
and p38MAPK (both low vs. both high) | 0.251
(0.108–0.588) | 0.001 |
 | Table IV.Multivariate analysis of variables
potentially associated with disease-free survival (Cox regression
model). |
Table IV.
Multivariate analysis of variables
potentially associated with disease-free survival (Cox regression
model).
| Variable | HR (95% CI) | P-value |
|---|
| TNF-α |
|
|
| Tumor
size (≤5 cm vs. >5 cm) | 2.340
(1.022–5.356) | 0.044 |
|
Microvascular tumor thrombus
(no vs. yes) | 2.411
(1.235–4.707) | 0.010 |
| TNF-α
(both low vs. TNF-α high) | 0.621
(0.444–0.869) | 0.005 |
| p38MAPK |
|
|
|
Microvascular tumor thrombus
(no vs. yes) | 2.057
(1.068–3.964) | 0.031 |
| p38MAPK
(both low vs. p38MAPK high) | 0.372
(0.193–0.716) | 0.003 |
| TNF-α and
p38MAPK |
|
|
|
Microvascular tumor thrombus
(no vs. yes) | 2.500
(1.230–5.079) | 0.011 |
| TNF-α
and p38MAPK (both low vs. both high) | 0.337
(0.166–0.681) | 0.002 |
Discussion
In the present study, datasets from the Oncomine™
database were used to demonstrate that TNF-α and/or p38MAPK
expression was not significantly associated with OS and DFS.
However, there was no detailed TNM staging information in the
Oncomine™ datasets; therefore, additional patients were screened
according to their TNM stage and only patients with
T1N0M0 HCC were selected. A
positive association between TNF-α and p38MAPK expression in the
HCC tumour microenvironment was revealed. p38MAPK expression was
negatively associated with AST levels. In addition, OS and DFS
rates were significantly improved in patients with
T1N0M0 HCC with high expression
levels of both TNF-α and p38MAPK in the tumour microenvironment
compared with patients in the other groups. On the basis of these
results, a pairwise comparison was conducted. It was revealed that
the OS and DFS rates of patients with
T1N0M0 HCC with high TNF-α and/or
p38MAPK expression were improved compared with those of patients
with low TNF-α and p38MAPK expression. In addition, a multivariate
survival analysis indicated that high expression levels of TNF-α
only or p38MAPK only, as well as both TNF-α and p38MAPK, in the
T1N0M0 HCC microenvironment were
independent predictive factors for OS and DFS rates.
TNF-α was identified in the 1970s as a cytokine
secreted by immune cells that inhibits tumour cell growth and
induces degenerative changes in tumours (22,23).
There is evidence that TNF-α can promote p38MAPK signalling pathway
activation (24,25). More notably, Sabio and Davis
(26) indicated that MAPK activation
by TNF-α can also increase TNF-α expression in target cells. These
studies support the conclusion of the present study that TNF-α and
p38MAPK are positively associated in the HCC tumour
microenvironment (r=0.253; P=0.021). Additionally, Ichijo et
al (15) indicated that TNF-α
can induce apoptosis through activating p38MAPK, which may affect
the prognosis of patients with cancer. Meldrum et al
(16) also suggested that p38MAPK
can induce apoptosis by enhancing TNF-α expression. Therefore, it
can be hypothesized that TNF-α and p38MAPK are involved in
apoptosis regulation and may thus have inhibitory effects on
tumours. However, the mechanism through which TNF-α and p38MAPK
induce apoptosis and further inhibit tumours remains unclear and
requires further investigation.
It has been suggested that TNF-α released by host
and tumour cells is an important factor in the initiation,
proliferation, angiogenesis and metastasis of various types of
cancer, and that it has a promoting effect on tumours (27,28).
These suggestions were derived through investigation of the
inhibitory effect of TNF-α on tumour cell apoptosis induced by
nuclear factor-κB; however, the cytokine network in the body is a
complex system. TNF-α does not exert its effects on tumours through
a single pathway, but rather through multiple signal transductions.
For example, Ichijo et al (15) and Donnahoo et al (29) identified that TNF-α can also promote
apoptosis in tumour cells by interacting with p38MAPK. In the
present study, the TNF-α/p38MAPK/TNF-α signalling pathway was
investigated, which has not been widely studied to date. However,
the current study has indicated that patients with
T1N0M0 HCC with high TNF-α and/or
p38MAPK expression had improved OS and DFS rates compared with the
patients with low expression of both TNF-α and p38MAPK (P<0.05).
In addition, a multivariate survival analysis revealed that high
TNF-α and/or p38MAPK expression in the
T1N0M0 HCC microenvironment was an
independent predictive factor for OS and DFS (P<0.05).
Therefore, to obtain improved OS and DFS rates, individualized
treatment plans may be developed for patients with
T1N0M0 HCC on the basis of
postoperative TNF-α and p38MAPK expression levels. Then, according
to the specific circumstances, a recombinant human TNF-α or p38MAPK
activator could be injected into the liver of patients with
T1N0M0 HCC. Van Horssen et
al (30) identified that the
topical use of TNF-α in combination with chemotherapeutic drugs can
produce potent antitumour effects. However, relevant clinical
trials should be designed to verify this treatment concept to
further benefit patients with
T1N0M0 HCC.
In summary, the results of the present study have
revealed a positive association between TNF-α and p38MAPK
expression in the T1N0M0 HCC
microenvironment. It was also identified that high TNF-α and/or
p38MAPK expression is an independent predictive factor of OS and
DFS rates and that patients with
T1N0M0 HCC with high TNF-α and/or
p38 MAPK expression have a favourable prognosis. TNF-α and p38MAPK
could act as predictive biomarkers or potential therapeutic targets
for treatment patients with T1N0M0
HCC. Importantly, the results from the present study indicate that
patients with T1N0M0 HCC with low
TNF-α and p38MAPK expression require more frequent follow-up
observations. It should be noted that the present study is a
single-centre study with a small sample size, and more conclusive
results may be obtained by including more patients. In addition,
the treatment for patients with
T1N0M0 HCC with low TNF-α and
p38MAPK expression also requires validation in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology for People's Livelihood Project of Qingdao
(no. 18-6-1-89-nsh), the Key Research and Development Plan of
Shandong Province (no. 2018GSF118233) and the Science and
Technology Plan of Qingdao City Shinan District (no.
2018-4-018-YY).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BH, LW and SZ contributed to the study design. MZ
and JH contributed to data analysis. HL and WH contributed to the
collection of the tissue samples and patient data. MZ and JH wrote
the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Qingdao University. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qin H, Du X, Zhang Y and Wang R:
Platycodin D, a triterpenoid saponin from Platycodon grandiflorum,
induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by
modulating the PI3K/Akt pathway. Tumor Biol. 35:1267–1274. 2014.
View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
65:69–90. 2011. View Article : Google Scholar
|
|
3
|
Josep ML: Hepatocellular carcinoma. Lancet
(London, England). 9399:2003.
|
|
4
|
Carr BI: Hepatocellular carcinoma: Current
management and future trends. Gastroenterology. 127:S218–S224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Altekruse SF, McGlynn KA, Dickie LA and
Kleiner DE: Hepatocellular carcinoma confirmation, treatment, and
survival in surveillance, epidemiology, and end results registries,
1992–2008. Hepatology. 55:476–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama T: Surgical treatment for
hepatocellular carcinoma. Japanese J Clin Oncol. 41:447–454. 2011.
View Article : Google Scholar
|
|
8
|
Poon RT: Prevention of recurrence after
resection of hepatocellular carcinoma: A daunting challenge.
Hepatology. 54:757–759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caminero A, Comabella M and Montalban X:
Tumor necrosis factor alpha (TNF-alpha), anti-TNF-alpha and
demyelination revisited: An ongoing story. J Neuroimmunol. 234:1–6.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore RJ, Owens DM, Stamp G, Arnott C,
Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M,
et al: Mice deficient in tumor necrosis factor-alpha are resistant
to skin carcinogenesis. Nat Med. 5:828–831. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joseph WR, Cao Z, Mountjoy KG, Marshall
ES, Baguley BC and Ching LM: Stimulation of tumors to synthesize
tumor necrosis factor-alpha in situ using
5,6-dimethylxanthenone-4-acetic acid: A novel approach to cancer
therapy. Cancer Res. 59:633–638. 1999.PubMed/NCBI
|
|
13
|
Mugami S, Dobkin-Bekman M, Rahamim-Ben
Navi L and Naor Z: Differential roles of PKC isoforms (PKCs) in
GnRH stimulation of MAPK phosphorylation in gonadotrope derived
cells. Mol Cell Endocrinol. 463:97–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokota T and Wang Y: p38 MAP kinases in
the heart. Gene. 575:369–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK That
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meldrum KK, Meldrum DR, Hile KL, Yerkes
EB, Ayala A, Cain MP, Rink RC, Casale AJ and Kaefer MA: p38 MAPK
mediates renal tubular cell TNF-alpha production and
TNF-alpha-dependent apoptosis during simulated ischemia. Am J
Physiol Cell physiol. 281:C563–C570. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valladares A, Alvarez AM, Ventura JJ,
Roncero C, Benito M and Porras A: p38 mitogen-activated protein
kinase mediates tumor necrosis factor-alpha-induced apoptosis in
rat fetal brown adipocytes. Endocrinology. 141:4383–4395. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamarajah SK, Frankel TL, Sonnenday C, Cho
CS and Nathan H: Critical evaluation of the American joint
commission on cancer (AJCC) 8th edition staging system for patients
with hepatocellular carcinoma (HCC): A surveillance, epidemiology,
end results (SEER) analysis. J Surg Oncol. 117:644–650. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Zhang S, Yang Z, Hu J, Hu W, Sun
P, Wu L and Han B: Association between the expression levels of
IL-6 and IL-6R in the hepatocellular carcinoma microenvironment and
postoperative recurrence. Oncol Lett. 16:7158–7165. 2018.PubMed/NCBI
|
|
22
|
Green S, Dobrjansky A and Chiasson MA:
Murine tumor necrosis-inducing factor: Purification and effects on
myelomonocytic leukemia cells. J Natl Cancer Inst. 68:997–1003.
1982.PubMed/NCBI
|
|
23
|
Matthews N and Watkins JF: Tumor-Necrosis
factor from the rabbit. I. Mode of action, specificity and
physicochemical properties. Br J Cancer. 38:302–309. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mose M, Kang Z, Raaby L, Iversen L and
Johansen C: TNFα-and IL-17A-mediated S100A8 expression is regulated
by p38 MAPK. Exp Dermatol. 22:476–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wang W, Wang L, Wang X and Xia J:
Regulatory mechanisms of interleukin-8 production induced by tumour
necrosis factor-α in human hepatocellular carcinoma cells. J Cell
Mol Med. 16:496–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabio G and Davis RJ: TNF and MAP kinase
signaling pathways. Semin Immunol. 26:237–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo JL, Maeda S, Hsu LC, Yagita H and
Karin M: Inhibition of NF-kappaB in cancer cells converts
inflammation-induced tumor growth mediated by TNFalpha to
TRAIL-mediated tumor regression. Cancer Cell. 6:297–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donnahoo KK, Shames BD, Harken AH and
Meldrum DR: Review article: The role of tumor necrosis factor in
renal ischemia-reperfusion injury. J Urol. 162:196–203. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|